Takotsubo Syndrome during COVID-19 Pandemic in the Veneto Region, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Aims of the Study
2.3. Statistical Analysis
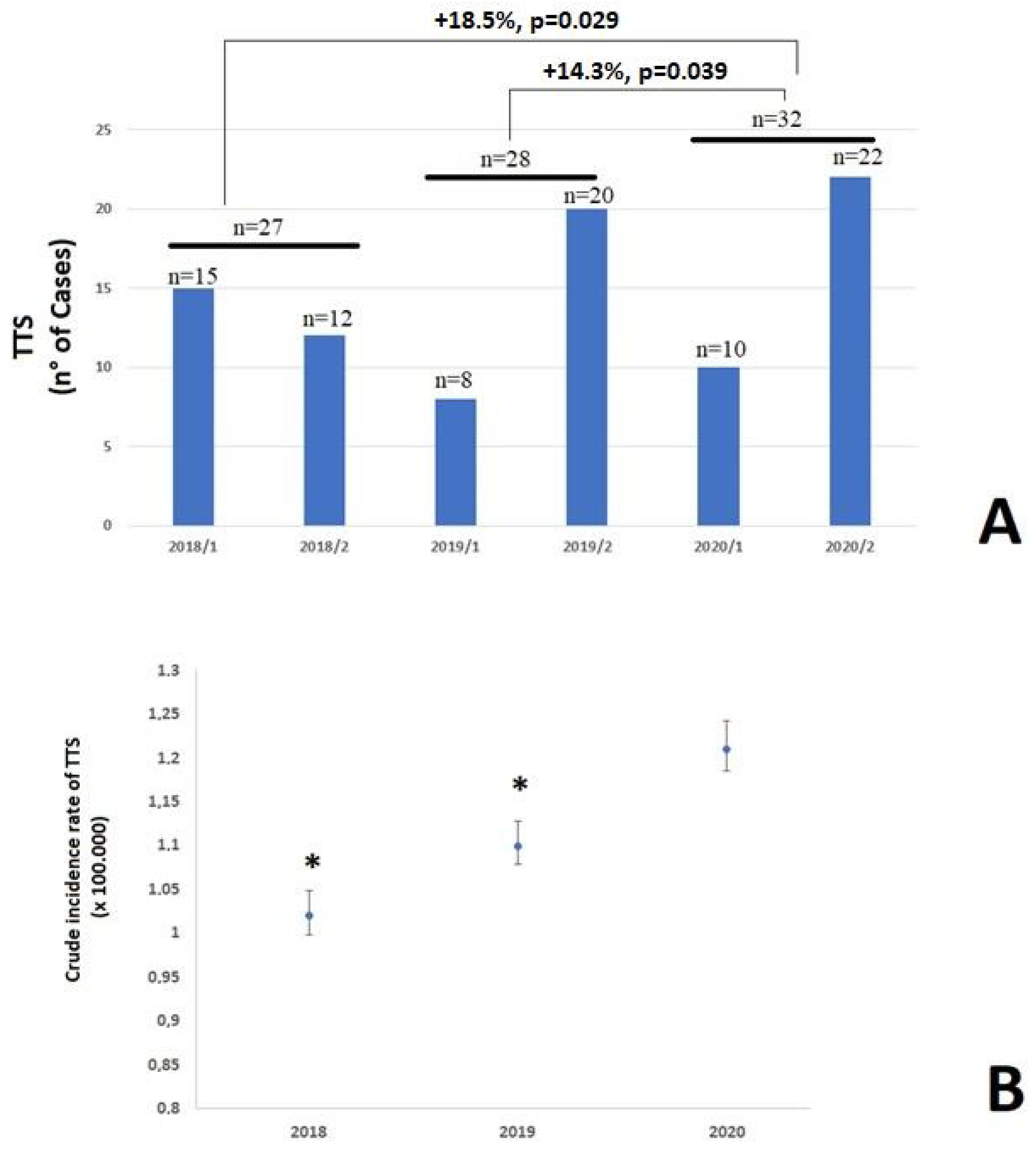
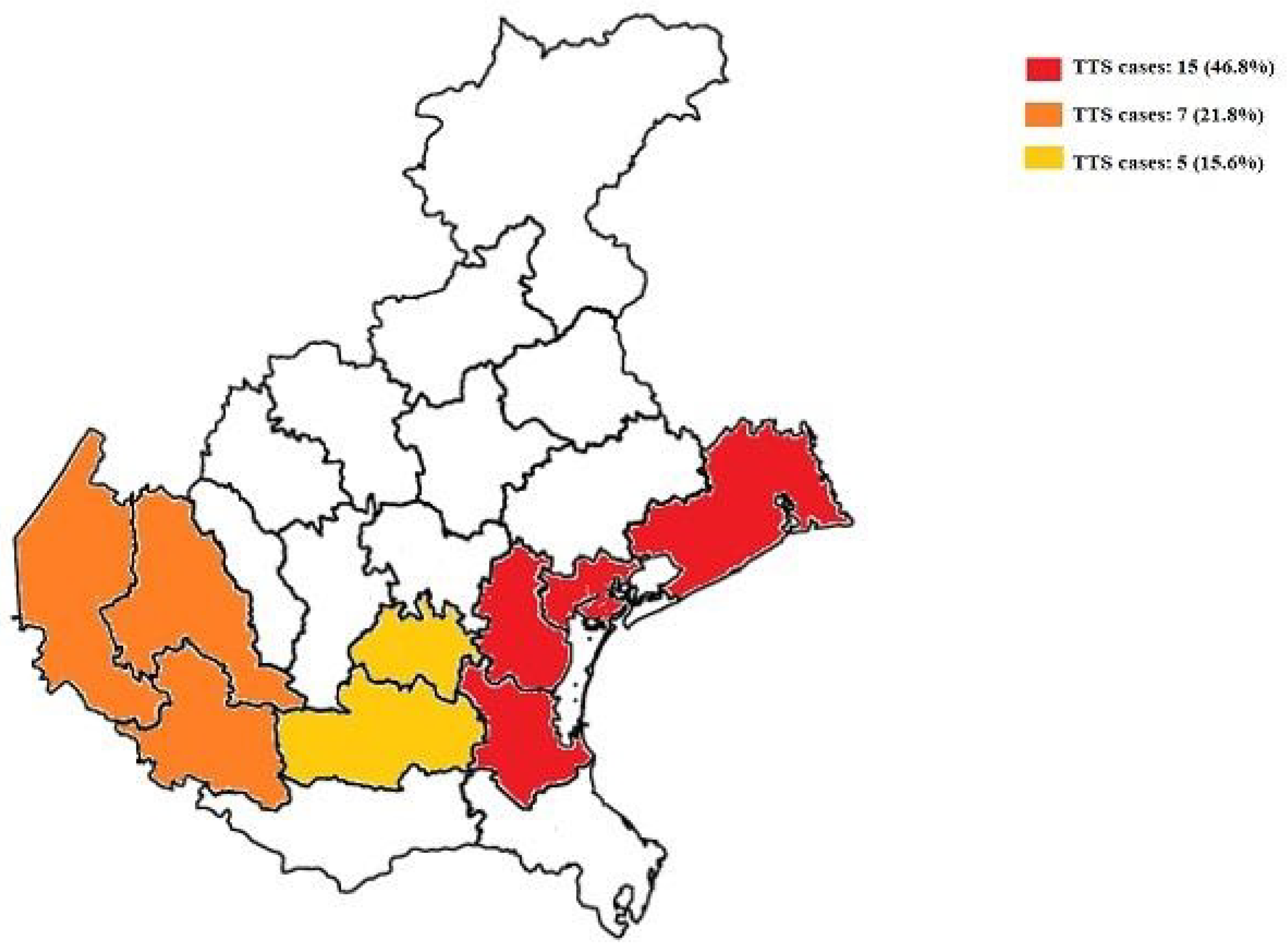
3. Results
3.1. Incidence of TTS
3.2. Clinical Characteristics
3.3. Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Indolfi, C.; Spaccarotella, C. The Outbreak of COVID-19 in Italy: Fighting the Pandemic. JACC Case Rep. 2020, 2, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Jabri, A.; Kalra, A.; Kumar, A.; Alameh, A.; Adroja, S.; Bashir, H.; Nowacki, A.S.; Shah, R.; Khubber, S.; Kanaa’N, A.; et al. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw. Open 2020, 3, e2014780. [Google Scholar] [CrossRef]
- Dabbagh, M.F.; Aurora, L.; D’Souza, P.; Weinmann, A.J.; Bhargava, P.; Basir, M.B. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020, 2, 1326–1330. [Google Scholar] [CrossRef]
- Dolci, G.; Prevedello, F.; Lobascio, I.; Mugnai, G.; Dalla Valle, C.; Bilato, C. Mid-ventricular Takotsubo syndrome ’lockdown’-related during coronavirus disease 2019 outbreak: A case report. J. Cardiovasc. Med. 2021, 22, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. In-ternational Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, G.; Cavedon, S.; Zuin, M.; Roncon, L.; Bilato, C. COVID-19: When more might be less. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2186–2188. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.M.; Shah, M.; Shah, S.; Li, A.; Jauhar, S. Takotsubo Syndrome and COVID-19: Associations and Implications. Curr. Probl. Cardiol. 2021, 46, 100763. [Google Scholar] [CrossRef] [PubMed]
- Migliore, F.; Zorzi, A.; Gregori, D.; Del Monte, A.; Falzone, P.V.; Verlato, R.; Siciliano, M.; Themistoclakis, S.; China, P.; Marchese, D.; et al. Padua School of Cardiology Network. Urgent Pacemaker Implantation Rates in the Veneto Region of Italy After the COVID-19 Outbreak. Circ. Arrhythm. Electrophysiol. 2020, 13, e008722. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Ferrara, P.; Mazzaglia, G.; D’Orso, M.I.; Ciampichini, R.; Fornari, C.; Madotto, F.; Magoni, M.; Sampietro, G.; Silenzi, A.; et al. Magnitude and time-course of excess mortality during COVID-19 out-break: Population-based empirical evidence from highly impacted provinces in north-ern Italy. ERJ Open Res. 2020, 6, 00458-2020. [Google Scholar] [CrossRef] [PubMed]
- Sala, S.; Peretto, G.; Gramegna, M.; Palmisano, A.; Villatore, A.; Vignale, D.; De Cobelli, F.; Tresoldi, M.; Cappelletti, A.M.; Basso, C.; et al. Acute myocarditis pre-senting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020, 41, 1861–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Desai, R.; Gandhi, Z.; Fong, H.K.; Doreswamy, S.; Desai, V.; Chockalingam, A.; Mehta, P.K.; Sachdeva, R.; Kumar, G. Takotsubo Syndrome in Patients with COVID-19: A Systematic Review of Published Cases. SN Compr. Clin. Med. 2020, 2, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabrò, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Società Italiana di Cardiologia and the CCU Academy investigators group. Reduction of hospitaliza-tions for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Region, V.; Zero, A. L’Infarto Miocardico Acuto (IMA) in Vene-to: Anni 2006–2020 [Myocardial infarction in the Veneto Region: An Analysis of Years 2006–2020]. 2021. Available online: https://www.ser-veneto.it/it (accessed on 10 December 2021).
- Kurowski, V.; Kaiser, A.; von Hof, K.; Killermann, D.P.; Mayer, B.; Hartmann, F.; Schunkert, H.; Radke, P.W. Apical and midventricular transient left ventricular dys-function syndrome (tako-tsubo cardiomyopathy): Frequency, mechanisms, and prognosis. Chest 2007, 132, 809–816. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Komiyama, T.; Kobayashi, H.; Ikari, Y. Gender Differences in Takotsubo Syndrome. Biology 2022, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Looi, J.-L.; Lee, M.; Grey, C.; Webster, M.; To, A.; Kerr, A.J. Seasonal variation in Takotsubo syndrome compared with myocardial infarction: ANZACS-QI 16. N. Z. Med. J. 2018, 131, 21–29. [Google Scholar] [PubMed]
- Kanaoka, K.; Okayama, S.; Terasaki, S.; Nakano, T.; Ishii, M.; Nakai, M.; Onoue, K.; Nishimura, K.; Yasuda, S.; Tsujita, K.; et al. Role of climatic factors in the incidence of Takotsubo syndrome: A nationwide study from 2012 to 2016. ESC Heart Fail. 2020, 7, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, C.; Maglietta, G.; Meschi, T.; Ticinesi, A.; Silva, M.; Sverzellati, N. Effects of the COVID-19 Epidemic on Hospital Admissions for Non-Communicable Diseases in a Large Italian University-Hospital: A Descriptive Case-Series Study. J. Clin. Med. 2021, 10, 880. [Google Scholar] [CrossRef] [PubMed]
| Annual Percentage Change (95% CI) | p | |
|---|---|---|
| Overall | +1.75 (1.72–1.78) | 0.006 |
| Men | +1.3 (1.1–1.4) | 0.02 |
| Women | +2.2% (2.0–2.4) | 0.001 |
| Variables | 2020 Cohort N = 32 | Historical Cohort (2018–2019) N = 55 | p |
|---|---|---|---|
| Age (years) | 73.5 ± 11.7 | 69.2 ± 11.1 | 0.941 |
| Age ≥65 years (%) | 27 (84.4) | 39 (70.9) | 0.163 |
| Female, n (%) | 30 (93.8) | 48 (87.3) | 0.332 |
| Arterial hypertension, n (%) | 26 (81.2) | 27 (49.1) | 0.042 |
| Diabetes mellitus, n (%) | 1 (3.1) | 8 (14.5) | 0.032 |
| Dyslipidemia, n (%) | 14 (43.8) | 28 (50.9) | 0.527 |
| TTS triggers | |||
| None | 11 (34.4) | 15 (27.3) | 0.488 |
| Emotional | 14 (43.8) | 19 (34.5) | 0.396 |
| Physical | 6 (18.6) | 17 (30.9) | 0.217 |
| Emotional and physical | 1 (3.1) | 4 (7.3) | 0.422 |
| Positive COVID-19 (at the admission for TTS), n (%) | 3 (9.4) | - | - |
| Presenting symptoms | |||
| None, n (%) | 1 (3.1) | 3 (5.5) | 0.606 |
| Chest pain, n (%) | 24 (75.0) | 38 (69.1) | 0.553 |
| Dyspnea, n (%) | 5 (15.6) | 9 (16.4) | 0.925 |
| Syncope, n (%) | 3 (9.4) | 5 (9.1) | 0.967 |
| ECG Features | |||
| Normal, n (%) | 9 (29.0) | 10 (18.2) | 0.247 |
| ST-segment elevation, n (%) | 10 (32.3) | 20 (36.4) | 0.705 |
| ST-segment depression, n (%) | 2 (6.5) | 4 (7.3) | 0.882 |
| Negative T waves, n (%) | 16 (32.3) | 21 (38.2) | 0.586 |
| Mean length of stay (days) | 5.4 ± 2.3 | 8.5 ± 2.8 | 0.021 |
| Coronary Angiography | |||
| Significant coronary artery disease | 2 (6.2) | 6 (10.9) | 0.464 |
| Troponin T, ng/L | 325 (51–1278) * | 76 (29–586) ** | 0.003 |
| Troponin I, ng/L | 675 (126–3675) § | 345 (38–3276) §§ | 0.002 |
| LVEF (%) | 49.6 ± 10.3 | 49.4 ± 8.7 | 0.918 |
| Arrhythmic events, n (%) | |||
| None, n (%) | 29 (90.3) | 47 (92.2) | 0.766 |
| AF, n (%) | 2 (6.5) | 2 (3.9) | 0.586 |
| NSVT, n (%) | 1 (3.2) | 0 | 0.216 |
| SVT, n (%) | 2 (3.9) | 0 | 0.166 |
| VF, n (%) | 0 | 0 | - |
| 2nd or 3rd AV blocks, n (%) | 0 | 0 | - |
| Cardiac Magnetic Resonance | |||
| Normal, n (%) | 16/21 (76.1) | 43/46 (93.4) | 0.041 |
| Edema, n (%) | 3 (14.2) | 2 (4.3) | 0.152 |
| LGE, n (%) | 2 (9.5) | 1 (2.1) | 0.172 |
| Outcomes | |||
| Re-hospitalization, n (%) | 1 (3.1) | 5 (9.1) | 0.282 |
| In-hospital mortality, n (%) | 0 | 2 (3.6) | 0.296 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuin, M.; Mugnai, G.; Anselmi, M.; Bonapace, S.; Bozzini, P.; Chirillo, F.; Cutolo, A.; Grassi, G.; Mancuso, D.; Meneghin, S.; et al. Takotsubo Syndrome during COVID-19 Pandemic in the Veneto Region, Italy. Viruses 2022, 14, 1971. https://doi.org/10.3390/v14091971
Zuin M, Mugnai G, Anselmi M, Bonapace S, Bozzini P, Chirillo F, Cutolo A, Grassi G, Mancuso D, Meneghin S, et al. Takotsubo Syndrome during COVID-19 Pandemic in the Veneto Region, Italy. Viruses. 2022; 14(9):1971. https://doi.org/10.3390/v14091971
Chicago/Turabian StyleZuin, Marco, Giacomo Mugnai, Maurizio Anselmi, Stefano Bonapace, Paolo Bozzini, Fabio Chirillo, Ada Cutolo, Giuseppe Grassi, Daniela Mancuso, Samuele Meneghin, and et al. 2022. "Takotsubo Syndrome during COVID-19 Pandemic in the Veneto Region, Italy" Viruses 14, no. 9: 1971. https://doi.org/10.3390/v14091971
APA StyleZuin, M., Mugnai, G., Anselmi, M., Bonapace, S., Bozzini, P., Chirillo, F., Cutolo, A., Grassi, G., Mancuso, D., Meneghin, S., Molon, G., Mugnolo, A., Pantano, I., Polo, A., Purita, P., Roncon, L., Saccà, S., Scarpa, D., Tavella, D., ... Bilato, C. (2022). Takotsubo Syndrome during COVID-19 Pandemic in the Veneto Region, Italy. Viruses, 14(9), 1971. https://doi.org/10.3390/v14091971








