Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition and Pre-Processing of Sequence Data
2.2. Computing Genetic Similarity
2.3. Acquisition of Epitope Data
2.4. Selection of Immunogenic Proteins
2.5. Visualization of Protein Crystal Structures
3. Results
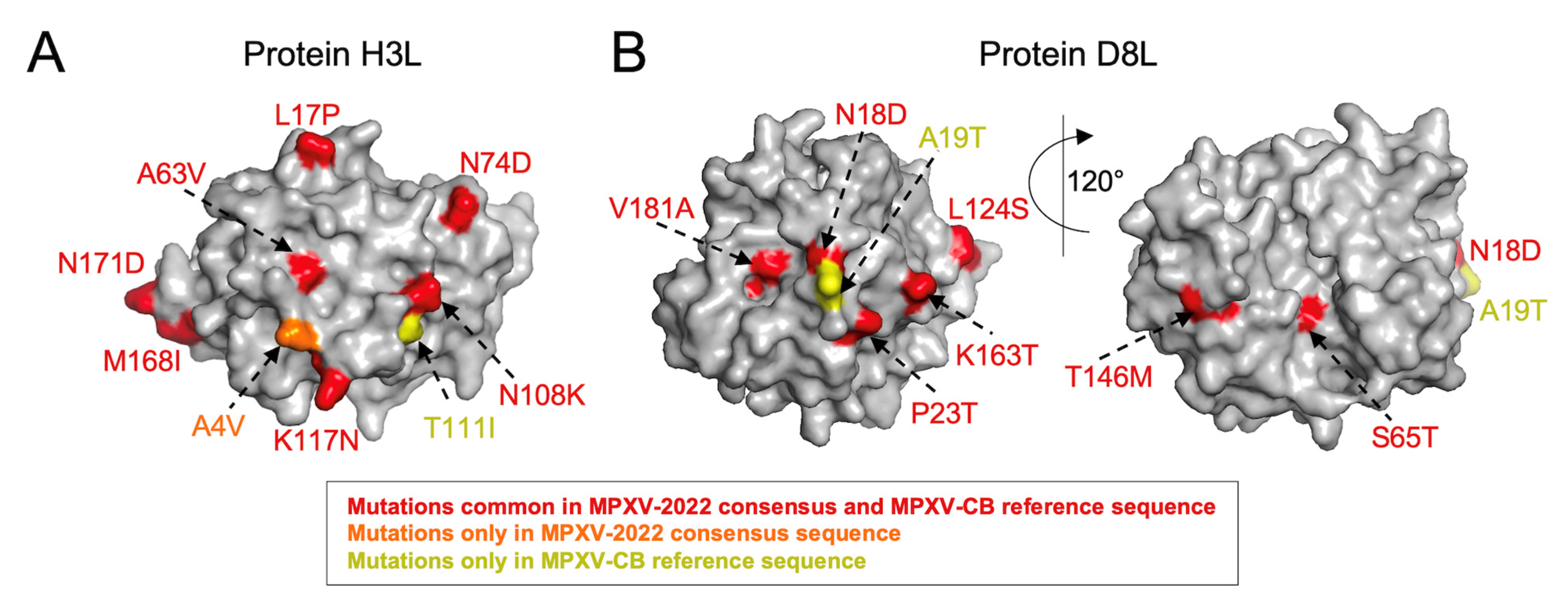
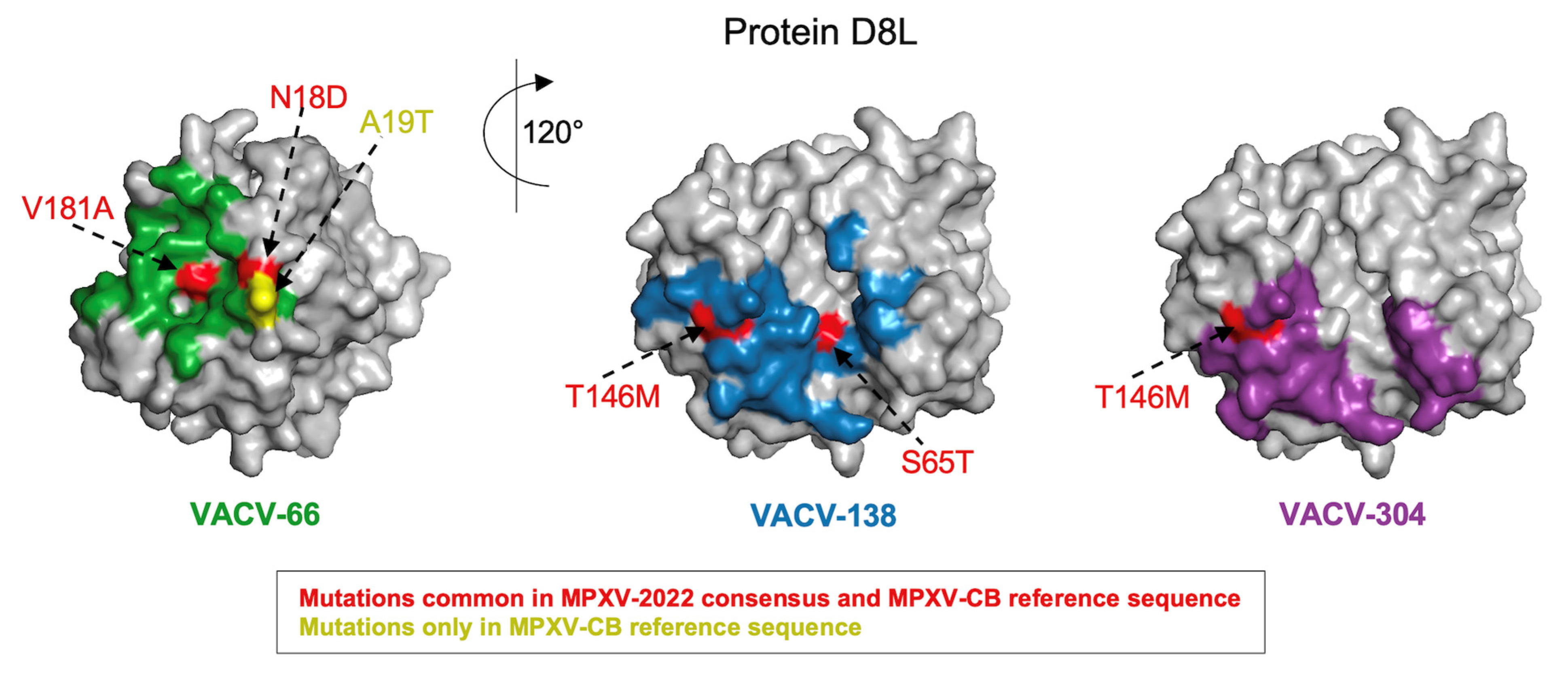
3.1. VACV Proteins Targeted by Neutralizing Antibodies Share High Sequence Similarity with MPXV-2022 Orthologs
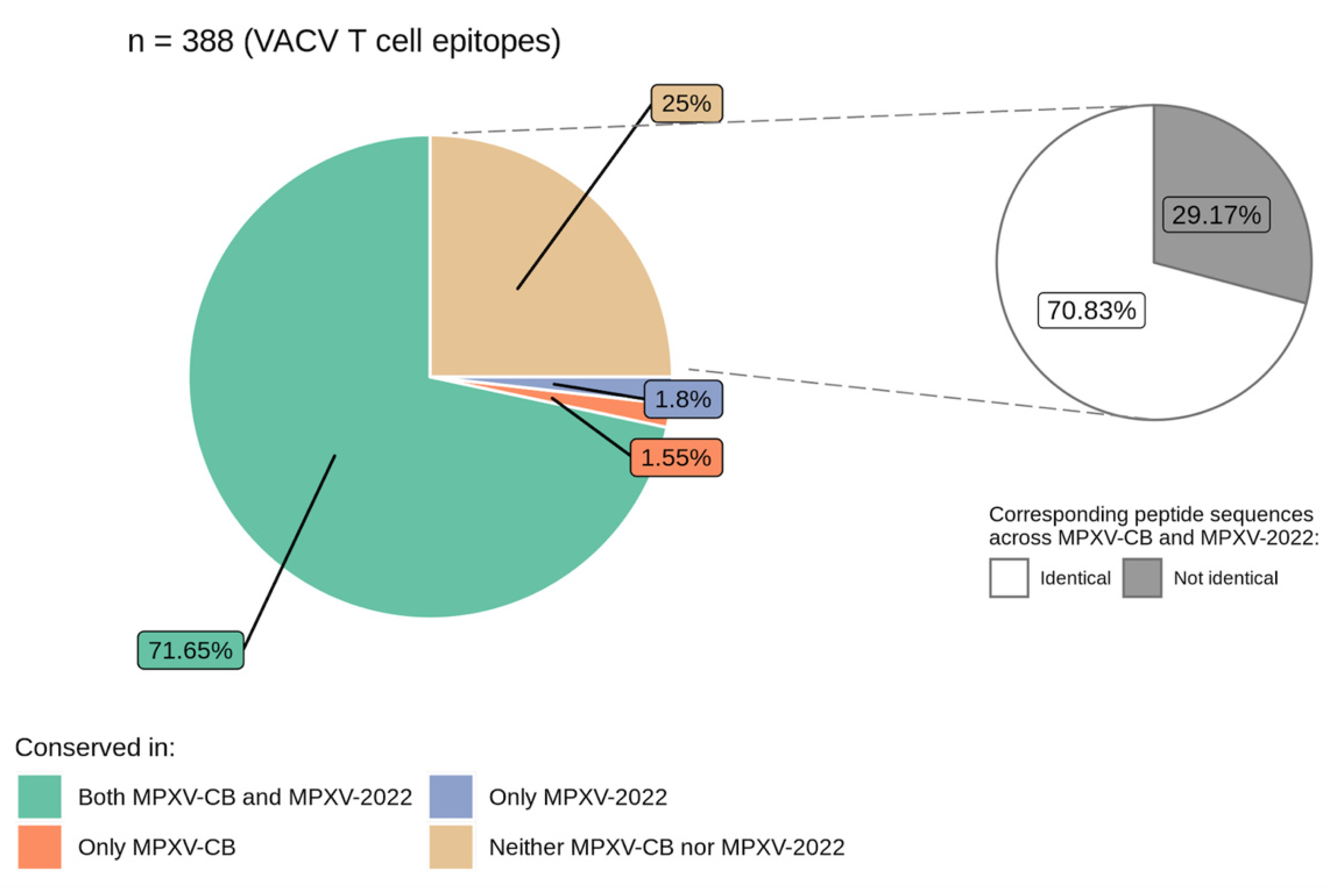
3.2. VACV Proteins and Epitopes Targeted by T Cells Are Largely Conserved in MPXV-2022
4. Limitations of the Study
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, M.G.; Damon, I.K. Outbreaks of Human Monkeypox after Cessation of Smallpox Vaccination. Trends Microbiol. 2012, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Beer, E.M.; Bhargavi Rao, V. A Systematic Review of the Epidemiology of Human Monkeypox Outbreaks and Implications for Outbreak Strategy. PLoS Negl. Trop. Dis. 2019, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.M.; Jezek, Z.; Grab, B.; Dixon, H. The Transmission Potential of Monkeypox Virus in Human Populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Grab, B.; Szczeniowski, M.V.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Secondary Attack Rates. Bull. World Health Organ. 1988, 66, 465–470. [Google Scholar] [PubMed]
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-Emergence of Monkeypox in Africa: A Review of the Past Six Years. Br. Med. Bull. 1998, 54, 693–702. [Google Scholar] [CrossRef]
- Mathieu, E.; Dattani, S.; Ritchie, H.; Roser, M. Monkeypox. Available online: https://ourworldindata.org/monkeypox (accessed on 2 September 2022).
- UK Health Security Agency. Monkeypox Cases Confirmed in England—Latest Updates. Available online: https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates (accessed on 22 June 2022).
- World Health Organization. WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Eeergency of International Concern. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 28 July 2022).
- World Health Organization. Multi-Country Monkeypox Outbreak: Situation Update June 4, 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390 (accessed on 13 June 2022).
- Isidro, J.; Borges, V.; Pinto, M.; Ferreira, R.; Sobral, D.; Nunes, A.; Santos, J.D.; Mixão, V.; Santos, D.; Duarte, S.; et al. Multi-Country Outbreak of Monkeypox Virus: Genetic Divergence and First Signs of Microevolution. Available online: https://virological.org/t/multi-country-outbreak-of-monkeypox-virus-genetic-divergence-and-first-signs-of-microevolution/806 (accessed on 22 June 2022).
- Elbe, S.; Buckland-Merrett, G. Data, Disease and Diplomacy: GISAID’s Innovative Contribution to Global Health. Glob. Chall. 2017, 1, 33–46. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A Tale of Two Clades: Monkeypox Viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update: Multistate Outbreak of Monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003, 52, 561–564. [Google Scholar]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence Differences between Monkeypox Virus Isolates from West Africa and the Congo Basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- World Health Organization. Vaccines and Immunization for Monkeypox: Interim Guidance, 14 June 2022. Available online: https://apps.who.int/iris/handle/10665/356120 (accessed on 27 July 2022).
- Nalca, A.; Zumbrun, E.E. ACAM2000: The New Smallpox Vaccine for United States Strategic National Stockpile. Drug Des. Devel. Ther. 2010, 4, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Considerations for Monkeypox Vaccination. Available online: https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html (accessed on 28 July 2022).
- Kennedy, J.S.; Greenberg, R.N. IMVAMUNE®: Modified Vaccinia Ankara Strain as an Attenuated Smallpox Vaccine. Expert Rev. Vaccines 2009, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Sobek, V.; Ennis, F.A.; Hill, H.; Yan, L.K.; Chaplin, P.; Vollmar, J.; Chaitman, B.R.; et al. Clinical and Immunologic Responses to Multiple Doses of IMVAMUNE® (Modified Vaccinia Ankara) Followed by Dryvax® Challenge. Vaccine 2007, 25, 8562–8573. [Google Scholar] [CrossRef] [PubMed]
- Damon, I.K.; Davidson, W.B.; Hughes, C.M.; Olson, V.A.; Smith, S.K.; Holman, R.C.; Frey, S.E.; Newman, F.; Belshe, R.B.; Yan, L.; et al. Evaluation of Smallpox Vaccines Using Variola Neutralization. J. Gen. Virol. 2009, 90, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Ennis, F.; Abate, G.; Hoft, D.F.; Monath, T.P. Comparison of the Safety and Immunogenicity of ACAM1000, ACAM2000 and Dryvax® in Healthy Vaccinia-Naive Adults. Vaccine 2009, 27, 1637–1644. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Overton, E.T.; Lawrence, S.J.; Stapleton, J.T.; Weidenthaler, H.; Schmidt, D.; Koenen, B.; Silbernagl, G.; Nopora, K.; Chaplin, P. A Randomized Phase II Trial to Compare Safety and Immunogenicity of the MVA-BN Smallpox Vaccine at Various Doses in Adults with a History of AIDS. Vaccine 2020, 38, 2600–2607. [Google Scholar] [CrossRef]
- Jezek, Z.; Marennikova, S.S.; Mutumbo, M.; Nakano, J.H.; Paluku, K.M.; Szczeniowski, M. Human Monkeypox: A Study of 2,510 Contacts of 214 Patients. J. Infect. Dis. 1986, 154, 551–555. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; van Amerongen, G.; Kondova, I.; Kuiken, T.; van Lavieren, R.F.; Pistoor, F.H.M.; Niesters, H.G.M.; van Doornum, G.; van der Zeijst, B.A.M.; Mateo, L.; et al. Modified Vaccinia Virus Ankara Protects Macaques against Respiratory Challenge with Monkeypox Virus. J. Virol. 2005, 79, 7845–7851. [Google Scholar] [CrossRef]
- Hatch, G.J.; Graham, V.A.; Bewley, K.R.; Tree, J.A.; Dennis, M.; Taylor, I.; Funnell, S.G.P.; Bate, S.R.; Steeds, K.; Tipton, T.; et al. Assessment of the Protective Effect of Imvamune and ACAM2000 Vaccines against Aerosolized Monkeypox Virus in Cynomolgus Macaques. J. Virol. 2013, 87, 7805–7815. [Google Scholar] [CrossRef]
- Demkowicz, W.E.; Littaua, R.A.; Wang, J.; Ennis, F.A. Human Cytotoxic T-Cell Memory: Long-Lived Responses to Vaccinia Virus. J. Virol. 1996, 70, 2627–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Murtadha, M.; Schnell, M.; Eisenlohr, L.C.; Hooper, J.; Flomenberg, P. Human T-Cell Responses to Vaccinia Virus Envelope Proteins. J. Virol. 2006, 80, 10010–10020. [Google Scholar] [CrossRef] [PubMed]
- Precopio, M.L.; Betts, M.R.; Parrino, J.; Price, D.A.; Gostick, E.; Ambrozak, D.R.; Asher, T.E.; Douek, D.C.; Harari, A.; Pantaleo, G.; et al. Immunization with Vaccinia Virus Induces Polyfunctional and Phenotypically Distinctive CD8+ T Cell Responses. J. Exp. Med. 2007, 204, 1405–1416. [Google Scholar] [CrossRef]
- Quadeer, A.A.; Barton, J.P.; Chakraborty, A.K.; McKay, M.R. Deconvolving Mutational Patterns of Poliovirus Outbreaks Reveals Its Intrinsic Fitness Landscape. Nat. Commun. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.S.; Louie, R.H.Y.; McKay, M.R.; Barton, J.P. MPL Resolves Genetic Linkage in Fitness Inference from Complex Evolutionary Histories. Nat. Biotechnol. 2021, 39, 472–479. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 Update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Sohail, M.S.; Quadeer, A.A.; McKay, M.R. Identification of Potential SARS-CoV-2 CD8+ T Cell Escape Mutants. Vaccines 2022, 10, 542. [Google Scholar] [CrossRef]
- Matho, M.H.; Schlossman, A.; Gilchuk, I.M.; Miller, G.; Mikulski, Z.; Hupfer, M.; Wang, J.; Bitra, A.; Meng, X.; Xiang, Y.; et al. Structure–Function Characterization of Three Human Antibodies Targeting the Vaccinia Virus Adhesion Molecule D8. J. Biol. Chem. 2018, 293, 390–401. [Google Scholar] [CrossRef]
- Moss, B. Smallpox Vaccines: Targets of Protective Immunity. Immunol. Rev. 2011, 239, 8–26. [Google Scholar] [CrossRef]
- Shchelkunov, S.; Shchelkunova, G.A. Genes That Control Vaccinia Virus Immunogenicity. Acta Nat. 2020, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fogg, C.; Lustig, S.; Whitbeck, J.C.; Eisenberg, R.J.; Cohen, G.H.; Moss, B. Protective Immunity to Vaccinia Virus Induced by Vaccination with Multiple Recombinant Outer Membrane Proteins of Intracellular and Extracellular Virions. J. Virol. 2004, 78, 10230–10237. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; Wilson, R.L.; Kirkwood-Watts, D.L.; King, D.S.; Warren, T.K.; Lund, S.A.; Brown, L.L.; Krupkin, A.K.; VanderMay, E.; Weimers, W.; et al. Vaccination of BALB/c Mice with Escherichia Coli-Expressed Vaccinia Virus Proteins A27L, B5R, and D8L Protects Mice from Lethal Vaccinia Virus Challenge. J. Virol. 2008, 82, 3517–3529. [Google Scholar] [CrossRef]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA Vaccine Protects Nonhuman Primates against Lethal Monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Heraud, J.-M.; Edghill-Smith, Y.; Ayala, V.; Kalisz, I.; Parrino, J.; Kalyanaraman, V.S.; Manischewitz, J.; King, L.R.; Hryniewicz, A.; Trindade, C.J.; et al. Subunit Recombinant Vaccine Protects against Monkeypox. J. Immunol. 2006, 177, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Aldaz-Carroll, L.; Ortiz, A.M.; Whitbeck, J.C.; Alexander, E.; Lou, H.; Davis, H.L.; Braciale, T.J.; Eisenberg, R.J.; Cohen, G.H.; et al. A Protein-Based Smallpox Vaccine Protects Mice from Vaccinia and Ectromelia Virus Challenges When given as a Prime and Single Boost. Vaccine 2007, 25, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.R.; Goudsmit, J.; Holterman, L.; Ewald, B.A.; Denholtz, M.; Devoy, C.; Giri, A.; Grandpre, L.E.; Heraud, J.-M.; Franchini, G.; et al. Differential Antigen Requirements for Protection against Systemic and Intranasal Vaccinia Virus Challenges in Mice. J. Virol. 2008, 82, 6829–6837. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the Monkeypox Virus Genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. COVIDep: A Web-Based Platform for Real-Time Reporting of Vaccine Target Recommendations for SARS-CoV-2. Nat. Protoc. 2020, 15, 2141–2142. [Google Scholar] [CrossRef]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika Virus Epitopes Reveals Immunodominant and Protective Roles for Dengue Virus Cross-Reactive CD8+ T Cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; Barton, J.P.; McKay, M.R. Cross-Serotypically Conserved Epitope Recommendations for a Universal T Cell-Based Dengue Vaccine. PLoS Negl. Trop. Dis. 2020, 14, e0008676. [Google Scholar] [CrossRef] [PubMed]
- Poran, A.; Harjanto, D.; Malloy, M.; Arieta, C.M.; Rothenberg, D.A.; Lenkala, D.; van Buuren, M.M.; Addona, T.A.; Rooney, M.S.; Srinivasan, L.; et al. Sequence-Based Prediction of SARS-CoV-2 Vaccine Targets Using a Mass Spectrometry-Based Bioinformatics Predictor Identifies Immunogenic T Cell Epitopes. Genome Med. 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.; Kitchen, A.; Shapiro, B.; Suchard, M.A.; Holmes, E.C.; Rambaut, A. Using Time-Structured Data to Estimate Evolutionary Rates of Double-Stranded DNA Viruses. Mol. Biol. Evol. 2010, 27, 2038–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. Initial Observations about Putative APOBEC3 Deaminase Editing Driving Short-Term Evolution of MPXV since 2017. Available online: https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830 (accessed on 22 June 2022).
| No. | VACV Protein | Protein Length (VACV) | PDB ID of 3D Structures | Protein Function [46] | MPXV Protein | Similarity (%) | |
|---|---|---|---|---|---|---|---|
| MPXV-CB (Reference Sequence) | MPXV-2022 (Consensus Sequence) | ||||||
| 1 | A17L | 203 | - | IMV surface membrane protein, early function in virion morphogenesis | A18 | 97.54% | 98.03% |
| 2 | A27L | 110 | 3VOP | IMV surface membrane 14-kDa fusion protein, binds cell surface heparan | A29 | 94.55% | 94.55% |
| 3 | A28L | 146 | - | - | A30 | 96.58% | 96.57% |
| 4 | A33R | 185 | 3K7B | EEV envelope glycoprotein, needed for formation of actin-containing microvilli and cell-to-cell spread | A35 | 96.22% | 95.48% |
| 5 | B5R | 317 | - | Palmitylated 42-kDa EEV glycoprotein required for efficient cell spread, complement control | B6 | 96.53% | 96.53% |
| 6 | D8L | 304 | 4E9O | IMV surface membrane 32 kDa protein, binds cell surface chondroitin sulfate, IMV adsorption to cell surface | E8 | 94.08% | 94.41% |
| 7 | L1R | 250 | 1YPY | Myristylated IMV surface membrane protein | M1 | 98.40% | 98.40% |
| 8 | H3L | 324 | 5EJ0 | IMV heparan-binding surface membrane protein | H3 | 93.83% | 93.83% |
| No. | VACV Protein | Length | Substitutions | ||
|---|---|---|---|---|---|
| Only in MPXV-CB | Only in MPXV-2022 | Common among MPXV-CB and MPXV-2022 | |||
| 1 | A17L | 203 | T183I | - | Y155F, R165K, T171P, V188I |
| 2 | A27L | 110 | - | - | K27N, A30T, D39Y, E40G, V61I, R74H |
| 3 | A28L | 146 | - | - | G23S, Q110R, V130I, V131A, A137T |
| 4 | A33R | 177 | L59Q | E67K, A88V | A73S, Q117K, L118S, S120E, T127A, I141T |
| 5 | B5R | 317 | - | - | Q50S, S55L, I82V, N87D, A166V, M188I, V233I, I236T, T240S, V283M, V296I |
| 6 | D8L | 304 | A19T | - | N18D, P23T, S65T, L66I, L114I, S118A, L124S, T146M, K163T, V181A, D209E, A246V, R253K, T261A, E272G, F293L, R296Q |
| 7 | L1R | 250 | - | - | L51I, K177R, V242I, M248I |
| 8 | H3L | 324 | T111I | A4V | L17P, P44Q, N48D, V51I, K53N, A63V, N74D, N108K, K117N, V124I, M143I, M168I, N171D, L230M, A233S, N251T, A263V, T265A, A274T |
| VACV Protein | Similarity (%) | ||
|---|---|---|---|
| MVA-BN | ACAM2000 | Dryvax | |
| A17L | 99.0 | 99.5 | 99.5 |
| A27L | 99.1 | 100.0 | 100.0 |
| A28L | 99.3 | 100.0 | 100.0 |
| A33R | 98.4 | 100.0 | 100.0 |
| B5R | 97.2 | 96.5 | 97.2 |
| D8L | 98.4 | 98.7 | 98.7 |
| H3L | 98.8 | 99.1 | 99.1 |
| LIR | 99.6 | 99.6 | 99.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.F.; Sohail, M.S.; Quadeer, A.A.; McKay, M.R. Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses 2022, 14, 1960. https://doi.org/10.3390/v14091960
Ahmed SF, Sohail MS, Quadeer AA, McKay MR. Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses. 2022; 14(9):1960. https://doi.org/10.3390/v14091960
Chicago/Turabian StyleAhmed, Syed Faraz, Muhammad Saqib Sohail, Ahmed Abdul Quadeer, and Matthew R. McKay. 2022. "Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus" Viruses 14, no. 9: 1960. https://doi.org/10.3390/v14091960
APA StyleAhmed, S. F., Sohail, M. S., Quadeer, A. A., & McKay, M. R. (2022). Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses, 14(9), 1960. https://doi.org/10.3390/v14091960








