Roles of Ambient Temperature and PM2.5 on Childhood Acute Bronchitis and Bronchiolitis from Viral Infection
Abstract
:1. Introduction
2. Methods and Materials
2.1. Data Source and Study Population
2.2. Environmental Condition Data
2.3. Statistical Analysis
3. Results
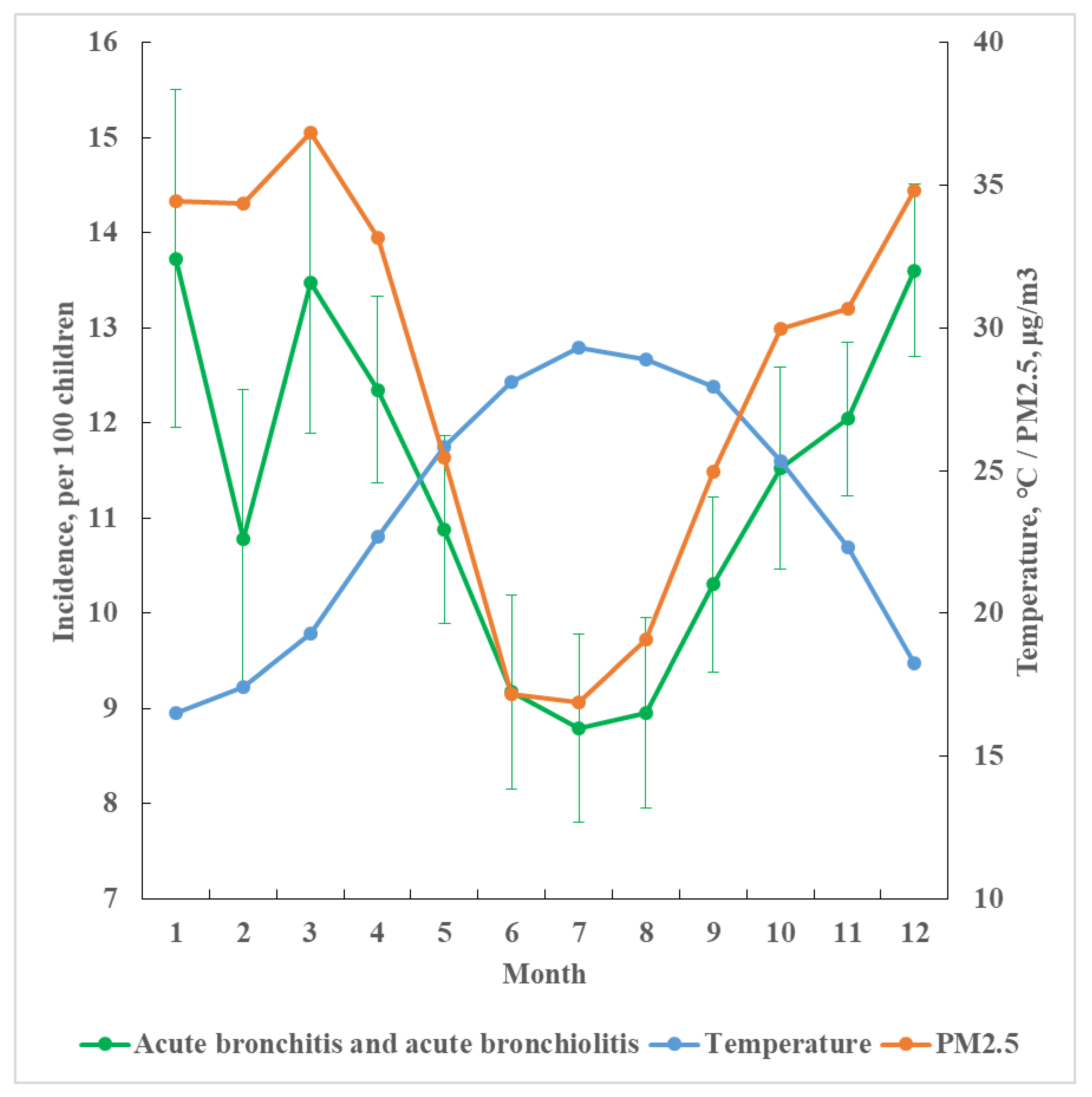
3.1. Monthly Incidence by Temperature and PM2.5 Levels and Daily Cases by Age, Temperature, and Sex
| Bronchitis | Bronchiolitis | Both | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boy | Girl | p | Boy | Girl | p | Boy | Girl | p | |||
| Age | Temperature | Days | n/Day | n/Day | n/Day | n/Day | n/Day | n/Day | |||
| <2 | <20 °C | 1017 | 46 | 36 | 0.0006 | 25 | 19 | 0.0002 | 71 | 55 | 0.0004 |
| 20–24 | 1036 | 47 | 37 | 23 | 18 | 70 | 55 | ||||
| 25–29 | 1654 | 41 | 32 | 20 | 15 | 61 | 47 | ||||
| 30+ | 249 | 43 | 34 | 22 | 16 | 65 | 50 | ||||
| 3–5 | <20 °C | 1017 | 104 | 88 | 0.0009 | 17 | 15 | 0.0002 | 121 | 103 | 0.0008 |
| 20–24 | 1036 | 96 | 82 | 15 | 13 | 111 | 95 | ||||
| 25–29 | 1654 | 82 | 69 | 13 | 11 | 95 | 80 | ||||
| 30+ | 249 | 84 | 71 | 13 | 11 | 97 | 82 | ||||
| 6–12 | <20 °C | 1017 | 145 | 124 | 0.0002 | 17 | 15 | 0.0004 | 162 | 139 | 0.0002 |
| 20–24 | 1036 | 120 | 101 | 14 | 12 | 134 | 113 | ||||
| 25–29 | 1654 | 90 | 75 | 11 | 9 | 101 | 84 | ||||
| 30+ | 249 | 89 | 74 | 11 | 9 | 100 | 83 | ||||
| All | <20 °C | 1017 | 295 | 249 | 0.0002 | 60 | 48 | 0.0003 | 355 | 297 | 0.0002 |
| 20–24 | 1036 | 263 | 220 | 53 | 43 | 316 | 263 | ||||
| 25–29 | 1654 | 213 | 177 | 44 | 35 | 257 | 212 | ||||
| 30+ | 249 | 216 | 179 | 46 | 37 | 261 | 216 | ||||
| Total | 3956 * | 987 | 825 | 203 | 163 | 1190 | 988 | ||||
3.2. Temperature, PM2.5, and Income Specific Daily Incident Cases
3.3. Daily Incidence and Risk by PM2.5 Level in Each Temperature Stratum
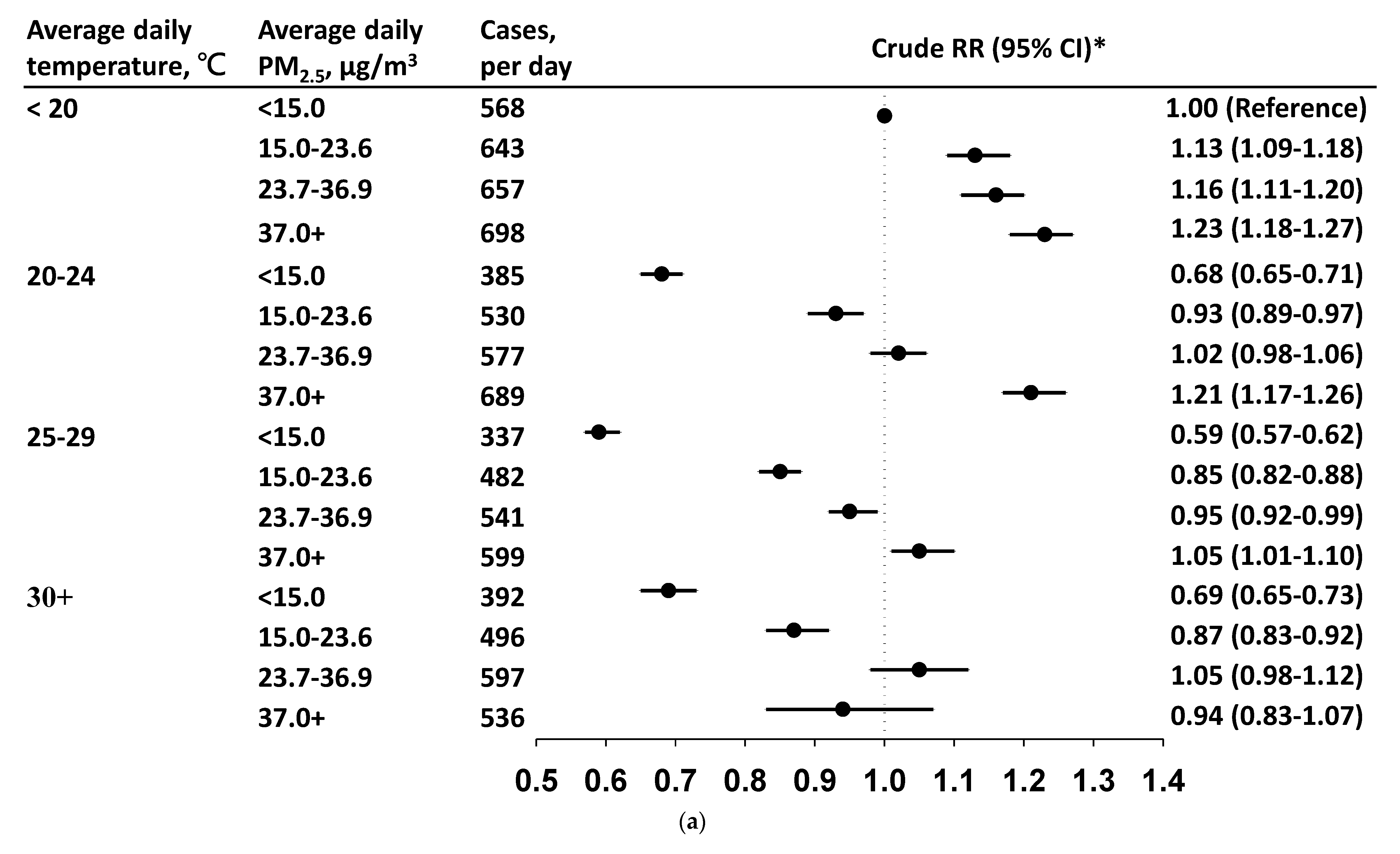
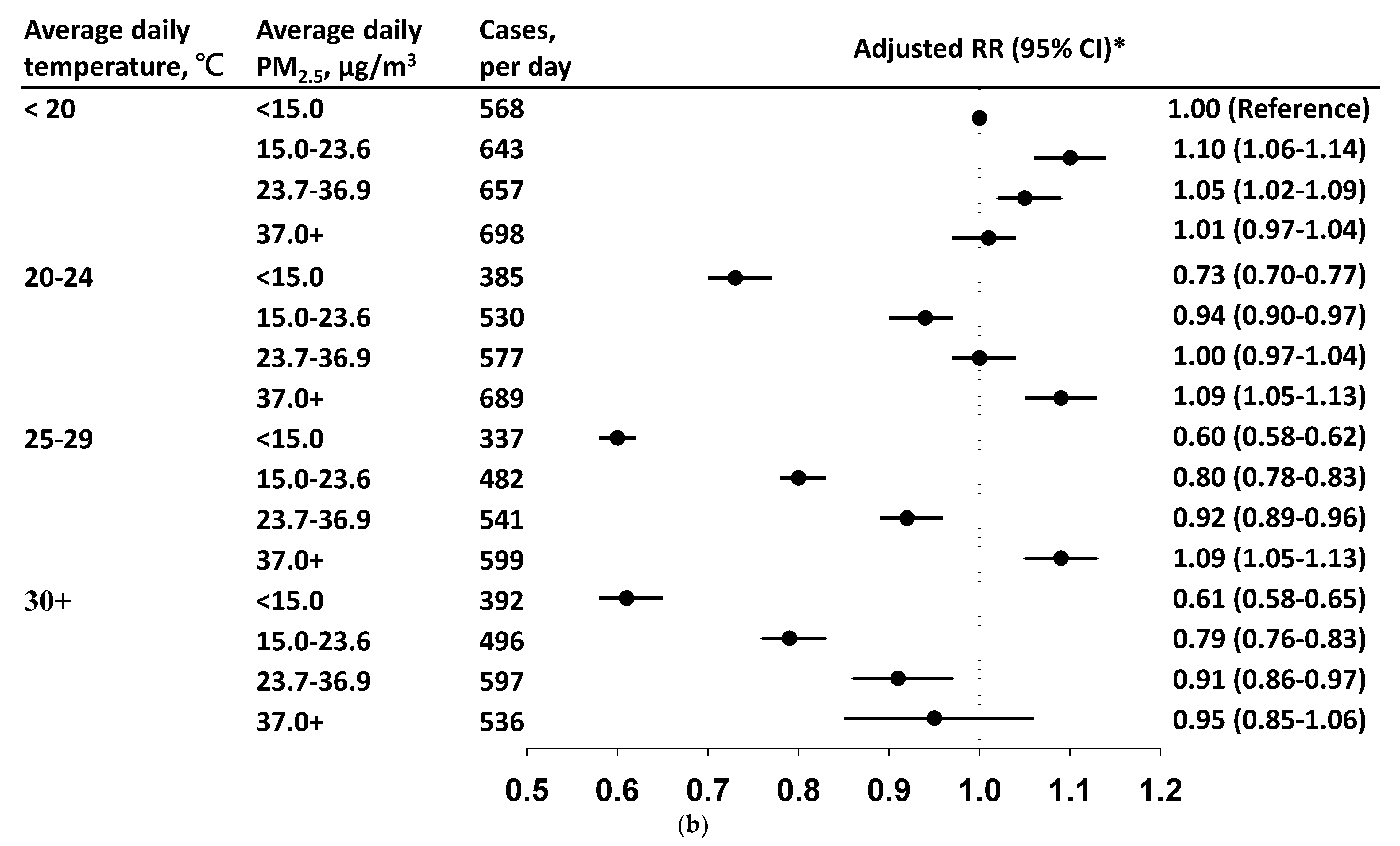
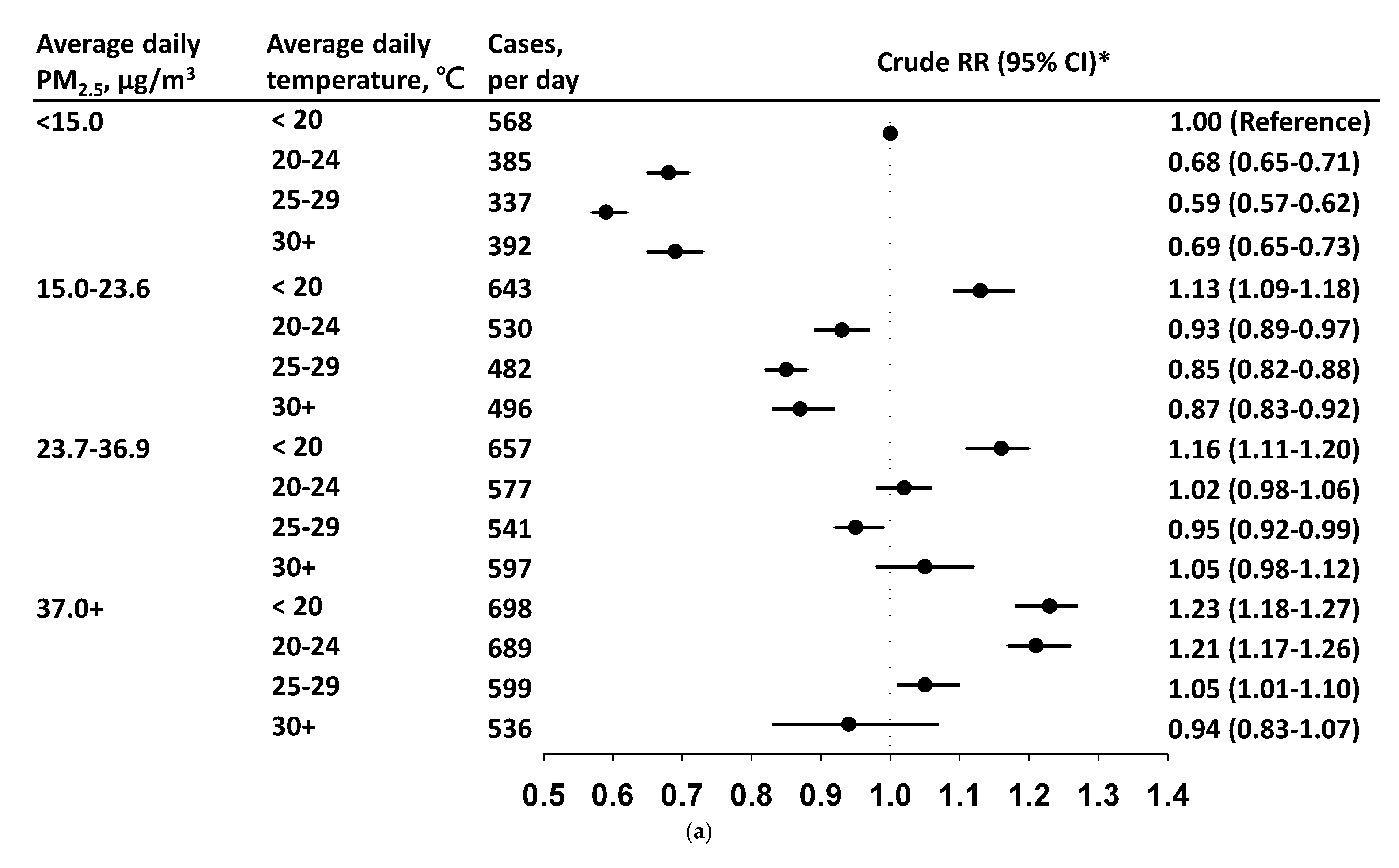
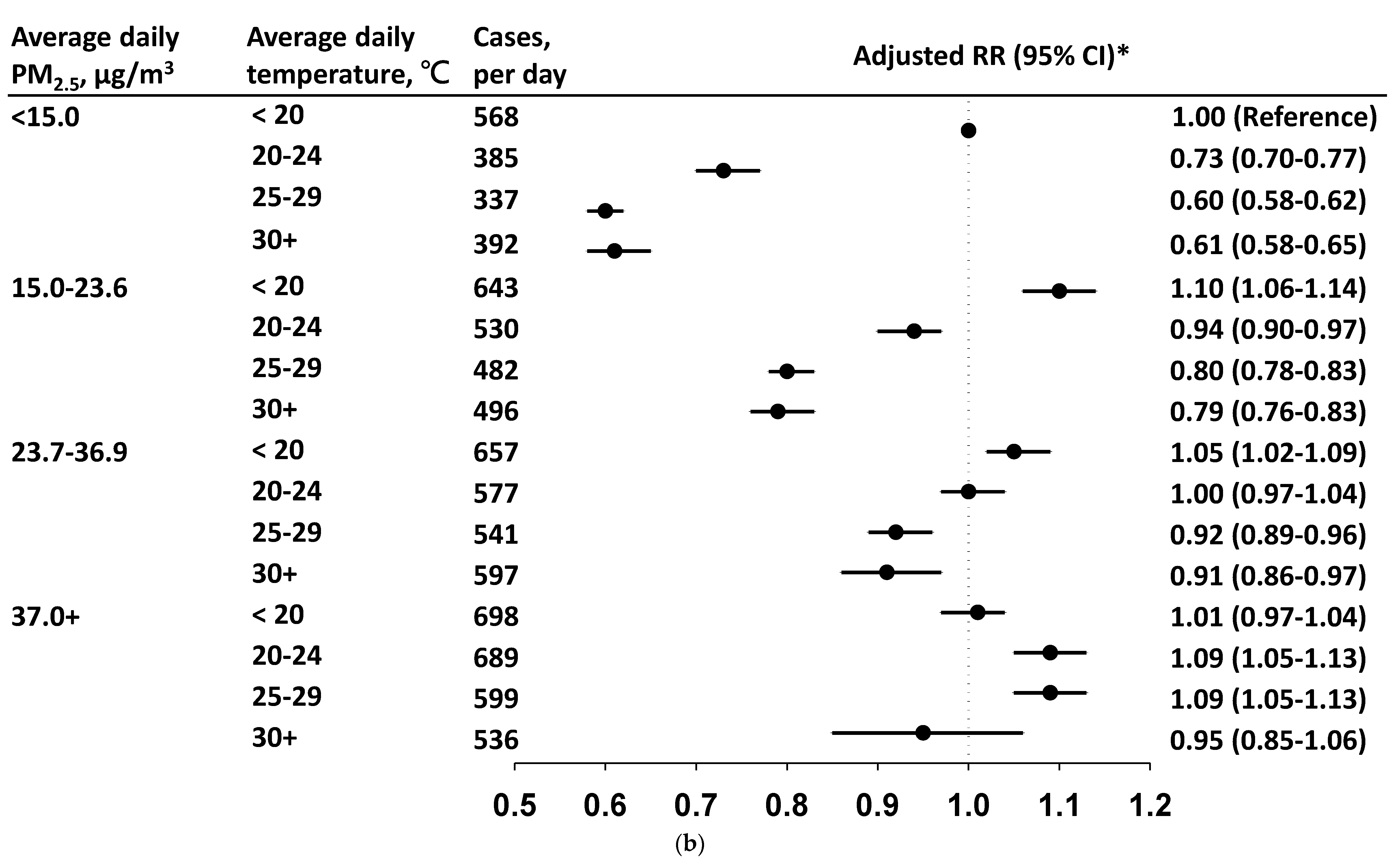
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aRR | adjusted relative risk |
| CABs | childhood acute bronchitis and bronchiolitis |
| CI | confidence interval |
| cRR | crude relative risk |
| EPA | Environmental Protection Administration |
| ICD-9-CM/ICD-10-CM | International Classification of Diseases, 9th/10th Revisions, Clinical Modification |
| O3 | ozone |
| PM2.5 | particulate matter of ≤2.5 µm |
| RR | relative risk |
| RSV | respiratory syncytial virus |
| SO2 | sulfur dioxide |
References
- Fleming, D.M.; Pannell, R.S.; Elliot, A.J.; Cross, K.W. Respiratory illness associated with influenza and respiratory syncytial virus infection. Arch. Dis. Child. 2005, 90, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.C. Viral bronchiolitis in children. N. Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- Rose, E.B.; Wheatley, A.; Langley, G.; Gerber, S.; Haynes, A. Respiratory Syncytial Virus Seasonality—United States, 2014–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Avendano Carvajal, L.; Perret Perez, P. Epidemiology of Respiratory Infections. In Pediatric Respiratory Diseases—A Comprehensive Textbook; Bertrand, P.J., Sánchez, I., Eds.; Springer: Cham, Switzerland, 2020; pp. 264–273. [Google Scholar] [CrossRef]
- Perez, M.; Piedmonte, G. Bronchiolitis. In Pediatric Respiratory Diseases—A Comprehensive Textbook; Bertrand, P.J., Sánchez, I., Eds.; Springer: Cham, Switzerland, 2020; pp. 283–297. [Google Scholar]
- Sato, M.; Saito, R.; Sakai, T.; Sano, Y.; Nishikawa, M.; Sasaki, A. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J. Clin. Microbiol. 2005, 43, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Hervás, D.; Reina, J.; Hervás, J.A. Meteorologic conditions and respiratory syncytial virus activity. Pediatr. Infect. Dis. J. 2012, 31, e176–e181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Shao, X.J.; Wang, J.; Guo, W.L. Temporal characteristics of respiratory syncytial virus infection in children and its correlation with climatic factors at a public pediatric hospital in Suzhou. J. Clin. Virol. 2013, 58, 666–670. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Zhang, X.; Zheng, M.; Kyaw, M.H. Burden of respiratory syncytial virus infections in China: Systematic review and meta-analysis. J. Glob. Health. 2015, 5, 020417. [Google Scholar] [CrossRef]
- Lopes, G.P.; Amorim, Í.P.S.; de Oliveira de Melo, B.; Maramaldo, C.E.C.; Bomfim, M.R.Q.; Neto, L.G.L.; Alves, M.S.; Silva, F.B.; Soeiro-Pereira, P.V.; Falcai, A. Identification and seasonality of rhinovirus and respiratory syncytial virus in asthmatic children in tropical climate. Biosci. Rep. 2020, 40, BSR20200634. [Google Scholar] [CrossRef]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E.; Strickland, M.J. Air pollution and acute respiratory infections among children 0–4 years of age: An 18-year time-series study. Am. J. Epidemiol. 2014, 180, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Picciotto, I.; Baker, R.J.; Yap, P.-S.; Dostál, M.; Joad, J.P.; Lipsett, M.; Greenfield, T.; Herr, C.E.; Beneš, I.; Shumway, R.H.; et al. Early childhood lower respiratory illness and air pollution. Environ. Health Perspect. 2007, 115, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Liu, Q.; Li, W.; Yang, B.; Chen, Y.; Lin, W.; Zhang, J.J. Changes in children’s respiratory morbidity and residential exposure factors over 25 years in Chongqing, China. J. Thorac. Dis. 2020, 12, 6356–6364. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Su, X.; Zhao, D.; Zhang, Y.; Cheng, Q.; Zhang, H.; Wang, S.; Xie, M.; Su, H. Exposure to traffic-related air pollution and acute bronchitis in children: Season and age as modifiers. J. Epidemiol. Community Health 2018, 72, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.Y.; Lau, S.F.; Kwok, K.L.; Mohammad, K.N.; Chan, P.K.S.; Chong, K.C. Short-term association among meteorological variation, outdoor air pollution and acute bronchiolitis in children in a subtropical setting. Thorax 2021, 76, 360–369. [Google Scholar] [CrossRef]
- Cheng, J.; Su, H.; Xu, Z. Intraday effects of outdoor air pollution on acute upper and lower respiratory infections in Australian children. Environ. Pollut. 2021, 268 Pt A, 115698. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Wu, P.J.; Cheng, I.J.; Mou, C.H.; Lan, Y.C.; Hsieh, D.P.H.; Sung, F.C. Spatiotemporal trend of fine particulate matter 2.5 μm in Taiwan from 2006-2016. Taiwan J. Public Health 2018, 37, 686–695. [Google Scholar]
- Meng, C.; Cheng, T.; Gu, X.; Hu, S.; Yuan, L.; Liu, Y.; Bao, F. Contribution of meteorological factors to particulate pollution during winters in Beijing. Sci. Total Environ. 2019, 656, 977–985. [Google Scholar] [CrossRef]
- Vecchi, R.; Marcazzan, G.; Valli, G.; Ceriani, M.; Antoniazzi, C. The role of atmospheric dispersion in the seasonal variation of PM1 and PM2.5 concentration and composition in the urban area of Milan (Italy). Atmos. Environ. 2004, 38, 4437–4446. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, M.; Lv, J.; Shi, T.; Liu, P.; Wu, Y.; Feng, W.; He, W.; Guo, P. Interactions between ambient air pollutants and temperature on emergency department visits: Analysis of varying-coefficient model in Guangzhou, China. Sci. Total Environ. 2019, 668, 825–834. [Google Scholar] [CrossRef]
- Ren, C.; O’Neill, M.S.; Park, S.K.; Sparrow, D.; Vokonas, P.; Schwartz, J. Ambient temperature, pollution, and heart rate variability in an aging population. Am. J. Epidemiol. 2011, 173, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Stafoggia, M.; Schwartz, J.; Forastiere, F.; Perucci, C.A.; SISTI Group. Does temperature modify the association between air pollution and mortality? A multicity case-crossover analysis in Italy. Am. J. Epidemiol. 2008, 167, 1476–1485. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, Y.K.; Chen, Y.J.; Hung, S.C.; Zafirah, Y.; Sung, F.C. Ambulance services associated with extreme temperatures and fine particles in a subtropical island. Sci. Rep. 2020, 10, 2855. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, Y.K.; Chuang, C.Y.; Li, M.H.; Chou, C.H.; Liao, C.H.; Sung, F.C. Associating emergency room visits with first and prolonged extreme temperature event in Taiwan: A population-based cohort study. Sci. Total Environ. 2012, 416, 97–104. [Google Scholar] [CrossRef]
- Chu, H.Y.; Kuypers, J.; Renaud, C.; Wald, A.; Martin, E.; Fairchok, M.; Magaret, A.; Sarancino, M.; Englund, J.A. Molecular epidemiology of respiratory syncytial virus transmission in childcare. J. Clin. Virol. 2013, 57, 343–350. [Google Scholar] [CrossRef]
- Frome, E.L.; Checkoway, H. Epidemiologic programs for computers and calculators. Use of Poisson regression models in estimating incidence rates and ratios. Am. J. Epidemiol. 1985, 121, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.A.; Yan, S.K. Two-year microwave radiometric observations of low-level boundary-layer temperature inversion signatures. In Proceedings of the 2006 IEEE International Symposium on Geoscience and Remote Sensing, Denver, CO, USA, 31 July–4 August 2006. [Google Scholar]
- Yin, P.Y.; Chang, R.I.; Day, R.F.; Lin, Y.C.; Hu, C.Y. Improving PM2.5 concentration forecast with the identification of temperature inversion. Appl. Sci. 2021, 12, 71. [Google Scholar] [CrossRef]
- Prussin, A.J., 2nd; Vikram, A.; Bibby, K.J.; Marr, L.C. Seasonal dynamics of the air borne bacterial community and selected viruses in a children’s day care center. PLoS ONE 2016, 11, e0151004. [Google Scholar] [CrossRef]
- Prussin, A.J., 2nd; Torres, P.J.; Shimashita, J.; Head, S.R.; Bibby, K.J.; Kelley, S.T.; Marr, L.C. Seasonal dynamics of DNA and RNA viral bioaerosol communities in a daycare center. Microbiome 2019, 7, 53. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hwang, D.; Chiu, N.C.; Weng, L.C.; Liu, H.F.; Mu, J.J.; Liu, C.P.; Chi, H. Increased Detection of Viruses in Children with Respiratory Tract Infection Using PCR. Int. J. Environ. Res. Public Health 2020, 17, 564. [Google Scholar] [CrossRef]
- Lee, A.H.I.; Yang, C.N.; Lin, C.Y. Evaluation of children’s after-school programs in Taiwan. Asia Pac. Educ. Rev. 2012, 13, 347–357. [Google Scholar] [CrossRef]
| Variable | Rate, n/Day | Crude RR(95% CI) |
|---|---|---|
| Average daily temperature, °C | ||
| <20 | 652 | 1.00 (Reference) |
| 20–24 | 578 | 0.89 (0.87–0.90) |
| 25–29 | 469 | 0.72 (0.71–0.73) |
| 30+ | 477 | 0.73 (0.71–0.76) |
| Average daily PM2.5, μg/m3 | ||
| <15 | 393 | 1.00 (Reference) |
| 15–23.6 | 528 | 1.34 (1.32–1.37) |
| 23.7–36.9 | 587 | 1.49 (1.46–1.52) |
| 37+ | 666 | 1.69 (1.66–1.73) |
| Income, NTD | ||
| <250,000 | 426 | 1.00 (Reference) |
| 250,000–299,999 | 515 | 1.21 (1.19–1.23) |
| 300,000+ | 838 | 1.97 (1.94–2.00) |
| SO2, 5ppb increment | 2.02 (2.00–2.04) | |
| O3, 5ppb increment | 0.98 (0.98–0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Mou, C.-H.; Chen, C.W.; Hsieh, D.P.H.; Tsai, S.P.; Wei, C.-C.; Sung, F.-C. Roles of Ambient Temperature and PM2.5 on Childhood Acute Bronchitis and Bronchiolitis from Viral Infection. Viruses 2022, 14, 1932. https://doi.org/10.3390/v14091932
Chen P-C, Mou C-H, Chen CW, Hsieh DPH, Tsai SP, Wei C-C, Sung F-C. Roles of Ambient Temperature and PM2.5 on Childhood Acute Bronchitis and Bronchiolitis from Viral Infection. Viruses. 2022; 14(9):1932. https://doi.org/10.3390/v14091932
Chicago/Turabian StyleChen, Pei-Chun, Chih-Hsin Mou, Chao W. Chen, Dennis P. H. Hsieh, Shan P. Tsai, Chang-Ching Wei, and Fung-Chang Sung. 2022. "Roles of Ambient Temperature and PM2.5 on Childhood Acute Bronchitis and Bronchiolitis from Viral Infection" Viruses 14, no. 9: 1932. https://doi.org/10.3390/v14091932
APA StyleChen, P.-C., Mou, C.-H., Chen, C. W., Hsieh, D. P. H., Tsai, S. P., Wei, C.-C., & Sung, F.-C. (2022). Roles of Ambient Temperature and PM2.5 on Childhood Acute Bronchitis and Bronchiolitis from Viral Infection. Viruses, 14(9), 1932. https://doi.org/10.3390/v14091932






