Detection of Apparent Early Rabies Infection by LN34 Pan-Lyssavirus Real-Time RT-PCR Assay in Pennsylvania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Direct Fluorescent Antibody (DFA) Test
2.3. Real-Time RT-PCR (RT-qPCR)
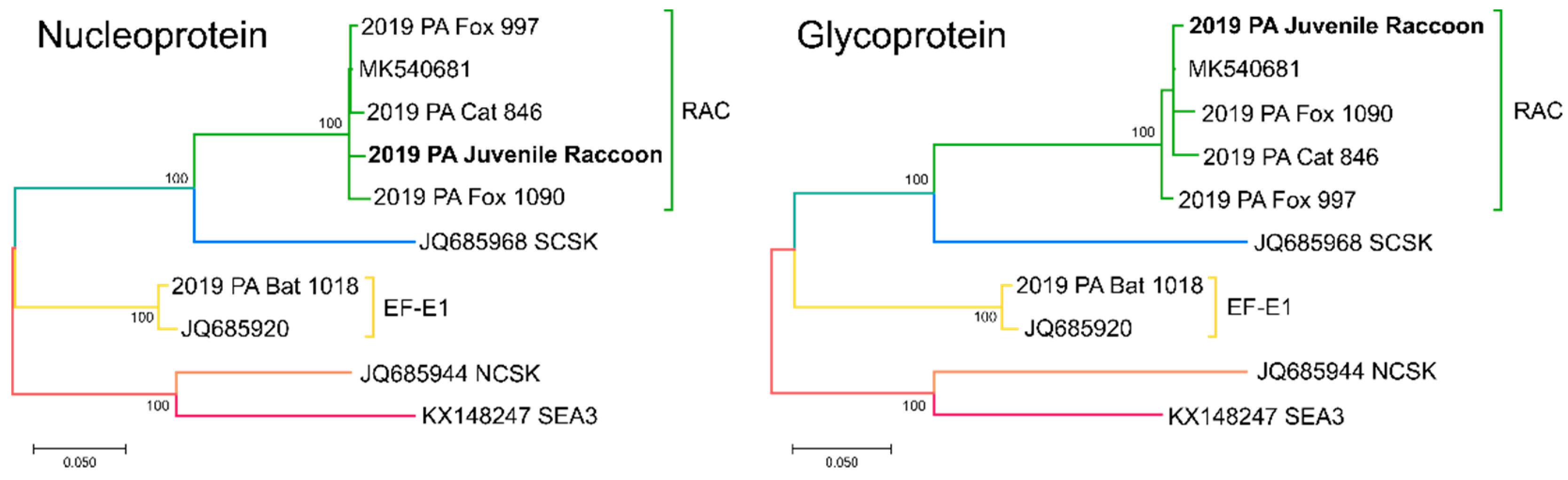
2.4. Sequencing
3. Results
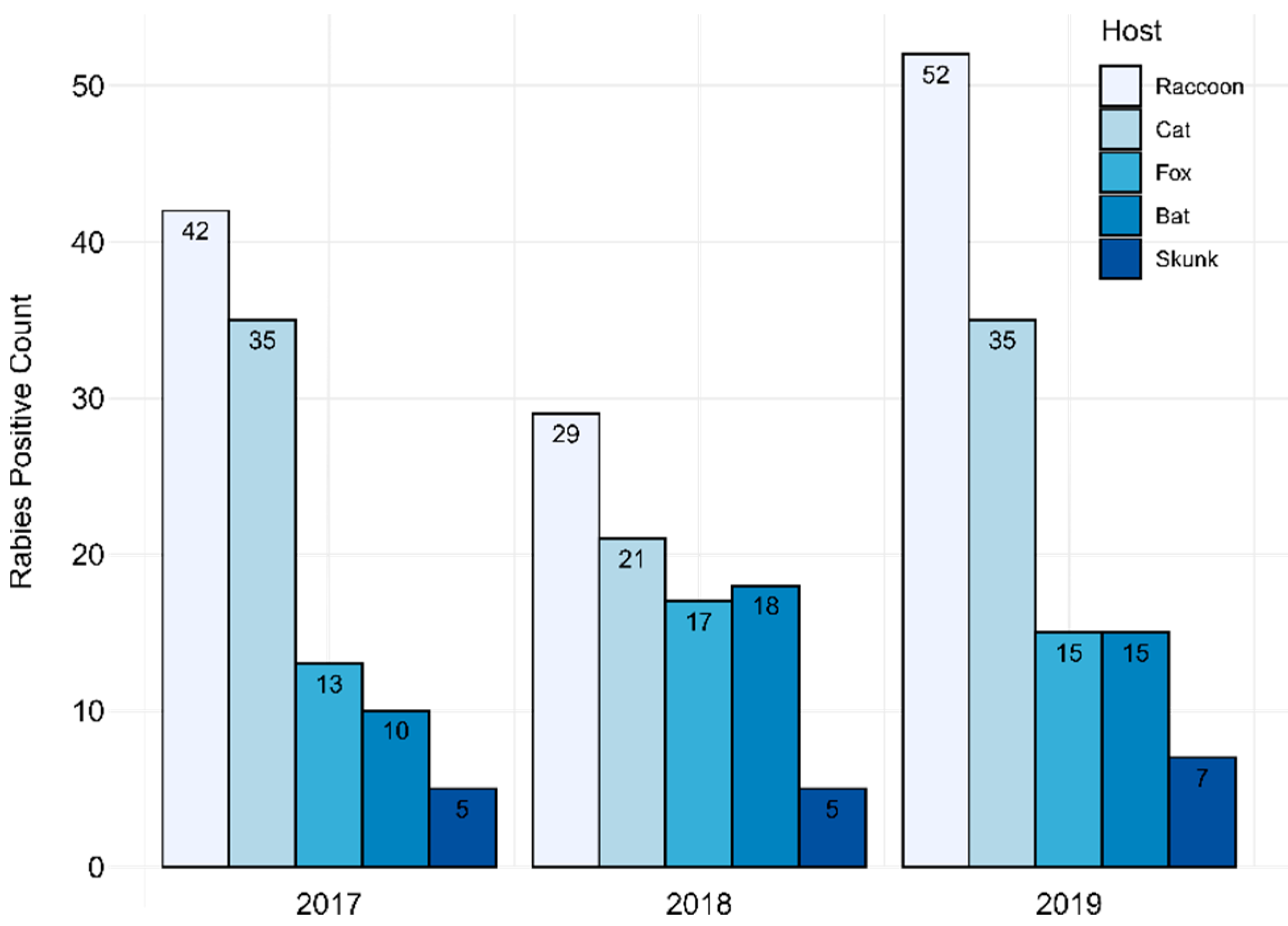
3.1. PABOL DFA and PCR Testing
3.2. CDC DFA and PCR Testing
3.3. Investigation into Potential Cross-Contamination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Belotto, A.; Leanes, L.F.; Schneider, M.C.; Tamayo, H.; Correa, E. Overview of rabies in the Americas. Virus Res. 2005, 111, 5–12. [Google Scholar] [CrossRef]
- Hanlon, C.A.; Childs, J.E.; Nettles, V.F. Recommendations of a National Working Group on Prevention and Control of Rabies in the United States. Article III: Rabies in wildlife. National Working Group on Rabies Prevention and Control. J. Am. Vet. Med. Assoc. 1999, 215, 1612–1618. [Google Scholar] [PubMed]
- Smith, J.S. New aspects of rabies with emphasis on epidemiology, diagnosis, and prevention of the disease in the United States. Clin. Microbiol. Rev. 1996, 9, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Monroe, B.P.; Cleaton, J.M.; Orciari, L.A.; Gigante, C.M.; Kirby, J.D.; Chipman, R.B.; Fehlner-Gardiner, C.; Gutierrez Cedillo, V.; Petersen, B.W.; et al. Public Veterinary Medicine: Public Health: Rabies surveillance in the United States during 2018. J. Am. Vet. Med. Assoc. 2020, 256, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Monroe, B.P.; Cleaton, J.M.; Orciari, L.A.; Li, Y.; Kirby, J.D.; Chipman, R.B.; Petersen, B.W.; Wallace, R.M.; Blanton, J.D. Rabies surveillance in the United States during 2017. J. Am. Vet. Med. Assoc. 2018, 253, 1555–1568. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. Laboratory Techniques in Rabies, 5th ed.; World Health Organization: Geneva, Switzerland, 2019; Volumes 1–2, Available online: https://apps.who.int/iris/handle/10665/310837 (accessed on 23 March 2020).
- World Organization for Animal Health (OIE). Rabies (Infection with Rabies Virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; Office International des Epizooties: Paris, France, 2018. [Google Scholar]
- Ronald, G.P.; Powell, J.; Raj, P.; Rudd, R.; Rupprecht, C.; Schnurr, D.; Smith, J.; Trimarchi, C. Protocol for Postmortem Diagnosis of Rabies in Animals by Direct Fluorescent Antibody Testing: A Minimum Standard for Rabies Diagnosis in the United States. 2003. Available online: https://www.cdc.gov/rabies/pdf/rabiesdfaspv2.pdf (accessed on 23 March 2020).
- Wadhwa, A.; Wilkins, K.; Gao, J.; Condori Condori, R.E.; Gigante, C.M.; Zhao, H.; Ma, X.; Ellison, J.A.; Greenberg, L.; Velasco-Villa, A.; et al. A Pan-Lyssavirus Taqman Real-Time RT-PCR Assay for the Detection of Highly Variable Rabies virus and Other Lyssaviruses. PLoS Negl. Trop. Dis. 2017, 11, e0005258. [Google Scholar] [CrossRef]
- Gigante, C.M.; Dettinger, L.; Powell, J.W.; Seiders, M.; Condori, R.E.C.; Griesser, R.; Okogi, K.; Carlos, M.; Pesko, K.; Breckenridge, M.; et al. Multi-site evaluation of the LN34 pan-lyssavirus real-time RT-PCR assay for post-mortem rabies diagnostics. PLoS ONE 2018, 13, e0197074. [Google Scholar]
- World Health Organization. The direct fluorescent antibody test. In Laboratory Techniques in Rabies, Volume 1, 5th ed.; Rupprecht, C.E., Fooks, A.R., Abela-Ridder, B., Eds.; World Health Organization: Geneva, Switzerland, 2019; Volume 2. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Gigante, C.M.; Yale, G.; Condori, R.E.; Costa, N.C.; Long, N.V.; Minh, P.Q.; Chuong, V.D.; Tho, N.D.; Thanh, N.T.; Thin, N.X.; et al. Portable Rabies Virus Sequencing in Canine Rabies Endemic Countries Using the Oxford Nanopore MinION. Viruses 2020, 12, 1255. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmin, I.V.; Shi, M.; Orciari, L.A.; Yager, P.A.; Velasco-Villa, A.; Kuzmina, N.A.; Streicker, D.G.; Bergman, D.L.; Rupprecht, C.E. Molecular inferences suggest multiple host shifts of rabies viruses from bats to mesocarnivores in Arizona during 2001–2009. PLoS Pathog. 2012, 8, e1002786. [Google Scholar] [CrossRef]
- Rudd, R.J.; Smith, J.S.; Yager, P.A.; Orciari, L.A.; Trimarchi, C.V. A need for standardized rabies-virus diagnostic procedures: Effect of cover-glass mountant on the reliability of antigen detection by the fluorescent antibody test. Virus Res. 2005, 111, 83–88. [Google Scholar] [CrossRef]
- Trimarchi, C.V.; Debbie, J.G. Standardization and quantitation of immunofluorescence in the rabies fluorescent-antibody test. Appl. Microbiol. 1972, 24, 609–612. [Google Scholar] [CrossRef]
- Murphy, F.A. Rabies pathogenesis. Arch. Virol. 1977, 54, 279–297. [Google Scholar] [CrossRef]
- Charlton, K.M.; Casey, G.A.; Wandeler, A.I.; Nadin-Davis, S. Early events in rabies virus infection of the central nervous system in skunks (Mephitis mephitis). Acta Neuropathol. 1995, 91, 89–98. [Google Scholar] [CrossRef]
- Aubert, M.F. Practical significance of rabies antibodies in cats and dogs. Rev. Sci. Tech. 1992, 11, 735–760. [Google Scholar] [CrossRef]
- Murray, K.O.; Holmes, K.C.; Hanlon, C.A. Rabies in vaccinated dogs and cats in the United States, 1997–2001. J. Am. Vet. Med. Assoc. 2009, 235, 691–695. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Wiktor, T.J.; Johnston, D.H.; Hamir, A.N.; Dietzschold, B.; Wunner, W.H.; Glickman, L.T.; Koprowski, H. Oral immunization and protection of raccoons (Procyon lotor) with a vaccinia-rabies glycoprotein recombinant virus vaccine. Proc. Natl. Acad. Sci. USA 1986, 83, 7947–7950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupprecht, C.E.; Hamir, A.N.; Johnston, D.H.; Koprowski, H. Efficacy of a vaccinia-rabies glycoprotein recombinant virus vaccine in raccoons (Procyon lotor). Rev. Infect. Dis. 1988, 10 (Suppl. 4), S803–S809. [Google Scholar] [CrossRef] [PubMed]
- Fehlner-Gardiner, C.; Rudd, R.; Donovan, D.; Slate, D.; Kempf, L.; Badcock, J. Comparing ONRAB(R) and raboral V-RG(R) oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA. J. Wildl. Dis. 2012, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.T.; Johnson, S.R.; Nelson, K.M.; Chipman, R.B.; VerCauteren, K.C.; Algeo, T.P.; Rupprecht, C.E.; Slate, D. Field Trials of Ontario Rabies Vaccine Bait in the Northeastern USA, 2012–2014. J. Wildl. Dis. 2018, 54, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Slate, D.; Chipman, R.B.; Algeo, T.P.; Mills, S.A.; Nelson, K.M.; Croson, C.K.; Dubovi, E.J.; Vercauteren, K.; Renshaw, R.W.; Atwood, T.; et al. Safety and immunogenicity of Ontario Rabies Vaccine Bait (ONRAB) in the first US field trial in raccoons (Procyon lotor). J. Wildl. Dis. 2014, 50, 582–595. [Google Scholar] [CrossRef] [Green Version]
- Blanton, J.D.; Niezgoda, M.; Hanlon, C.A.; Swope, C.B.; Suckow, J.; Saidy, B.; Nelson, K.; Chipman, R.B.; Slate, D. Evaluation of Oral Rabies Vaccination: Protection against Rabies in Wild Caught Raccoons (Procyon Lotor). J. Wildl. Dis. 2018, 54, 520–527. [Google Scholar] [CrossRef]
- Bell, J.F.; Sancho, M.I.; Diaz, A.M.; Moore, G.J. Nonfatal rabies in an enzootic area: Results of a survey and evaluation of techniques. Am. J. Epidemiol. 1972, 95, 190–198. [Google Scholar] [CrossRef]
- Botros, B.A.; Lewis, J.C.; Kerkor, M. A study to evaluate non-fatal rabies in animals. J. Trop. Med. Hyg. 1979, 82, 137–141. [Google Scholar]
- McLean, R.G. Rabies in raccoons in the Southeastern United States. J. Infect. Dis. 1971, 123, 680–681. [Google Scholar] [CrossRef]
- Ramey, P.C.; Blackwell, B.F.; Gates, R.J.; Slemons, R.D. Oral rabies vaccination of a northern Ohio raccoon population: Relevance of population density and prebait serology. J. Wildl. Dis. 2008, 44, 553–568. [Google Scholar] [CrossRef]
- Bigler, W.J.; McLean, R.G.; Trevino, H.A. Epizootiologic aspects of raccoon rabies in Florida. Am. J. Epidemiol. 1973, 98, 326–335. [Google Scholar] [CrossRef] [PubMed]
- East, M.L.; Hofer, H.; Cox, J.H.; Wulle, U.; Wiik, H.; Pitra, C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proc. Natl. Acad. Sci. USA 2001, 98, 15026–15031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.C.; Reimer, D.L. Pathogenesis of experimental rabies in mice: An immunohistochemical study. Acta Neuropathol. 1989, 78, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Dietzschold, B.; Koprowski, H. Direct entry of rabies virus into the central nervous system without prior local replication. J. Virol. 1991, 65, 2736–2738. [Google Scholar] [CrossRef] [Green Version]
- Tirawatnpong, S.; Hemachudha, T.; Manutsathit, S.; Shuangshoti, S.; Phanthumchinda, K.; Phanuphak, P. Regional distribution of rabies viral antigen in central nervous system of human encephalitic and paralytic rabies. J. Neurol. Sci. 1989, 92, 91–99. [Google Scholar] [CrossRef]
- Bingham, J.; van der Merwe, M. Distribution of rabies antigen in infected brain material: Determining the reliability of different regions of the brain for the rabies fluorescent antibody test. J. Virol. Methods 2002, 101, 85–94. [Google Scholar] [CrossRef]
- Juntrakul, S.; Ruangvejvorachai, P.; Shuangshoti, S.; Wacharapluesadee, S.; Hemachudha, T. Mechanisms of escape phenomenon of spinal cord and brainstem in human rabies. BMC Infect. Dis. 2005, 5, 104. [Google Scholar] [CrossRef] [Green Version]
- Murphy, F.A.; Bauer, S.P.; Harrison, A.K.; Winn, W.C., Jr. Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab. Investig. 1973, 28, 361–376. [Google Scholar]
- Tang, Y.; Rampin, O.; Giuliano, F.; Ugolini, G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: Retrograde transneuronal tracing with rabies virus. J. Comp. Neurol. 1999, 414, 167–192. [Google Scholar] [CrossRef]
- Meslin, F.X.; Kaplin, M.; Koprowski, H. Laboratory Techniques in Rabies; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Hicks, D.J.; Nunez, A.; Healy, D.M.; Brookes, S.M.; Johnson, N.; Fooks, A.R. Comparative pathological study of the murine brain after experimental infection with classical rabies virus and European bat lyssaviruses. J. Comp. Pathol. 2009, 140, 113–126. [Google Scholar] [CrossRef]
- Stein, L.T.; Rech, R.R.; Harrison, L.; Brown, C.C. Immunohistochemical study of rabies virus within the central nervous system of domestic and wildlife species. Vet. Pathol. 2010, 47, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.J.; Abelseth, M.K. Laboratory techniques in rabies: The fluorescent antibody test. In Monograph Series; World Health Organization: Geneva, Switzerland, 1973; pp. 73–84. [Google Scholar]
- Chopra, J.S.; Banerjee, A.K.; Murthy, J.M.; Pal, S.R. Paralytic rabies: A clinico-pathological study. Brain 1980, 103, 789–802. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence |
|---|---|
| Nucleoprotein Forward | TTTCTGTTGGTGCTGATATTGCACGCTTAACAACCAGATCAAAGAA TTTCTGTTGGTGCTGATATTGCACGCTTAACAACAAAATCADAGAAG |
| Nucleoprotein Reverse | ACTTGCCTGTCGCTCTATCTTCAGGAGGRGTGTTAGTTTTTTTC |
| Glycoprotein Forward | TTTCTGTTGGTGCTGATATTGCGATGTGAAAAAACTATYAACATCCCTC |
| Glycoprotein Reverse | ACTTGCCTGTCGCTCTATCTTCTGTGAKCTATTGCTTRTGTYCTTCA |
| DFA Results | PCR Results | ||||||
|---|---|---|---|---|---|---|---|
| Result | Replicates | Antigen | Result | Replicates | Cq Value | ||
| 2019 Juvenile Raccoon | Minced tissue * | Negative | 0/1 | ND | Positive | 2/2 | 34.1 |
| Hippocampus | Negative | 0/1 | ND | NA | NA | NA | |
| Cerebellum | Negative | 0/1 | ND | Negative | 0/2 | ND | |
| Brain stem | Negative | 0/1 | ND | Indeterminate | 2/2 | 35.5 | |
| Spinal cord | Negative | 0/1 | ND | Positive | 2/2 | 32.7 | |
| 2017 Adult Raccoon | Minced tissue * | Negative | 0/1 | ND | Positive | 8/8 | 32.1 |
| Cerebellum | Negative | 0/1 | ND | NA | NA | NA | |
| Brain stem | Negative | 0/1 | ND | NA | NA | NA | |
| Spinal cord | Negative | 0/1 | ND | Positive | 2/2 | 34.2 | |
| DFA Results | PCR Results | ||||||
|---|---|---|---|---|---|---|---|
| Result | Replicates | Antigen | Result | Replicates | Cq Value | ||
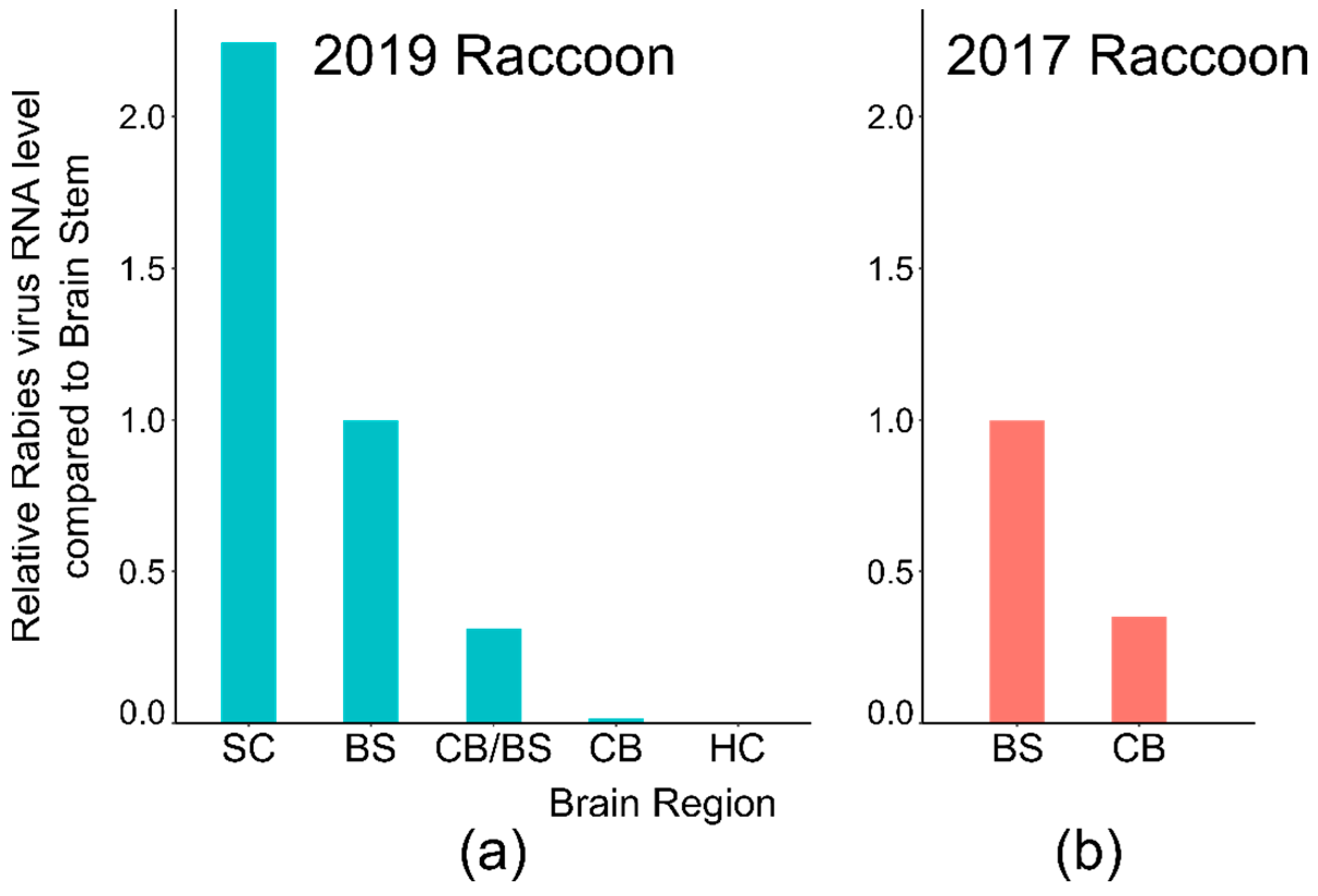
| 2019 Juvenile Raccoon | Cortex/HC | Indeterminate | 1/6 | Atypical | Indeterminate | 2/3 | 42.6 * |
| Cerebellum | Positive | 3/5 | <10% | Indeterminate ** | 4/6 | 39.0 ** | |
| Brain stem | Positive | 3/3 | <10% | Positive | 3/3 | 35.0 | |
| Spinal cord | NA | NA | NA | Positive | 3/3 | 32.4 | |
| 2017 Adult Raccoon | Cerebellum | Positive | 2/6 | <10% | Positive | 3/3 | 31.8 |
| Brain stem | Positive | 1/1 | <10% | Positive | 3/3 | 31.4 | |
| Spinal cord | Positive | 1/1 | <10% | NA | NA | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettinger, L.; Gigante, C.M.; Sellard, M.; Seiders, M.; Patel, P.; Orciari, L.A.; Yager, P.; Lute, J.; Regec, A.; Li, Y.; et al. Detection of Apparent Early Rabies Infection by LN34 Pan-Lyssavirus Real-Time RT-PCR Assay in Pennsylvania. Viruses 2022, 14, 1845. https://doi.org/10.3390/v14091845
Dettinger L, Gigante CM, Sellard M, Seiders M, Patel P, Orciari LA, Yager P, Lute J, Regec A, Li Y, et al. Detection of Apparent Early Rabies Infection by LN34 Pan-Lyssavirus Real-Time RT-PCR Assay in Pennsylvania. Viruses. 2022; 14(9):1845. https://doi.org/10.3390/v14091845
Chicago/Turabian StyleDettinger, Lisa, Crystal M. Gigante, Maria Sellard, Melanie Seiders, Puja Patel, Lillian A. Orciari, Pamela Yager, James Lute, Annette Regec, Yu Li, and et al. 2022. "Detection of Apparent Early Rabies Infection by LN34 Pan-Lyssavirus Real-Time RT-PCR Assay in Pennsylvania" Viruses 14, no. 9: 1845. https://doi.org/10.3390/v14091845
APA StyleDettinger, L., Gigante, C. M., Sellard, M., Seiders, M., Patel, P., Orciari, L. A., Yager, P., Lute, J., Regec, A., Li, Y., & Xia, D. (2022). Detection of Apparent Early Rabies Infection by LN34 Pan-Lyssavirus Real-Time RT-PCR Assay in Pennsylvania. Viruses, 14(9), 1845. https://doi.org/10.3390/v14091845





