Prognostic Value of Mid-Region Proadrenomedullin and In Vitro Interferon Gamma Production for In-Hospital Mortality in Patients with COVID-19 Pneumonia and Respiratory Failure: An Observational Prospective Study
Abstract
:1. Introduction
2. Methods
2.1. Mid-Regional Proadrenomedullin (MR-proADM)
2.2. In Vitro Interferon Gamma (IFNγ) Production
2.3. Statistical Analysis
3. Results
3.1. Study Population and Comparison between Survivors and Deceased
| Overall (n = 100) | Survivors (n = 87) | Deceased (n = 13) | p Value | |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age | 65 (54–75) | 63 (53–73) | 77 (73–82) | 0.003 |
| Gender female | 36 (36.0) | 31 (35.6) | 5 (38.5) | 0.843 |
| BMI | 25.8 (23.7–29.7) | 25.5 (23.4–29.4) | 29.3 (27–31.9) | 0.066 |
| CCI | 0 (0–1) | 0 (0–1) | 1 (1–3) | <0.001 |
| Hypertension | 43 (43.0) | 35 (40.2) | 8 (61.5) | 0.148 |
| Ever smoker | 19 (19.0) | 17 (19.5) | 2 (15.4) | 1.00 |
| Any comorbidity # | 58 (58.0) | 46 (52.9) | 12 (92.3) | 0.007 |
| Days from symptom onset to hospitalization | 7 (5–10) | 7 (5–10) | 8.5 (4.5–10) | 0.604 |
| At least one dose of COVID-19 vaccine before admission | 0.006 | |||
| No | 74 (74.0) | 67 (77.0) | 7 (53.9) | |
| Yes | 16 (16.0) | 15 (17.2) | 1 (7.7) | |
| Missing | 10 (10.0) | 5 (5.8) | 5 (38.5) | |
| Clinical and Laboratory Characteristics at T0 | ||||
| Steroid intake before admission | 38 (38.0) | 36 (41.9) | 2 (15.4) | 0.123 |
| Body temperature, °C | 36.5 (36.1–37.5) | 36.5 (36.0–37.5) | 37.0 (36.2–38.0) | 0.254 |
| PaO2:FiO2 ratio | 241 (157–309) | 248 (167–314) | 150 (111–247) | 0.023 |
| Respiratory support ^ | 0.026 | |||
| None/low-flow oxygen | 64 (64.0) | 60 (69.0) | 4 (30.8) | |
| Non-invasive ventilation | 31 (31.0) | 23 (26.4) | 8 (61.5) | |
| Mechanical ventilation | 5 (5.0) | 4 (4.6) | 1 (7.7) | |
| NIH ordinal scale † | 0.051 | |||
| 4 | 2 (2.0) | 2 (2.3) | 0 (0.0) | |
| 5 | 62 (62.0) | 58 (66.7) | 4 (30.8) | |
| 6 | 31 (31.0) | 23 (26.4) | 8 (61.5) | |
| 7 | 5 (5.0) | 4 (4.6) | 1 (7.7) | |
| Steroid intake § | 0.001 | |||
| Standard dose | 79 (79.0) | 73 (84.9) | 6 (46.1) | |
| High dose | 20 (20.0) | 13 (15.1) | 7 (43.9) | |
| C-reactive protein, mg/dL | 7.1 (4.4–12.1) | 7.3 (3.6–12.4) | 7.0 (5.1–8.5) | 0.918 |
| Lymphocyte, cell/µL | 860 (600–1290) | 900 (600–1300) | 700 (500–1200) | 0.118 |
| Creatinine, mg/dL | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 1.0 (0.9–1.2) | 0.386 |
| D dimer, µg/L (n = 84) | 770 (559–1335) | 733 (537–1278) | 1116 (841–1543) | 0.077 |
| Clinical and Laboratory Characteristics at T1 | ||||
| PaO2:FiO2 ratio (n = 48) | 215 (143–260) | 223 (194–284) | 113 (98–170) | 0.001 |
| Respiratory support ^ | 0.001 | |||
| None | 23 (28.4) | 23 (33.3) | 0 (0.0) | |
| Low-flow oxygen | 14 (17.3) | 14 (20.3) | 0 (0.0) | |
| Non-invasive ventilation | 40 (49.4) | 30 (43.5) | 10 (83.3) | |
| Mechanical ventilation | 4 (4.9) | 2 (2.9) | 2 (16.7) | |
| NIH ordinal scale † | 0.003 | |||
| 3 | 9 (11.1) | 9 (13.0) | 0 (0.0) | |
| 4 | 14 (17.3) | 14 (20.3) | 0 (0.0) | |
| 5 | 14 (17.3) | 14 (20.3) | 0 (0.0) | |
| 6 | 40 (49.4) | 30 (43.5) | 10 (83.3) | |
| 7 | 4 (4.9) | 2 (2.9) | 2 (16.7) | |
| Steroid intake § | 0.057 | |||
| No steroid | 2 (2.5) | 2 (2.9) | 0 (0.0) | |
| Standard dose | 47 (58.0) | 43 (61.8) | 4 (33.3) | |
| High dose | 32 (39.5) | 24 (35.3) | 8 (66.7) | |
| C-reactive protein, mg/dL | 1.0 (0.4–2.8) | 0.8 (0.4–2.1) | 5.4 (0.9–12) | 0.013 |
| Lymphocyte, cell/µL | 1290 (820–1920) | 1400 (1100–2000) | 600 (400–1000) | <0.001 |
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.8 (0.8–1.0) | 0.442 |
| D dimer, µg/L (n = 58) | 1005 (624–1980) | 898 (541–1785) | 1693 (1105–4073) | 0.028 |
| Days of hospitalization | 12.5 (8.5–21.0) | 12.0 (8.0–20.0) | 20 (11.0–25.0) | 0.063 |
3.2. Association of MR-proADM and IFNγ Production Levels with In-Hospital Mortality
3.3. Prognostic Values of MR-proADM and In Vivo IFNγ Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Team E. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Z.; Jiang, Y.; Shi, O.; Zhang, X.; Xu, K.; Suo, C.; Wang, Q.; Song, Y.; Yu, K.; et al. Early prediction of mortality risk among patients with severe COVID-19, using machine learning. Int. J. Epidemiol. 2020, 49, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef] [PubMed]
- Ruetsch, C.; Brglez, V.; Crémoni, M.; Zorzi, K.; Fernandez, C.; Boyer-Suavet, S.; Benzaken, S.; Demonchy, E.; Risso, K.; Courjon, J.; et al. Functional Exhaustion of Type I and II Interferons Production in Severe COVID-19 Patients. Front. Med. 2021, 7, 603961. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, S.; Ranzani, V.; Bandera, A.; Gruarin, P.; Bevilacqua, V.; Putignano, A.R.; Gobbini, A.; Galeota, E.; Manara, C.; Bombaci, M.; et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci. Immunol. 2021, 6, eabg5021. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Vincent, J.L.; Levi, M.; Hunt, B.J. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir. Med. 2022, 10, 214–220. [Google Scholar] [CrossRef]
- Sood, S.; Yu, L.; Visvanathan, K.; Angus, P.W.; Gow, P.J.; Testro, A.G. Immune function biomarker QuantiFERON-monitor is associated with infection risk in cirrhotic patients. World J. Hepatol. 2016, 8, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mian, M.; Natori, Y.; Ferreira, V.; Selzner, N.; Husain, S.; Singer, L.; Kim, S.J.; Humar, A.; Kumar, D. Evaluation of a Novel Global Immunity Assay to Predict Infection in Organ Transplant Recipients. Clin. Infect. Dis. 2018, 66, 1392–1397. [Google Scholar] [CrossRef] [Green Version]
- Sood, S.; Cundall, D.; Yu, L.; Miyamasu, M.; Boyle, J.S.; Ong, S.Y.; Gow, P.J.; Jones, R.M.; Angus, P.W.; Visvanathan, K.; et al. A novel biomarker of immune function and initial experience in a transplant population. Transplantation 2014, 97, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Cremoni, M.; Ruetsch, C.; Zorzi, K.; Fernandez, C.; Boyer-Suavet, S.; Benzaken, S.; Demonchy, E.; Dellamonica, J.; Ichai, C.; Esnault, V.; et al. Humoral and Cellular Response of Frontline Health Care Workers Infected by SARS-CoV-2 in Nice, France: A Prospective Single-Center Cohort Study. Front. Med. 2021, 7, 608804. [Google Scholar] [CrossRef] [PubMed]
- Krintus, M.; Kozinski, M.; Braga, F.; Kubica, J.; Sypniewska, G.; Panteghini, M. Plasma midregional proadrenomedullin (MR-proADM) concentrations and their biological determinants in a reference population. Clin. Chem. Lab. Med. 2018, 56, 1161–1168. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, F.; Valenzuela-Méndez, B.; Rodríguez-Gutiérrez, J.F.; Estella-García, Á.; González-García, M.Á. New role of biomarkers: Mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann. Transl. Med. 2016, 4, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal-Morell, E.; García-Villalba, E.; del Carmen Vera, M.; Medina, B.; Martinez, M.; Callejo, V.; Valero, S.; Cinesi, C.; Piñera, P.; Alcaraz, A.; et al. Usefulness of midregional pro-adrenomedullin as a marker of organ damage and predictor of mortality in patients with sepsis. J. Infect. 2018, 76, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Jarczak, D.; Fischer, M.; Haddad, M.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Karakas, M.; et al. MR-proAdrenomedullin as a predictor of renal replacement therapy in a cohort of critically ill patients with COVID-19. Biomarkers 2021, 26, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Sales, G.; Rumbolo, F.; Palmesino, F.; Fanelli, V.; Urbino, R.; Filippini, C.; Mengozzi, G.; Brazzi, L. Effectiveness of mid-regional proadrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: An observational prospective study. PLoS ONE 2021, 16, e0246771. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Martínez Martínez, M.; Rodríguez Mulero, M.D.; Esteban-Torrella, P.; Hernández Olivo, M.; Alcaraz García, M.J.; Campos-Rodríguez, V.; Sancho-Rodríguez, N.; Galindo Martínez, M.; Alcaraz, A.; et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Int. J. Infect. Dis. 2021, 111, 211–218. [Google Scholar] [CrossRef]
- Gregoriano, C.; Koch, D.; Kutz, A.; Haubitz, S.; Conen, A.; Bernasconi, L.; Hammerer-Lercher, A.; Saeed, K.; Mueller, B.; Schuetz, P. The vasoactive peptide MR-pro-adrenomedullin in COVID-19 patients: An observational study. Clin. Chem. Lab. Med. 2021, 59, 995–1004. [Google Scholar] [CrossRef]
- van Oers, J.A.H.; Kluiters, Y.; Bons, J.A.P.; de Jongh, M.; Pouwels, S.; Ramnarain, D.; de Lange, D.W.; de Grooth, H.-J.; Girbes, A.R.J. Endothelium-associated biomarkers mid-regional proadrenomedullin and C-terminal proendothelin-1 have good ability to predict 28-day mortality in critically ill patients with SARS-CoV-2 pneumonia: A prospective cohort study. J. Crit. Care 2021, 66, 173–180. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 2 June 2022).
- Girona-Alarcon, M.; Bobillo-Perez, S.; Sole-Ribalta, A.; Hernandez, L.; Guitart, C.; Suarez, R.; Balaguer, M.; Cambra, F.J.; Jordan, I. The different manifestations of COVID-19 in adults and children: A cohort study in an intensive care unit. BMC Infect. Dis. 2021, 21, 4–11. [Google Scholar] [CrossRef]
- Benedetti, I.; Spinelli, D.; Callegari, T.; Bonometti, R.; Molinaro, E.; Novara, E.; Cassinari, M.; Frino, C.; Guaschino, R.; Boverio, R.; et al. High levels of mid-regional proadrenomedullin in ARDS COVID-19 patients: The experience of a single, Italian center. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Calvo Nieves, M.D.; Rodríguez Mulero, M.D.; Calcerrada Alises, I.; Hernández Olivo, M.; Trapiello Fernández, W.; González Morales, M.; Bolado Jiménez, C.; Albaladejo-Otón, M.D.; Fernández Ovalle, H.; et al. MR-proADM as marker of endotheliitis predicts COVID-19 severity. Eur. J. Clin. Investig. 2021, 51, e13511. [Google Scholar] [CrossRef] [PubMed]
- Méndez, R.; González-Jiménez, P.; Latorre, A.; Piqueras, M.; Bouzas, L.; Yépez, K.; Ferrando, A.; Zaldívar-Olmeda, E.; Moscardó, A.; Alonso, R.; et al. Acute and sustained increase in endothelial biomarkers in COVID-19. Thorax 2021, 77, 400–403. [Google Scholar] [CrossRef]
- Moore, N.; Williams, R.; Mori, M.; Bertolusso, B.; Vernet, G.; Lynch, J.; Philipson, P.; Ledgerwood, T.; Kidd, S.P.; Thomas, C.; et al. Mid-regional proadrenomedullin (MR-proADM), C-reactive protein (CRP) and other biomarkers in the early identification of disease progression in patients with COVID-19 in the acute NHS setting. J. Clin. Pathol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Minieri, M.; Di Lecce, V.N.; Lia, M.S.; Maurici, M.; Bernardini, S.; Legramante, J.M. Role of MR-proADM in the risk stratification of COVID-19 patients assessed at the triage of the Emergency Department. Crit. Care 2021, 25, 407. [Google Scholar] [CrossRef] [PubMed]
- Sacco, C.; Mateo-Urdiales, A.; Petrone, D.; Spuri, M.; Fabiani, M.; Vescio, M.F.; Bressi, M.; Riccardo, F.; Del Manso, M.; Bella, A.; et al. Estimating averted COVID-19 cases, hospitalisations, intensive care unit admissions and deaths by COVID-19 vaccination, Italy, january−september 2021. Eurosurveillance 2021, 26, 2101001. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, G.; Mangioni, D.; Minoia, F.; Aliberti, S.; Grasselli, G.; Barbetta, L.; Castelli, V.; Palomba, E.; Alagna, L.; Lombardi, A.; et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: An observational cohort study. J. Allergy Clin. Immunol. 2021, 147, 561–566.e4. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; Grondman, I.; de Nooijer, A.H.; Boahen, C.K.; Koeken, V.A.; Matzaraki, V.; Kumar, V.; He, X.; Kox, M.; Koenen, H.J.; et al. Dysregulated Innate and Adaptive Immune Responses Discriminate Disease Severity in COVID-19. J. Infect. Dis. 2021, 223, 1322–1333. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.; Faivre, V.; Miatello, J.; Leentjens, J.; Brumpt, C.; Tissières, P.; Dupuis, C.; Pickkers, P.; Lukaszewicz, A.C. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect. Dis. 2019, 19, 931. [Google Scholar] [CrossRef] [Green Version]
- Van Laarhoven, A.; Kurver, L.; Overheul, G.J.; Kooistra, E.J.; Abdo, W.F.; van Crevel, R.; Duivenvoorden, R.; Kox, M.; Ten Oever, J.; Schouten, J.; et al. Interferon gamma immunotherapy in five critically ill COVID-19 patients with impaired cellular immunity: A case series. Med 2021, 2, 1163–1170.e2. [Google Scholar] [CrossRef] [PubMed]
- Blot, M.; Bour, J.B.; Quenot, J.P.; Bourredjem, A.; Nguyen, M.; Guy, J.; Monier, S.; Georges, M.; Large, A.; Dargent, A.; et al. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J. Transl. Med. 2020, 18, 457. [Google Scholar] [CrossRef]
- Dhanda, A.D.; Felmlee, D.; Boeira, P.; Moodley, P.; Tan, H.; Scalioni, L.D.; Lilly, K.; Sheridan, D.A.; Cramp, M.E. Patients with moderate to severe COVID-19 have an impaired cytokine response with an exhausted and senescent immune phenotype. Immunobiology 2022, 227, 152185. [Google Scholar] [CrossRef]

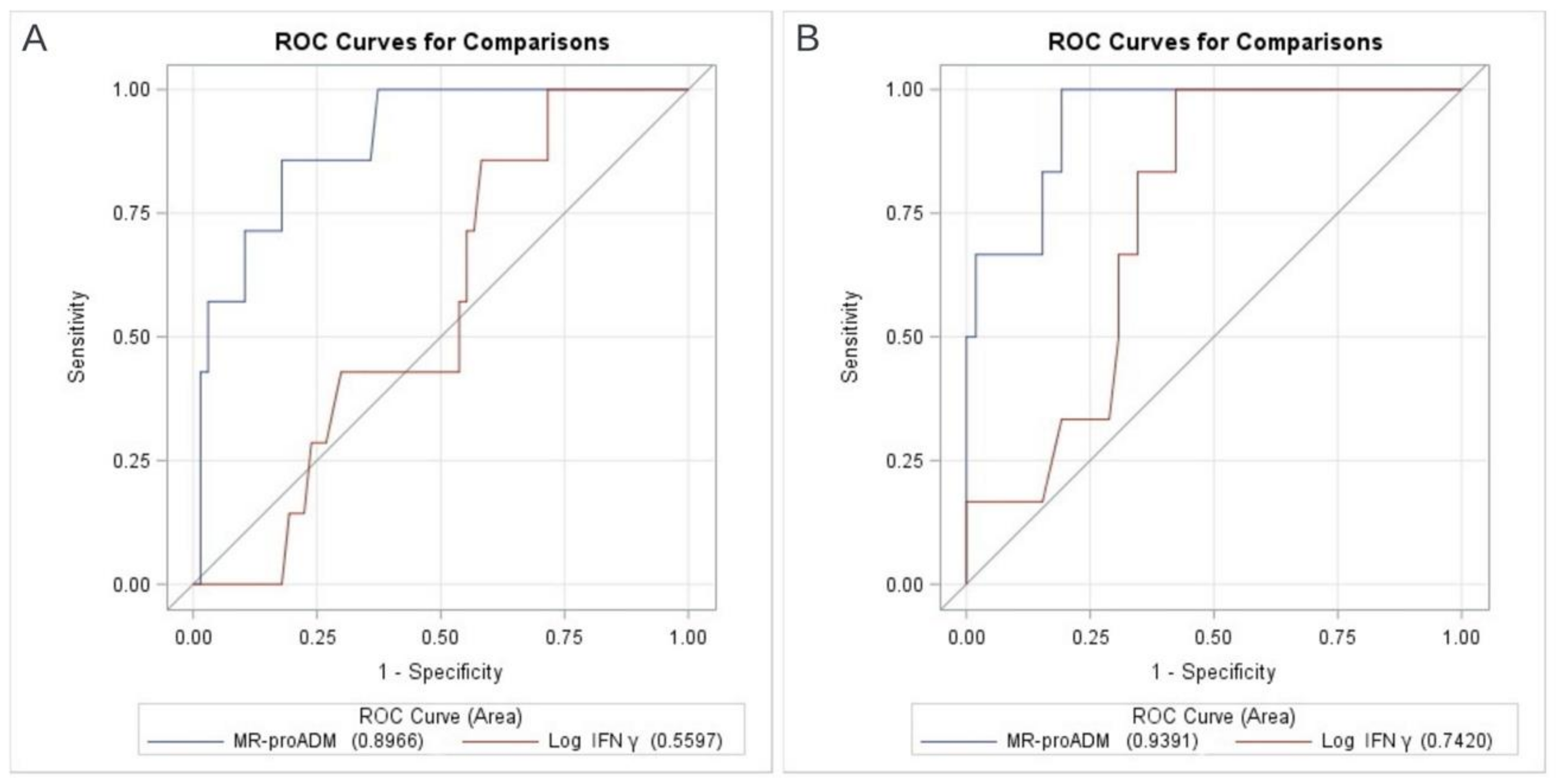
| Overall | Survivors | Deceased | OR (95% CI), p Value # | OR (95% CI), p Value § | |
|---|---|---|---|---|---|
| T0 (n = 100, 13 deceased) | |||||
| MR-proADM, nmol/L | 0.84 (0.66–1.20) | 0.79 (0.63–1.03) | 1.41 (1.12–1.77) | 5.03 (1.77–16.32), <0.001 | 3.39 (1.01–11.96), 0.048 |
| In vitro IFNγ production, IU/mL Log IFNγ production | 4.50 (0.85–17.60) 1.50 (−0.16–2.87) | 3.90 (0.80–16.80) 1.36 (−0.22–2.82) | 5.30 (1.10–20.10) 1.67 (0.10–3.00) | 1.04 (0.84–1.27), 0.773 | 0.86 (0.64–1.12), 0.289 |
| T1 (n = 81, 12 deceased) | |||||
| MR-proADM, nmol/L | 0.72 (0.55–1.10) | 0.66 (0.53–0.95) | 1.67 (1.08–1.96) | 9.98 (3.09–39.52), <0.001 | 11.80 (2.73–78.77), <0.001 |
| In vitro IFNγ production, IU/mL Log IFNγ production | 4.35 (0.75–17.25) 1.47 (−0.29–2.85) | 5.80 (0.75–20.95) 1.76 (−0.29–3.04) | 1.20 (0.65–3.10) 0.17 (−0.46–1.11) | 0.81 (0.60–1.03), 0.083 | 0.73 (0.49–1.01), 0.057 |
| Subset of non-vaccinated patients * | |||||
| T0 (n = 74, 7 deceased) | |||||
| MR-proADM, nmol/L | 0.82 (0.63–1.05) | 0.79 (0.61–0.98) | 1.51 (1.12–1.90) | 30.25 (4.35–346.36), <0.001 | N/A |
| In vitro IFNγ production, IU/mL Log IFNγ production | 3.60 (0.90–15.40) 1.28 (−0.11–2.73) | 3.50 (0.90–19.10) 1.25 (−0.11–2.95) | 4.50 (0.80–5.80) 1.50 (−0.22–1.76) | 0.89 (0.62–1.20), 0.536 | N/A |
| T1 (n = 59, 6 deceased) | |||||
| MR-proADM, nmol/L | 0.69 (0.54–1.01) | 0.64 (0.53–0.88) | 1.66 (1.07–1.95) | 64.65 (6.77–900), <0.001 | N/A |
| In vitro IFNγ production, IU/mL Log IFNγ production | 3.85 (0.60–17.90) 1.32 (−0.51–2.82) | 6.20 (0.65–20.95) 1.82 (−0.43–3.04) | 0.95 (0.50–1.40) −0.05 (.0.69–0.34) | 0.60 (0.29–0.96), 0.028 | N/A |
| First Author/DOI | Study Design | Study Period | Population at Enrolment | Mortality Rate | Time for MR-proADM Dosing | Endpoint | MR-proADM Cut-Off Value/Performance |
|---|---|---|---|---|---|---|---|
| Spoto S. 10.1002/jmv.26676 | prospective cohort study | 04/2020–06/2020 | 69 hospitalized COVID-19 patients - 39 (56.5%) admitted to medical ward - 30 (43.5%) admitted to ICU | 16/69 (23.2%) | N/A | - 30-day mortality - ARDS | - for mortality prediction: 2 nmol/L - for ARDS development: 3.04 nmol/L |
| Roedl K. 10.1080/1354750X.2021.1905067 | prospective cohort study | 03/2020–09/2020 | 64 COVID-19 ICU patients - 29 (45%) required RRT - 35 (55%) without RRT | 17/64 (26.5%) | ICU admission | RRT requirement | 1.26 nmol/L AUC 0.685 (95% CI: 0.543–0.828) |
| Montrucchio G. 10.1371/journal.pone.0246771 | prospective cohort study | 03/2020–06/2020 | 57 COVID-19 ICU patients | 31/57 (54.4%) | – T0 (≤48 h from ICU admission) – T1 (day 3) – T2 (day 7) – T3 (day 14) | in-hospital mortality | 1.8 nmol/L AUC 0.85 (95% CI: 0.78–0.90) |
| Lo Sasso B. 10.1093/labmed/lmab032 | retrospective cohort study | 09/2020–10/2020 | 110 hospitalized COVID-19 patients | 14/110 (12.7%) | hospital admission | in-hospital mortality | 1.73 nmol/L AUC 0.95 (95% CI: 0.86–0.99, 90% sensitivity and 95% specificity) |
| Gregoriano C. 10.1515/cclm-2020-1295 | prospective cohort study | 02/2020–04/2020 | 89 hospitalized COVID-19 patients | 17/89 (19.1%) | – T0 (initial blood draw upon hospital admission) – T1 (day 3/4) – T2 (day 5/6) – T3 (day 7/8) | in-hospital mortality | 0.93 nmol/L (at T0) AUC 0.78 (93% sensitivity, 60% specificity and 97% negative predictive value) |
| Sozio E. 10.1038/s41598-021-84478-1 | retrospective cohort study | 03/2020–05/2020 | 111 hospitalized COVID-19 patients | negative outcome (death or orotracheal intubation): 28/111 (25.2%) | hospital admission | negative outcome (death and/or orotracheal intubation) | 0.895 nmol/L AUC 0.849 (95% CI: 0.77–0.73, 86% sensitivity and 69% specificity) |
| Benedetti I. 10.26355/eurrev_202102_24885 | prospective observational study | 03/2020–04/2020 | 21 hospitalized COVID-19 patients with ARDS | 11/21 (52.4%) | - T0 (admission)- T1 (24 h)- T3 (day 3)- T5 (day 5) | 30-day mortality | 1.07 nmol/L (at T0) AUC 0.81 (91% sensitivity, 71% specificity) |
| García de Guadiana-Romualdo L. 10.1111/eci.13511 | prospective cohort study | 03/2020–04/2020 | 99 hospitalized COVID-19 patients | 14/99 (14.1%) | hospital admission | - 28-day mortality - severe COVID-19 progression (composite of admission to ICU and/or need for mechanical ventilation and/or 28-day mortality) | 1.01 nmol/L AUC for 28-day mortality 0.905 (95% CI: 0.829–0.955) and AUC for progression to severe disease 0.829 (95% CI: 0.740–0.897) |
| van Oers J.A.H. 10.1016/j.jcrc.2021.07.017 | prospective cohort study | 03/2020–05/2020 | 105 hospitalized COVID-19 patients with pneumonia | 30/105 (28.6%) | hospital admission and daily in the first 7 days | 28-day mortality | 1.57 nmol/L AUC 0.84 (95% CI: 0.76–0.92) |
| Girona-Alarcon M. 10.1186/s12879-021-05786-5 | prospective cohort study | 03/2020–06/2020 | 20 COVID-19 ICU patients -16 adults with ARDS -4 children with MIS-C | 0/20 (0%) | N/A | N/A | N/A |
| Zaninotto M. 10.1016/j.cca.2021.09.016 | retrospective cohort study | 11/2020 | 135 hospitalized COVID-19 patients - Group 1, n = 20, MR-proADM ≤ 0.55 nmol/L - Group 2, n = 82, MR-proADM > 0.55 nmol/L ≤ 1.50 nmol/L - Group 3, n = 33, MR-proADM > 1.50 nmol/L | 14/135 (10.4%) | single specimen collection within hospitalization (median time elapsed from hospital admission to MR-proADM measurement = 7 days) | - in-hospital mortality - ICU/sub-ICU admission | N/A |
| García de Guadiana-Romualdo L. 10.1016/j.ijid.2021.08.058 | multicenter prospective cohort study | 09/2020–10/2020 | 359 hospitalized COVID-19 patients | 90-day mortality: 32/359 (8.9%) | hospital admission | 90-day mortality | 0.8 nmol/L AUC 0.832 (95% CI: 0.770–0.894, 96.9% sensitivity, 58.4% specificity and 99.5% negative predictive value) |
| Mendez R. 10.1136/thoraxjnl-2020-216797 | prospective observational study | 03/2020–06/2020 | 210 COVID-19 patients at the ED (23 discharged and managed as outpatients, 179 with initial ward admission, 8 with initial ICU admission). Of these, 97 patients with biomarkers at day 1 and follow-up visit | 27/210 (12.8%) | - T1 (ED admission) - T2 (post-hospitalization follow-up visit, median time = 65 days) | in-hospital mortality | 1.16 nmol/L |
| Moore N. 10.1136/jclinpath-2021-207750 | prospective cohort study | 04/2020–06/2020 | 135 hospitalized COVID-19 patients | 30/135 (22.2%) | hospital admission | 30-day all-cause mortality, intubation and ventilation, critical care admission and NIV use | N/A (applied external cut-off values) AUC 0.8441 for 30-day mortality |
| Minieri M. 10.1186/s13054-021-03834-9 | retrospective cohort study | N/A | 321 COVID-19 patients at the ED | 97/321 (30.2%) | ED admission | in-hospital mortality | 1.105 nmol/L AUC 0.85 |
| Oblitas C.M. 10.3390/v13122445 | prospective cohort study | 08/2020–11/2020 | 95 COVID-19 ICU patients | 12/95 (12.6%) | ≤72 h from ICU admission | 30-day mortality and combined event (mortality, venous or arterial thrombosis, orotracheal intubation) | 1.0 nmol/L AUC for mortality 0.73 (95% CI: 0.63–0.81, positive likelihood ratio and negative likelihood ratio 2.40 and 0.46, respectively), AUC for combined event 0.72 (95% CI: 0.62–0.81, positive likelihood ratio and negative likelihood ratio 3.16 and 0.63, respectively) |
| Indirli R. 10.1111/eci.13753 | retrospective cohort study | 03/2020–06/2020 | 116 hospitalized COVID-19 patients | 21/116 (18.1%) | hospital admission | - in-hospital mortality - composite outcome (death, ICU admission, in-hospital complications), length of stay | 1.0 nmol/L AUC 0.79 (71.3% sensitivity, 85.7% specificity, 5.0 positive likelihood ratio and 0.33 negative likelihood ratio) |
| First Author /DOI | Study Design | Study Period | Population at Enrolment | Mortality Rate | Time of in vitro IFNγ Production Dosing | Endpoint | In Vitro IFNγ Production Cut-Off Value/Performance |
|---|---|---|---|---|---|---|---|
| Blot M. 10.1186/s12967-020-02646-9 | prospective cohort study | 11/2018–02/2020 | 63 hospitalized patients with severe pneumonia - 27 COVID-19 - 36 non-COVID-19 CAP 7 healthy controls | - COVID-19: 1/27 (3.7%) - non-COVID-19 CAP: 2/36 (5.5%) | ≤48 h from hospital admission | 30-day mortality | N/A |
| Ruetsch C. 10.3389/fmed.2020.603961 | prospective cohort study | 03/2020–04/2020 | 101 COVID-19 patients - 41 mild disease (outpatients) - 30 moderate disease (medical wards) - 30 severe disease (ICU) 50 healthy controls | 6/101 (5.9%) | at baseline (day 0) and follow-up time points up to 2 months after admission to the hospital (not further specified) | disease progression and complications (deep vein thrombosis, secondary bacterial infections, organ failure, ICU access and death) | 15 IU/mL |
| Cremoni M. 10.3389/fmed.2020.608804 | prospective cohort study | 04/2020–05/2020 | 29 HCWs with SARS-CoV-2 infection -13 asymptomatic -15 mild disease (outpatients) -1 moderate disease (hospitalized) 60 COVID-19 patients -30 moderate disease -30 severe disease (ICU) | N/A | Blood samples were collected at day 0 of the admission (patients) and at inclusion for HCWs | hospitalization | 12.1 IU/mL AUC 0.92 (51% sensitivity, 96% specificity) |
| Dhanda A.D. 10.1016/j.imbio.2022.152185 | prospective cohort study | 04/2020–05/2020, 02/2021 | 41 hospitalized COVID-19 patients - 11 with oxygen support - 1 in ICU at admission 12 healthy controls | 7/41 (17.1%) | at baseline | in-hospital mortality | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangioni, D.; Oggioni, M.; Chatenoud, L.; Liparoti, A.; Uceda Renteria, S.; Alagna, L.; Biscarini, S.; Bolis, M.; Di Modugno, A.; Mussa, M.; et al. Prognostic Value of Mid-Region Proadrenomedullin and In Vitro Interferon Gamma Production for In-Hospital Mortality in Patients with COVID-19 Pneumonia and Respiratory Failure: An Observational Prospective Study. Viruses 2022, 14, 1683. https://doi.org/10.3390/v14081683
Mangioni D, Oggioni M, Chatenoud L, Liparoti A, Uceda Renteria S, Alagna L, Biscarini S, Bolis M, Di Modugno A, Mussa M, et al. Prognostic Value of Mid-Region Proadrenomedullin and In Vitro Interferon Gamma Production for In-Hospital Mortality in Patients with COVID-19 Pneumonia and Respiratory Failure: An Observational Prospective Study. Viruses. 2022; 14(8):1683. https://doi.org/10.3390/v14081683
Chicago/Turabian StyleMangioni, Davide, Massimo Oggioni, Liliane Chatenoud, Arianna Liparoti, Sara Uceda Renteria, Laura Alagna, Simona Biscarini, Matteo Bolis, Adriana Di Modugno, Marco Mussa, and et al. 2022. "Prognostic Value of Mid-Region Proadrenomedullin and In Vitro Interferon Gamma Production for In-Hospital Mortality in Patients with COVID-19 Pneumonia and Respiratory Failure: An Observational Prospective Study" Viruses 14, no. 8: 1683. https://doi.org/10.3390/v14081683
APA StyleMangioni, D., Oggioni, M., Chatenoud, L., Liparoti, A., Uceda Renteria, S., Alagna, L., Biscarini, S., Bolis, M., Di Modugno, A., Mussa, M., Renisi, G., Ungaro, R., Muscatello, A., Gori, A., Ceriotti, F., & Bandera, A. (2022). Prognostic Value of Mid-Region Proadrenomedullin and In Vitro Interferon Gamma Production for In-Hospital Mortality in Patients with COVID-19 Pneumonia and Respiratory Failure: An Observational Prospective Study. Viruses, 14(8), 1683. https://doi.org/10.3390/v14081683






