Abstract
Human group A rotaviruses (RVA) are important enteric pathogens, as they are a leading cause of acute gastroenteritis (AGE) in children worldwide. Since 2013, the German Standing Committee on vaccination recommended the routine rotavirus vaccination for infants in Germany. While vaccination has significantly decreased RVA cases and worldwide mortality, in some cases, infants can develop acute gastroenteritis as an adverse reaction after immunization with an attenuated live vaccine. Pediatricians, as well as clinicians and diagnostic laboratories, contacted the Consultant Laboratory for Rotaviruses and inquired whether cases of RVA-positive AGE after vaccination were associated with vaccine or with wild-type RVA strains. A testing algorithm based on distinguishing PCRs and confirmative sequencing was designed, tested, and applied. Diagnostic samples from 68 vaccinated children and six cases where horizontal transmission was suspected were investigated in this study. Using a combination of real-time PCR, fragment-length analysis of amplicons from multiplex PCRs and confirmative sequencing, vaccine-like virus was detected in 46 samples and wild-type RVA was detected in 6 samples. Three mixed infections of vaccine and wild-type RVA were detectable, no RVA genome was found in 19 samples. High viral loads (>1.0 × 107 copies/g stool) were measured in most RVA-positive samples. Furthermore, information on co-infections with other AGE pathogens in the vaccinated study population was of interest. A commercial multiplex PCR and in-house PCRs revealed three co-infections of vaccinated infants with bacteria (two samples with Clostridioides difficile and one sample with enteropathogenic E. coli) and six co-infections with norovirus in a subset of the samples. Human astrovirus was detected in one sample, with suspected horizontal transmission. The cases of suspected horizontal transmission of vaccine RVA strains could not be confirmed, as they either involved wild-type RVA or were RVA negative. This study shows that RVA-positive AGE after vaccination is not necessarily associated with the vaccine strain and provides a reliable workflow to distinguish RVA vaccine strains from wild-type strains.
1. Introduction
Group A rotaviruses (RVA) are a leading cause of acute gastroenteritis (AGE) worldwide, particularly in children. A high percentage of children have been infected with RVA by three years of age, but older children and adults can also be affected [1]. RVA infections can cause severe diarrheal disease and dehydration in infants and young children and are highly associated with mortality in low-income countries [2,3]. It was estimated that 128,500 deaths caused by diarrhea were attributable to rotavirus infection among children younger than 5 years of age in 2016 [4].
Rotaviruses are classified as the genus Rotavirus in the family Reoviridae. The genome of 11 segments encodes for six viral structural proteins, VP1 to VP4 and VP6 to VP7, as well as six non-structural proteins (NSP1 to NSP6) [5]. Classification of RVA is standardized for all 11 genome segments, including a binary G+P-genotyping system based on VP7 (G type) and VP4 (P type) [6]. Currently, 42 different VP7 G types and 58 different VP4 P-types have been accepted by the Rotavirus Classification Working Group, and at least 16 G-types and 19 VP4 P-types have been identified for RVA strains infecting humans [7,8]. A small number of RVA genotype combinations circulate globally in the human population at higher frequencies, including combinations of G1, G3, G4, G9, G12 with P[8], and G2 with P[4]. Additionally, in some regions, P[6] is relevant in different genotype constellations [9].
The German Standing Committee on Vaccination (STIKO) has recommended the rotavirus vaccination for infants in Germany since 2013 [10]. Vaccination mimics natural RVA infections and has been identified as a major strategy to decrease the burden associated with severe and fatal rotavirus-induced diarrhea [11,12]. So far, two commercial vaccines have been licensed in Germany since 2006. The monovalent Rotarix® vaccine (RV1) contains a live attenuated human G1P1A[8] RVA strain. RotaTeq® (RV5) is a pentavalent bovine–human vaccine of five reassortant bovine strains containing genes that express outer capsid proteins of five common circulating human RVA strains (G1, G2, G3, G4, and P[8]), together with the antigens originating from the bovine RVA strain used as a vector (G6 and P7[5]). The vaccines RV5 and RV1 are administered orally to infants in two or three doses, respectively [10]. RVA vaccinations, as well as previous natural RVA infections [13], do not induce full protection against re-infection with wild-type RVA strains. However, AGE hospitalization rates significantly decreased after vaccination, as did episodes of severe AGE [10,14,15,16].
Adverse reactions can follow after RVA immunization in infants, especially AGE. Shedding of both live vaccines has been detected in stool samples for several days or weeks [17,18]. Shed vaccine strains can be transmitted to unvaccinated children, adults, and the elderly, where they can cause AGE [19,20,21,22]. The methods in the present study were developed to answer inquiries from pediatricians, as well as clinicians and diagnostic laboratories, regarding whether cases of AGE after vaccination were associated with vaccine strains or were due to coincidental infection with wild-type RVA strains. Molecular methods which reliably discriminate between vaccine-like strains and common wild-type RVA are useful for monitoring the rate of occurrence and clinical relevance of vaccine strains in symptomatic pediatric RVA infections in the relevant age group worldwide [23,24].
Therefore, a PCR-based algorithm including sensitive and highly specific PCRs was developed to identify RVA infections and to distinguish wild-type RVA strains from vaccine-like strains in human specimens, even in mixed infections.
2. Materials and Methods
2.1. Samples
A total of 72 stool samples, one cerebrospinal fluid sample (CSF), and one serum sample were investigated in this study, sent in for diagnostic characterization during the years 2009 to 2019. Sixty-eight out of seventy-four samples were from RV-vaccinated children with AGE, and six samples were from cases with suspected horizontal transmission. Stool samples were diluted 1:10 in phosphate-buffered saline before RNA extraction.
2.2. Design of the Workflow for Molecular Diagnostics and Discrimination between RVA Wild-Type and RVA Vaccine-like Strains
RNA was extracted from 140 µL of stool suspension, serum, or CSF using a QIAcube device (Qiagen, Hilden, Germany) with the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) and eluted in a total volume of 60 µL AVE (supplied elution buffer).
The first step of the RVA-discriminating workflow was the screening of samples for RVA genome with a sensitive and highly specific real-time RT-PCR method, as described previously [25]. Only RVA-positive samples were subjected to further analysis for the differentiation of wild-type RVA and vaccine-derived RVA.
Two highly specific and sensitive RT-PCR assay systems were implemented in the workflow of the RVA discrimination algorithm to identify wild-type RVA strains versus RV5-, or versus RV1 vaccine-like strains (Figure 1). The PCRs were applied according to known vaccination with RV5 or RV1. If no information on vaccination was available, both PCR systems were applied.
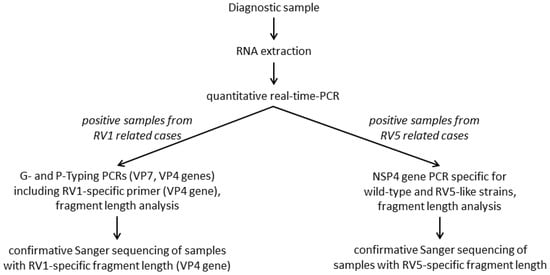
Figure 1.
Workflow of the molecular differentiation of wild-type RVA and vaccine-like strains from RV1 and RV5 related cases.
2.3. Discrimination of Wild-Type RVA and RV5
Discrimination between human wild-type RVA and RV5 was achieved using multiplex nested RT-PCR with primer sets located in the NSP4 gene of the RVA genome, producing amplicons of 119 bp to 122 bp in the second PCR round for RVA wild-type viruses and 197 bp for RV5 vaccine-like strains. First set of outer primers (Table 1): RV5-like (RoA71; RoA74); wild-type strains: (RoA61; RoA64c, RoA64d; RoA64e; RoA64f). Second PCR round: RV5-like (RoA72; RoA73), wild-type strains (RoA63a; RoA63b; RoA63c). All primers were purchased from TIB Molbiol (Berlin, Germany). After denaturation of RNA at 95 °C for 1 min, RT-PCR was performed with 5 µL of RNA in a total reaction volume of 12.5 µL with 240 nmol/l primers using a One-step RT-PCR kit (Qiagen, Hilden, Germany). Reverse transcription (RT)-reaction was carried out at 50 °C for 30 min and 15 min at 95 °C, followed by 30 cycles at 94 °C for 30 s, 42 °C for 30 s, 30 s at 72 °C, and a final elongation step at 72 °C for 5 min. The second PCR round was as follows: PCR products were diluted in water (1:20) and 1 µL (of the dilution) was added to the second PCR round in a reaction volume of 12.5 µL using HotStarTaq Master Mix Kit (Qiagen, Hilden, Germany) and 240 nmol/l primers. PCR conditions were as follows: 15 min at 95 °C, 30 cycles of 30 s at 94 °C, 30 s at 42 °C, 30 s at 72 °C, and a final elongation step at 72 °C for 5 min. PCR amplicons were visualized by agarose gel electrophoresis and the PCR results were confirmed by direct sequencing.

Table 1.
Primers and probes used for algorithm of RVA discrimination.
The validation of the workflow included testing of the specificity, sensitivity, and reproducibility in intra- and inter-assay comparison of three independent runs. Selected pre-characterized samples of RVA-positive patients (RVA wild-type virus strains) and RV5 vaccine were used to test the system. First, RVA genome in the samples was quantified by a previously described real-time RT-PCR [25]. After creating serial 1:10 dilutions of the quantified samples, the detection limit was estimated with endpoint titration and quantification of the endpoint dilution with the real time RT-PCR system. Determination of the detection limit was achieved using virus dilutions at the estimated detection limit, as well as dilutions 10-fold higher, 3-fold higher, 0.3-fold lower, and 0.1-fold lower than the estimated detection limit. The detection limit of the multiplex RV5/wild-type RT-PCR was defined as the lowest concentration where at least 95% of 24 samples tested positive (8 replicates tested in 3 independent runs). The determined detection limit was 600 RNA copies/reaction (1.3 × 106 copies/g stool) of wild-type virus and 60 RNA copies/reaction (1.3 × 105 copies/g stool) of RV5. Additionally, 100% reproducibility was found via intra- and inter-assay comparison of samples with the RV5 and a G1P[8] wild-type strain.
The specificity of the assay was tested with samples from patients with AGE which were known to be positive for human norovirus (3 samples) or positive for sapovirus (3 samples) or positive for three enteroviruses (echovirus 18, coxsackie B5 virus, and coxsackie A9 virus). All of these samples were negative.
2.4. Discrimination of Wild-Type RVA and RV1
Differentiation of wild-type RVA and RV1 was performed via a multiplex nested RT-PCR reaction using VP4-genotyping primers based on a published P typing assay [26] complemented with a new RV1-specific primer (published primer set [27], added RV1-specific sense primer, Table 1: RoA83; all primers from TIB Molbiol (Berlin, Germany). The different genotype-specific amplicon sizes of RVA P-types ([27], Table 1) can be determined by agarose gel electrophoresis.
In order to distinguish between wild-type P[8]- and vaccine-derived strains more reliably, especially in mixed infections, capillary fragment-length analysis was used to increase the overall specificity of amplicon size calling (ABI 3500xL Dx device and GeneMapper 5.0 software: Applied Biosystems, Forster City, CA, USA) [26]. Fragments of 246 bp for RV1-like strain and 224 bp for RVA wild-type strains could be distinguished reliably by two sharply distinct peaks. For confirmation, the RV1-like fragments were sequenced directly (Sanger) with PCR primers.
2.5. Detecting Co-Infections with Other AGE Pathogens
Samples from 2009 to 2015 that were PCR positive for RVA vaccine-like strains (RV1, RV5) and negative for wild-type RVA viruses were additionally investigated for the presence of other gastroenteritis pathogens using the multiplex PCR system GastroFinder™ SMART 17 FAST (PathoFinder B.V., Maastricht, Netherlands), detecting: Campylobacter jejuni, Campylobacter spp., Clostridioides difficile toxin A/B, Escherichia coli O157:H7, Salmonella spp., Shiga toxin-producing E. coli (STEC), Shigella spp., Yersinia enterocolitica, Aeromonas spp., Giardia lamblia, Entamoeba histolytica, Cryptosporidium spp., Dientamoeba fragilis, adenovirus (F40/41), astrovirus, RVA, norovirus (NV) (GI/GII/GIV), and in-house PCR assays for NV GI and GII, sapovirus (SaV), and all human astrovirus (HAstV) [28,29].
Samples from 2016 onwards were tested for NV, SaV, and HAstV using in-house PCR assays as previously described [30].
3. Results
3.1. Differentiation between Wild-Type RVA and RV5-like Strains
The differentiation of RV5-like strains and RVA wild-type virus strains was based on a multiplex reverse-transcription (RT) nested-PCR of the NSP4 gene, resulting in different fragment lengths for RV5-like and wild-type strains in both PCR rounds (Table 1).
This assay was used to test diagnostic samples for RV5-like strains using fragment-length analysis (Table 2). RV5-like PCR fragments were sequenced for verification. No discrepancies between fragment length analysis and sequencing were found. Mixed infections could be successfully sequenced using RV5- or wild-type specific primers, respectively.
3.2. Differentiation between Wild-Type RVA and RV1-like Strains
For specific detection of RV1-like strains, a well described and widely used P-typing method [26,27] was modified by addition of a primer with a higher melting temperature specific to RV1-like strains (RoA83, Table 1). Fragment length analysis was used to distinguish wild-type P[8] and RV1-like P[8] strains, followed by confirmative sequencing (Table 2). By sequencing all diagnostic samples, specificity of 100% for distinguishing wild-type and RV1-like strains was found. The RoA83 primer was also included in P typing for molecular surveillance of more than 5000 samples (data not shown), and RV1-positive results were verified via sequencing.
3.3. Patients
In this study, 74 patients with AGE were included: 68 vaccinees with suspected adverse reactions after vaccination, and 6 relatives with suspected horizontal transmission (Table 2). The median age of vaccinees was three months (range 1–10 months, two patients without data) and the rate of male and female vaccinees in this study was 56% and 43%, respectively (no gender was communicated in one case). Thirty-six children were vaccinated with RV1 and thirty-two were vaccinated with RV5.

Table 2.
Patients and results of samples from 2009 to 2019.
Table 2.
Patients and results of samples from 2009 to 2019.
| Patient No. | Sampling Year | Age [mo.] | Gender | Vaccine Used | d.p.v. | RV1/RV5 Detected | Wild-Type RVA Detected | Other AGE Pathogen Detected | ID |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2009 | 2 | Female | RV1 | 5 | + | − | NV | |
| 2 | 2009 | 5 | Male | RV5 | n.d. | − | − | NV | |
| 3 | 2009 | 5 | Female | RV5 | 34 | − | − | Not tested | |
| 4 | 2009 | 4 | Female | RV5 | >30 | − | − | Not tested | |
| 5 | 2009 | 4 | Male | RV5 | n.d. | + | − | − | SCID |
| 6 | 2009 | 6 | Male | RV1 | 76 | − | − | Not tested | |
| 7 | 2010 | 4 | Male | RV1 | 6 | + | − | − | SCID |
| 8 | 2010 | 2 | Female | RV5 | 7 | + | − | − | |
| 9 | 2011 | 2 | Female | RV1 | 16 | + | − | − | |
| 10 | 2011 | 6 | Male | RV1 | 6 | + | G1P[8] | C. diff. | |
| 11 | 2011 | 10 | Male | RV1 | n.d. | − | G9P[8] | Not tested | Unspecif. |
| 12 | 2011 | n.d. | Male | RV5 | n.d. | − | − | Not tested | |
| 13 | 2011 | n.d. | N.d. | RV5 | n.d. | + | − | − | |
| 14 | 2012 | 2 | Female | RV5 | 7 | + | − | − | |
| 15 | 2013 | 5 | Male | RV5 | n.d. | + | − | − | SCID |
| 16 | 2013 | 2 | Female | RV1 | 5 | − | G9P[8] | Not tested | |
| 17 | 2013 | 3 | Female | RV5 | 7 | + | − | − | |
| 18 | 2014 | 6 | Male | RV5 | n.d. | + | − | − | |
| 19 | 2014 | 3 | Male | RV1 | 7 | + | − | − | |
| 20 | 2014 | 7 | Female | RV5 | 6 | − | − | Not tested | |
| 21 | 2014 | 1 | Male | RV1 | 14 | − | − | Not tested | |
| 22 | 2014 | 4 | Female | RV5 | n.d. | − | − | Not tested | |
| 23 | 2014 | 3 | Female | RV1 | n.d. | + | − | − | |
| 24 | 2014 | 4 | Female | RV1 | n.d. | − | − | Not tested | |
| 25 | 2014 | 4 | Female | RV1 | 29 | − | − | Not tested | |
| 26 | 2014 | 3 | Female | RV1 | n.d. | + | − | − | |
| 27 | 2014 | 3 | Female | RV1 | n.d. | + | − | − | |
| 28 | 2015 | 2 | Male | RV5 | n.d. | + | − | C. diff. | |
| 29 | 2015 | 5 | Male | RV5 | n.d. | + | − | − | |
| 30 | 2015 | 2 | Female | RV5 | 3 | + | G9P[8] | EPEC | |
| 31 | 2015 | 12 | Female | Not vaccinated § | - | − | G9P[8] | Not tested | |
| 32 | 2015 | 5 | Male | RV5 | n.d. | + | − | − | |
| 33 | 2015 | 4 | Female | RV5 | n.d. | − | G3Px | Not tested | |
| 34 | 2015 | 8 | Male | RV5 | n.d. | + | − | − | |
| 35 | 2015 | 2 | Male | RV1 | 9 | + | − | − | |
| 36 | 2016 | 9 | Male | RV1 | 200 | + | − | − | SCID |
| 37 | 2016 | 4 | Male | RV5 | 3 | − | − | − | |
| 38 | 2016 | 2 | Male | RV1 | 10 | + | − | NV | |
| 39 | 2016 | 3 | Male | RV5 | 7 | − | − | − | Suspected |
| 40 | 2016 | 5 | Male | RV1 | 33 | + | − | − | |
| 41 | 2016 | 1 | Male | RV1 | 17 * | + | − | − | |
| 42 | 2016 | 4 | Male | RV5 | 34 | − | − | − | |
| 43 | 2016 | 3 | Female | RV1 | 34 | + | − | − | |
| 44 | 2016 | 3 | Male | RV1 | 23 | + | − | − | |
| 45 | 2016 | 2 | Female | RV1 | 17 | + | − | NV | |
| 46 | 2017 | 3 | Female | RV1 | 30 * | − | − | − | |
| 47 | 2017 | 6 | Male | RV1 | n.d. | − | − | − | |
| 48 | 2017 | 5 | Male | RV5 | 5 | + | − | − | |
| 49 | 2017 | 2 | Male | RV1 | 8 | + | − | − | |
| 50 | 2017 | 2 | Female | RV5 | 15 | + | − | − | |
| 51 | 2017 | 2 | Male | RV5 | 9 | + | − | − | |
| 52 | 2017 | 7 | Female | RV5 | 60 * | + | − | − | SCID |
| 53 | 2017 | 765 | Female | Not vacc. ° | n.d. | − | G2P[4] | − | |
| 54 | 2017 | 3 | Male | RV5 | 49 | − | − | − | |
| 55 | 2017 | 2 | Male | RV5 | 6 | + | − | − | |
| 56 | 2017 | 2 | Female | RV1 | 12 | − | − | − | |
| 57 | 2017 | 2 | Female | RV5 | 35 | + | − | − | |
| 58 | 2017 | 2 | Male | RV1 | 17 | + | − | − | |
| 59 | 2017 | 2 | Male | RV1 | 15 | + | − | − | |
| 60 | 2018 | 4 | Female | RV1 | 9 | − | − | NV | |
| 61 | 2018 | 2 | Male | RV1 | 12 | + | G3P[8] | − | |
| 62 | 2018 | 352 | Female | Not vacc. §§ | n.d. | − | G3P[8] | − | |
| 63 | 2018 | 19 | Female | Not vacc. §§ | n.d. | − | G3P[8] | − | |
| 64 | 2018 | 92 | Female | Not vacc. §§ | n.d. | − | G3P[8] | HAstV | |
| 65 | 2018 | 2 | Male | RV5 | 10 | + | − | − | |
| 66 | 2018 | 3 | Female | RV5 | 45 | + | − | − | |
| 67 | 2018 | 6 | Female | RV1 | 72 | + | − | − | Suspected |
| 68 | 2019 | 4 | Male | RV1 | 11 | + | − | − | |
| 69 | 2019 | 2 | Female | RV1 | 7 | − | G2P[4] | − | |
| 70 | 2019 | 2 | Female | RV1 | 12 | + | − | − | |
| 71 | 2019 | 7 | Male | RV5 | 195* | + | − | − | |
| 72 | 2019 | 3 | Male | RV1 | 36 | + | − | − | |
| 73 | 2019 | <1 | Male | Not vacc. ° | 28 | - | − | − | |
| 74 | 2019 | 4 | Male | RV1 | 47 | + | − | NV |
Not vacc.—not vaccinated, d.p.v.—days post vaccination, ID—immunodeficiency, n.d.—no data were present, * approximation (only month of vaccination available), § sibling to vaccinated patient 30, §§ relatives to vaccinated patient 62, ° no sample from suspected source received.
3.4. Shedding of Vaccine and Wild-Type Strains
Vaccine strains were detected in 46 of 68 vaccinees, but not in patients with suspected horizontal transmission (Table 3). Three vaccinees were positive for both vaccine and wild-type virus. Mixed infections were positive for RV1 and wild-type G1P[8], RV1 and G3P[8], and RV5 and G9P[8], respectively. Four vaccinees were negative for vaccine strains but positive for wild-type virus: two for G9P[8], one for G2P[4], and one for G3 (P not typeable).

Table 3.
Frequency of RVA vaccine and wild-type strains detected in the study group.
Regarding virus shedding after vaccination, a correlation analysis was performed with detected viral loads (RVA genome copies/g stool) and the time interval between vaccination and collection of the stool. Information on the exact date of vaccination and the sample collection date was available for 28 RVA-positive vaccinees (Figure 2), excluding samples from patients with known immunodeficiencies (prolonged shedding) and patients with mixed infection (vaccine strain and wild-type strain). RV1 vaccinated children had virus loads between 1.2 × 105 to 3.0 × 1012 copies/g stool with a median of 1.1 × 108 copies/g stool. RV5 vaccinated children showed copy numbers from 1.6 × 104 to 4.2 × 1011 copies/g stool with a median of 8.0 × 106 copies/g stool. The earliest day of sampling was three days after vaccination. The highest copy numbers were found in the first week after the last vaccination with RV1 (3.8 × 106 to 9.4 × 1010, median 2.1 × 109 copies/g stool) and RV5 (1.6 × 104 to 4.2 × 1011, median 2.7 × 1010 copies/g stool). After seven days, the median for RV1 and RV5 was 1.2 × 107 copies/g stool (range 1.2 × 105 to 9.7 × 109 copies/g stool) and 7.0 × 106 copies/g stool (range 4.6 × 105 to 5.8 × 107 copies/g stool), respectively. RVA genome could be detected up to 45 days after vaccination.
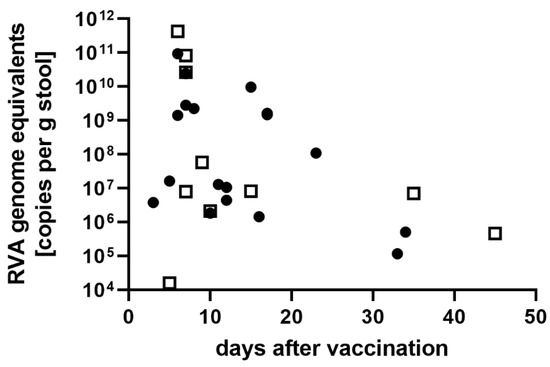
Figure 2.
RVA genome equivalents (viral load) detected in samples from immune-competent vaccinees (no known immune deficiencies). Closed circles: samples from RV1-vaccinated children, open squares: samples from RV5-vaccinated children.
3.5. RVA Shedding in Patients with Immunodeficiencies
Five children had severe combined immunodeficiencies (SCID), whereas immunodeficiency disease was unspecified in one case and suspected in two cases (Table 2). Four of these infants were vaccinated with RV5 and another four with RV1. The course of infection was described in detail for patient 36 [31]. This RV1-vaccinated patient, who suffered from SCID, persistently shed RV1 and only became RVA negative six months after hematopoietic stem cell transplantation [31].
Detected viral loads of vaccinees with SCID were in the range of 1.4 × 109 copies/g stool to 3.0 × 1012 copies/g stool. In the five vaccinated children with SCID, vaccine-derived RVA was detectable, while the child with unspecified immunodeficiency disease was positive for wild-type G9P[8].
3.6. Suspected Horizontal Transmission
Suspected horizontal transmission of vaccine strains was also analyzed in the study group (Table 3). Two samples were from siblings: an unvaccinated girl of 12 months and her sister of 2 months of age, who was vaccinated with RV5 (patients 30 and 31, Table 2). Both children were positive for RVA, with viral loads of 3.9 × 1011 copies/g stool for the vaccinated child and 1.9 × 1011 copies/g stool for the unvaccinated sibling. In both children, wild-type RVA G9P[8] was detectable. Additionally, the vaccinated child was positive for vaccine-derived RVA and EPEC.
Another case of suspected horizontal transmission was that of patient 53, where the grandchild (no sample received) was vaccinated and the grandmother, who had an autoimmune disease, developed diarrhea and acute renal failure. A wild-type G2P[4] RVA was detected, but no vaccine strain was found.
In the case of patient 61, horizontal transmission after vaccination to his mother and two siblings (patients 62, 63 and 64, Table 2) was suspected. Both RV1 vaccine strain and wild-type G3P[8] strain were found in the sample of patient 62. However, the samples of all relatives contained only the wild-type G3P[8] strain.
Finally, horizontal transmission was suspected in patient 73, a premature infant with necrotizing enterocolitis where the mother (no sample received) had contact with an RV1-vaccinated child. No RVA whatsoever was detected in the sample of the premature infant.
3.7. Screening for Co-Infection with Other AGE Pathogens
To analyze if vaccine strains were the only possible factor in AGE after vaccination, the 22 specimens that tested positive for RVA vaccine strains from 2009 to 2015 and 1 RVA-negative sample were also screened for co-infections with other causative agents of AGE using a commercial kit for gastrointestinal bacterial and viral pathogens (Table 2). Furthermore, all 37 samples from 2016 to 2019 were tested for norovirus (NV), human astroviruses (HAstV), and sapovirus (SaV). Co-infections of vaccine strains with NV were detected in four samples. Two NV-positive samples were negative for any RVA strain, and HAstV was detected in a sample positive for a RVA wild-type strain. No SaV was found. In three samples, human pathogenic bacterial genome was present, showing two samples with Clostridioides difficile (C. diff) and one with enteropathogenic E. coli (EPEC) (Table 2).
4. Discussion
In this study, an algorithm was developed and applied for diagnostic differentiation between vaccine-like RVA viruses and wild-type RVA strains. A panel of 68 samples from children with AGE who were vaccinated with either RV1 or RV5 was analyzed. In six additional cases, horizontal transmission of vaccine strains was suspected. Shedding of RVA was detectable in the majority of the samples. It is well known that vaccination with live attenuated RVA vaccines comes along with virus shedding. In this regard, a review of different studies estimated virus shedding of RVA to be approximately 10% (RV5) to 50% (RV1) of vaccine recipients when analyzed by ELISA [32]. Asymptomatic shedding of RV5 was detected in vaccinated children for up to 84 days after the third immunization dose [18,33]. In children with immune deficiency, shedding was detectable for a prolonged time [23,31,34,35]. One study demonstrated that the peak of virus shedding after administration of the first dose occurred between day 4 and day 7, with highest viral loads at day 6 and 7 [17]. It was reported that, after RV1 vaccination, the mean titer of virus shedding was 1.7 × 109 copies/g stool, and for RV5, the mean titer was 2.6 × 107 copies/g stool [17], which is lower than the virus loads in the present study. In this study with symptomatic vaccinees, the highest viral loads were also found within the first week after vaccination, peaking at day 7. However, the median viral load of RV5 strains was one order of magnitude higher than RV1 and more than two orders of magnitude higher than the previously determined peak of viral load for RV5 strains in healthy vaccinees at 6–7 days [17]. Due to the limited number of samples in the present study, this finding should be interpreted carefully. Moreover, while shedding of high viral loads of wild-type RVA has been associated with AGE and low viral loads with asymptomatic infection [36,37], healthy vaccinees shed high amounts of attenuated vaccine strains [17]. This is in line with a recently published study, in which no correlation between shedding of RVA vaccine strain and symptoms of AGE was observed [38]. Thus, shedding of high viral loads of RVA vaccine strains does not have a clear correlation with symptomatic infection, in contrast with RVA wild-type infection. Due to attenuation, vaccine strains can replicate at high levels without causing symptoms. A recent study showed no significant difference concerning the duration and amount of shedding of RV5 strains between asymptomatic and symptomatic vaccinees [24].
The situation is different with primary immune deficiencies. Five infants with SCID shed vaccine-like RVA while one child with unspecified immune deficiency disease suffered from wild-type virus infection. SCID often has only been diagnosed when a more severe infection or generally high morbidity became obvious in infants. However, live attenuated vaccines are of risk for children with SCID [39]. Shedding of vaccine strains at high viral loads can persist for months until immunocompetence has been established by hematopoietic stem cell transplantation or other therapies [31,35,40]. The WHO and STIKO emphasize the contra-indication of RVA vaccination in SCID patients [10,11]. In Germany, detection of SCID was added to the scope of general examinations of neonates by the Federal Joint Committee (G-BA) in July of 2019, which will help to prevent further cases of severe disease in vaccinees with SCID.
As duration of shedding and viral load of vaccine strains do not clearly correlate with disease in immunocompetent vaccinees, the detection of unspecified RVA or vaccine strain in particular is not a fully reliable indicator for the etiological agent. Therefore, screening for other possible pathogens should be considered. Co-infection of RVA with other gastroenteritis pathogens has been described. Depending on the published study design, RVA was detected as co-infection with, e.g., adenovirus, norovirus, astrovirus, sapovirus, bocavirus, Clostridioides difficile, enterotoxigenic Escherichia coli, Shigella, Campylobacter jejuni, Giardia, and Entamoeba histolytica [41,42,43,44]. In the present study, co-infections of RVA with other AGE pathogens was demonstrable in 8 out of 51 tested samples (16%). Mixed infection of RVA vaccine and wild-type strains was found in three of the 50 RVA-positive vaccinees (6%). Thus, differentiation of vaccine and wild-type strains is needed to identify co-incidental infection with wild-type RVA as a possible etiological agent of AGE, and complements screening for pathogens.
For diagnostic differentiation, a set of RT-PCRs specific for the vaccines RV1 or RV5 were designed and applied, as shown in the present study. These assays have been used for more than 10 years. They rely on a basic RT-PCR setup with fragment-length analysis for both vaccine strains, and do not include real-time PCR, sequencing, or digestion by restriction enzymes for differentiation, and therefore differ from previously published studies with respect to methodology [20,23,24,45]. The sensitivity for detection of mixed infections with vaccine and wild-type strains is higher than Sanger sequencing, still distinguishing up to three orders of magnitude difference in vaccine and wild-type copy numbers. Discrimination of the strains can be achieved via gel electrophoresis with acceptable specificity. However, due to higher-resolution fragment-length analysis, using a capillary sequencer for the highly multiplexed P typing assay (RV1) is helpful to exclude false-positive signals that may result from multiplex PCR of nucleic extracts from stool samples. Additionally, sequencing confirms if the detected vaccine-like strains from fragment-length analysis are actually vaccine derived or (very rarely) just closely related wild-types, and identifies possible genetic drift after longer periods of shedding. However, confirmative sequencing of RV1-like strains revealed no case of mistyping by fragment-length analysis. This is in line with the finding that the vast majority of circulating P[8] strains are of lineage III [46], and therefore differ significantly from the RV1 strain (lineage I). The same applies to the RV5 vaccine strains, all of which contain the NSP4 gene originating from a bovine rotavirus. Thus, confirmative sequencing might be added as additional layer of information, but is not needed in every setting.
Specific detection of wild-type and vaccine strains was also used in the cases of suspected horizontal transmission of vaccine strains by relatives. If RVA was found in samples from relatives, it was wild-type RVA, whereas vaccinees had mixed infections with wild-type and vaccine strains. While no horizontal transmission of vaccine strains was detected in the present study, vaccine strains can be transmitted and may cause symptoms. A possible dose dependency was discussed in the case of an unvaccinated two-year-old sibling with AGE, who was infected with RV1 after handling the stool-discharged diaper of the younger sibling that had been vaccinated [47].
In the present study, RV1 and RV5 vaccine strains and wild-type strains with common genotypes were detected (VP7: G1, G2, G3 and G9, VP4: P[4] and P[8]). With the implementation of live attenuated RVA vaccines, surveillance and monitoring studies started to trace the impact of RV immunization to the prevalence of common RVA genotypes and the emergence of immune escape RVA strains [20,48,49]. Worldwide, there is great diversity in wild-type RVA strains. This diversity is mainly influenced by several mechanisms: accumulation of point mutations, reassortment, direct transmission of animal strains into humans, and gene rearrangement into coding or non-coding regions [50]. Due to differences in the diversity of RVA genotypes in developing and developed countries, it is important to monitor genotype variation worldwide to detect vaccine-reassortant strains [45,50,51,52]. The specific detection of RV1 strains described in the present study, enabled by addition of primer RoA83 to a published P typing assay, is also being used for molecular surveillance [27].
With regard to the introduction of rotavirus vaccines such as Rotavac® and Rotasiil®, further vaccine development [53] and the impact of different vaccines on the herd immunity, it is mandatory to monitor virus variability [54]. A previously published study investigated the effectiveness of mixed rotavirus vaccinations [55]. Administration of mixed vaccines will pose a challenge for discrimination between wild-type RVA and vaccine strains in the future.
Previous studies reported on different methods of discriminating RVA strains from vaccine-like strains [20,45,56,57,58]. An assay distinguished between RV1 vaccine-like strain and RVA wild-type virus targeting the NSP3 gene using multiplex RT-PCR and BspHI-endonuclease restriction-length polymorphism [20]. Another RT-PCR method discriminated between RV5 vaccine-like and RVA wild-type, targeting the NSP3 gene [56]. A previously described method is based on real-time RT-PCR technology. This assay focused on the differentiation of RV5, RV1, and wild-type RVA strains in a multiplex reaction, detecting RVA G/P types G12, G9, G4, G3, G2, P[4], P[8], and P[6], respectively. However, the authors recommended confirming RV-positive real-time PCR results with direct sequencing or next-generation sequencing [57]. Bucardo et al. applied full-genome sequencing and detected reassortants of wild-type strains with a NSP2 gene identical to the RV5 strains, which would not be detectable with assays focusing on specific other genes, including the assays presented here. To prevent reassortment with vaccine strains and reduce the risk of adverse events, non-replicating RVA vaccines could be an alternative in the future, if they prove to be at least as efficacious as current live vaccines [59,60].
Since the workflow in the present study is mainly based on nested RT-PCR techniques and fragment-length analysis, it is economical and easy to implement with basic laboratory equipment and therefore useful in any setting. It is also low budget. A capillary sequencer for fragment-length analysis and Sanger sequencing is helpful for confirmation and increased specificity, but is not required.
In conclusion, this study provides relevant data concerning the detection of RVA vaccine and wild-type strains, as well as viral loads in cases of suspected adverse reactions and horizontal transmission. An algorithm for use in differential diagnosis and molecular surveillance has been developed to discriminate reliably between RVA wild-type and RVA vaccine-like strains in patient samples using molecular methods. The emphasis was on an easy-to-follow protocol including application of common molecular methods.
The specific detection of RVA wild-type strains as likely etiological agents instead of vaccine strains in some cases added important data for differential diagnosis. It is not sufficient to apply common RVA assays to cases of AGE with suspected involvement of RVA vaccine strains. The detection of RVA should include discrimination between vaccine and wild-type strains.
Author Contributions
Conceptualization, A.M.M.; methodology, R.L. and A.M.M.; validation, R.L. and A.M.M.; formal analysis, S.J. and A.M.M.; investigation, S.J., S.N., R.L. and A.M.M.; resources, C.-T.B.; data curation, S.J., S.N., R.L. and A.M.M.; writing—original draft preparation, S.J.; writing—review and editing, S.N. and A.M.M.; visualization, S.J. and A.M.M.; supervision, S.J., S.N., C.-T.B. and A.M.M.; project administration, A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Stool and CSF samples were from patients with acute gastroenteritis (AGE) which were sent for genotyping purposes to the Consultant Laboratory for Rotaviruses at the Robert Koch-Institute, Berlin, Germany, by local health authorities, pediatricians, hospitals and diagnostic laboratories for diagnostic clarification. Further investigations using these samples were carried out on the basis of and in accordance with the regulations of section 13 paragraph 3 of the German Infection Protection Act. The analyses of all patient data were done anonymously and retrospectively. For the reasons explained an ethics committee approval was not required.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Kathrin Stanossek, Ute Obst, Anja Lerch, and Sonja Zimmermann for their excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clarke, E.; Desselberger, U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U. Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.; Ramani, S.; Lopman, B.A.; Church, J.A.; Iturriza-Gomara, M.; Prendergast, A.J.; Grassly, N.C. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018, 13, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Banyai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef]
- Rotavirus Classification Working Group: List of Accepted RVA Genotypes. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 6 July 2022).
- Banga-Mingo, V.; Esona, M.D.; Betrapally, N.S.; Gautam, R.; Jaimes, J.; Katz, E.; Waku-Kouomou, D.; Bowen, M.D.; Gouandjika-Vasilache, I. Whole gene analysis of a genotype G29P[6] human rotavirus strain identified in Central African Republic. BMC Res. Notes 2021, 14, 218. [Google Scholar] [CrossRef]
- Doro, R.; Laszlo, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Banyai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Koch, J.; Wiese-Posselt, M.; Remschmidt, C.; Wichmann, O.; Bertelsmann, H.; Garbe, E.; Hengel, H.; Meerpohl, J.J.; Mas Marques, A.; Oppermann, H.; et al. Background paper to the recommendation for routine rotavirus vaccination of infants in Germany. Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 957–984. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Rotavirus vaccines: WHO position paper—July 2021. Weekly Epidemiol. Record. 2021, 96, 219–301. Available online: https://www.who.int/publications/i/item/WHO-WER9628 (accessed on 6 July 2022).
- Soares-Weiser, K.; Bergman, H.; Henschke, N.; Pitan, F.; Cunliffe, N. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2019, 3, CD008521. [Google Scholar] [CrossRef]
- Velazquez, F.R.; Matson, D.O.; Calva, J.J.; Guerrero, L.; Morrow, A.L.; Carter-Campbell, S.; Glass, R.I.; Estes, M.K.; Pickering, L.K.; Ruiz-Palacios, G.M. Rotavirus infections in infants as protection against subsequent infections. N. Engl. J. Med. 1996, 335, 1022–1028. [Google Scholar] [CrossRef]
- Bergman, H.; Henschke, N.; Hungerford, D.; Pitan, F.; Ndwandwe, D.; Cunliffe, N.; Soares-Weiser, K. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2021, 11, CD008521. [Google Scholar] [CrossRef]
- Marquis, A.; Koch, J. Impact of Routine Rotavirus Vaccination in Germany: Evaluation Five Years After Its Introduction. Pediatr. Infect. Dis. J. 2020, 39, e109–e116. [Google Scholar] [CrossRef]
- Dettori, S.; Cortesia, I.; Mariani, M.; Opisso, A.; Mesini, A.; Saffioti, C.; Castagnola, E. Impact of rotavirus vaccine in reducing hospitalization rates in pediatric patients: A single center experience in Italy. Hum. Vaccines Immunother. 2021, 17, 5646–5649. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Wu, F.T.; Hsiung, C.A.; Wu, H.S.; Chang, K.Y.; Huang, Y.C. Comparison of virus shedding after lived attenuated and pentavalent reassortant rotavirus vaccine. Vaccine 2014, 32, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Markkula, J.; Hemming, M.; Vesikari, T. Detection of vaccine-derived rotavirus strains in nonimmunocompromised children up to 3-6 months after RotaTeq(R) vaccination. Pediatr. Infect. Dis. J. 2015, 34, 296–298. [Google Scholar] [CrossRef]
- Payne, D.C.; Edwards, K.M.; Bowen, M.D.; Keckley, E.; Peters, J.; Esona, M.D.; Teel, E.N.; Kent, D.; Parashar, U.D.; Gentsch, J.R. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics 2010, 125, e438–e441. [Google Scholar] [CrossRef]
- Rose, T.L.; Miagostovich, M.P.; Leite, J.P. Rotavirus A genotype G1P[8]: A novel method to distinguish wild-type strains from the Rotarix vaccine strain. Mem. Inst. Oswaldo Cruz. 2010, 105, 1068–1072. [Google Scholar] [CrossRef]
- Roczo-Farkas, S.; Bines, J.E.; Australian Rotavirus Surveillance, G. Australian Rotavirus Surveillance Program: Annual Report, 2018. Commun. Dis. Intell. 2021, 45, 1–18. [Google Scholar] [CrossRef]
- Thomas, S.; Donato, C.M.; Roczo-Farkas, S.; Hua, J.; Bines, J.E.; Australian Rotavirus Surveillance, G. Australian Rotavirus Surveillance Program: Annual Report, 2019. Commun. Dis. Intell. 2021, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gower, C.M.; Dunning, J.; Nawaz, S.; Allen, D.; Ramsay, M.E.; Ladhani, S. Vaccine-derived rotavirus strains in infants in England. Arch. Dis. Child. 2020, 105, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Whiley, D.M.; Ware, R.S.; Kirkwood, C.D.; Lambert, S.B.; Grimwood, K. Multivalent Rotavirus Vaccine and Wild-type Rotavirus Strain Shedding in Australian Infants: A Birth Cohort Study. Clin. Infect. Dis. 2018, 66, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Japhet, M.O.; Famurewa, O.; Iturriza-Gomara, M.; Adesina, O.A.; Opaleye, O.O.; Niendorf, S.; Bock, C.T.; Mas Marques, A. Group A rotaviruses circulating prior to a national immunization programme in Nigeria: Clinical manifestations, high G12P[8] frequency, intra-genotypic divergence of VP4 and VP7. J. Med. Virol. 2018, 90, 239–249. [Google Scholar] [CrossRef]
- Mas Marques, A.; Diedrich, S.; Huth, C.; Schreier, E. Group A rotavirus genotypes in Germany during 2005/2006. Arch. Virol. 2007, 152, 1743–1749. [Google Scholar] [CrossRef]
- Iturriza-Gomara, M.; Kang, G.; Gray, J. Rotavirus genotyping: Keeping up with an evolving population of human rotaviruses. J. Clin. Virol. 2004, 31, 259–265. [Google Scholar] [CrossRef]
- Oka, T.; Katayama, K.; Hansman, G.S.; Kageyama, T.; Ogawa, S.; Wu, F.T.; White, P.A.; Takeda, N. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 2006, 78, 1347–1353. [Google Scholar] [CrossRef]
- Bernard, H.; Hohne, M.; Niendorf, S.; Altmann, D.; Stark, K. Epidemiology of norovirus gastroenteritis in Germany 2001-2009: Eight seasons of routine surveillance. Epidemiol. Infect. 2014, 142, 63–74. [Google Scholar] [CrossRef]
- Japhet, M.O.; Famurewa, O.; Adesina, O.A.; Opaleye, O.O.; Wang, B.; Hohne, M.; Bock, C.T.; Mas Marques, A.; Niendorf, S. Viral gastroenteritis among children of 0-5 years in Nigeria: Characterization of the first Nigerian aichivirus, recombinant noroviruses and detection of a zoonotic astrovirus. J. Clin. Virol. 2019, 111, 4–11. [Google Scholar] [CrossRef]
- Rosenfeld, L.; Mas Marques, A.; Niendorf, S.; Hofmann, J.; Gratopp, A.; Kuhl, J.S.; Schulte, J.H.; von Bernuth, H.; Voigt, S. Life-threatening systemic rotavirus infection after vaccination in severe combined immunodeficiency (SCID). Pediatr. Allergy Immunol. 2017, 28, 841–843. [Google Scholar] [CrossRef]
- Anderson, E.J. Rotavirus vaccines: Viral shedding and risk of transmission. Lancet Infect. Dis. 2008, 8, 642–649. [Google Scholar] [CrossRef]
- Markkula, J.; Hemming-Harlo, M.; Vesikari, T. Shedding of oral pentavalent bovine-human reassortant rotavirus vaccine indicates high uptake rate of vaccine and prominence of G-type G1. Vaccine 2020, 38, 1378–1383. [Google Scholar] [CrossRef]
- Patel, N.C.; Hertel, P.M.; Estes, M.K.; de la Morena, M.; Petru, A.M.; Noroski, L.M.; Revell, P.A.; Hanson, I.C.; Paul, M.E.; Rosenblatt, H.M.; et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N. Engl. J. Med. 2010, 362, 314–319. [Google Scholar] [CrossRef]
- Kaplon, J.; Cros, G.; Ambert-Balay, K.; Leruez-Ville, M.; Chomton, M.; Fremy, C.; Pothier, P.; Blanche, S. Rotavirus vaccine virus shedding, viremia and clearance in infants with severe combined immune deficiency. Pediatr. Infect. Dis. J. 2015, 34, 326–328. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Sarkar, R.; Menon, V.K.; Babji, S.; Paul, A.; Rajendran, P.; Sowmyanarayanan, T.V.; Moses, P.D.; Iturriza-Gomara, M.; Gray, J.J.; et al. Rotavirus shedding in symptomatic and asymptomatic children using reverse transcription-quantitative PCR. J. Med. Virol. 2013, 85, 1661–1668. [Google Scholar] [CrossRef]
- Bennett, A.; Bar-Zeev, N.; Jere, K.C.; Tate, J.E.; Parashar, U.D.; Nakagomi, O.; Heyderman, R.S.; French, N.; Iturriza-Gomara, M.; Cunliffe, N.A. Determination of a Viral Load Threshold To Distinguish Symptomatic versus Asymptomatic Rotavirus Infection in a High-Disease-Burden African Population. J. Clin. Microbiol. 2015, 53, 1951–1954. [Google Scholar] [CrossRef][Green Version]
- Zhuo, R.; Tarr, G.A.M.; Xie, J.; Freedman, S.B.; Payne, D.C.; Lee, B.E.; McWilliams, C.; Chui, L.; Ali, S.; Pang, X.; et al. Detection and Clinical Implications of Monovalent Rotavirus Vaccine-Derived Virus Strains in Children with Gastroenteritis in Alberta, Canada. J. Clin. Microbiol. 2021, 59, e0115421. [Google Scholar] [CrossRef]
- Bakare, N.; Menschik, D.; Tiernan, R.; Hua, W.; Martin, D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: Reports to the Vaccine Adverse Events Reporting System (VAERS). Vaccine 2010, 28, 6609–6612. [Google Scholar] [CrossRef]
- Palau, M.J.; Vescina, C.M.; Regairaz, L.; Cabanillas, D.; Stupka, J.A.; Degiuseppe, J.I. Persistent infection with a rotavirus vaccine strain in a child suffering from Severe Combined Immunodeficiency in Argentina. Rev. Argent. Microbiol. 2021, 53, 216–219. [Google Scholar] [CrossRef]
- Imade, P.E.; Eghafona, N.O. Viral Agents of Diarrhea in Young Children in Two Primary Health Centers in Edo State, Nigeria. Int. J. Microbiol. 2015, 2015, 685821. [Google Scholar] [CrossRef]
- Li, L.L.; Liu, N.; Humphries, E.M.; Yu, J.M.; Li, S.; Lindsay, B.R.; Stine, O.C.; Duan, Z.J. Aetiology of diarrhoeal disease and evaluation of viral-bacterial coinfection in children under 5 years old in China: A matched case-control study. Clin. Microbiol. Infect. 2016, 22, 381 e9–381 e16. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Khurshid, A.; Shaukat, S.; Sharif, S.; Suleman, R.M.; Angez, M.; Nisar, N.; Aamir, U.B.; Naeem, M.; Zaidi, S.S. Human bocavirus in Pakistani children with gastroenteritis. J. Med. Virol. 2015, 87, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Vasco, G.; Trueba, G.; Atherton, R.; Calvopina, M.; Cevallos, W.; Andrade, T.; Eguiguren, M.; Eisenberg, J.N. Identifying etiological agents causing diarrhea in low income Ecuadorian communities. Am. J. Trop. Med. Hyg. 2014, 91, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Esona, M.D.; Mijatovic-Rustempasic, S.; Ian Tam, K.; Gentsch, J.R.; Bowen, M.D. Real-time RT-PCR assays to differentiate wild-type group A rotavirus strains from Rotarix((R)) and RotaTeq((R)) vaccine strains in stool samples. Hum. Vaccines Immunother. 2014, 10, 767–777. [Google Scholar] [CrossRef]
- Pietsch, C.; Schuster, V.; Liebert, U.G. A hospital based study on inter- and intragenotypic diversity of human rotavirus A VP4 and VP7 gene segments, Germany. J. Clin. Virol. 2011, 50, 136–141. [Google Scholar] [CrossRef]
- Sakon, N.; Miyamoto, R.; Komano, J. An infant with acute gastroenteritis caused by a secondary infection with a Rotarix-derived strain. Eur. J. Pediatr. 2017, 176, 1275–1278. [Google Scholar] [CrossRef]
- Gibory, M.; Bruun, T.; Flem, E.; Dembinski, J.L.; Haltbakk, I.; Stordal, K.; Nordbo, S.A.; Jakobsen, K.; Haarr, E.; Leegaard, T.M.; et al. Genetic diversity of rotavirus strains circulating in Norway before and after the introduction of rotavirus vaccination in children. J. Med. Virol. 2021, 96, 2624–2631. [Google Scholar] [CrossRef]
- Degiuseppe, J.I.; Stupka, J.A. Genotype distribution of Group A rotavirus in children before and after massive vaccination in Latin America and the Caribbean: Systematic review. Vaccine 2020, 38, 733–740. [Google Scholar] [CrossRef]
- Kirkwood, C.D. Genetic and antigenic diversity of human rotaviruses: Potential impact on vaccination programs. J. Infect. Dis. 2010, 202, S43–S48. [Google Scholar] [CrossRef]
- Boom, J.A.; Sahni, L.C.; Payne, D.C.; Gautam, R.; Lyde, F.; Mijatovic-Rustempasic, S.; Bowen, M.D.; Tate, J.E.; Rench, M.A.; Gentsch, J.R.; et al. Symptomatic infection and detection of vaccine and vaccine-reassortant rotavirus strains in 5 children: A case series. J. Infect. Dis. 2012, 206, 1275–1279. [Google Scholar] [CrossRef][Green Version]
- Donato, C.M.; Ch’ng, L.S.; Boniface, K.F.; Crawford, N.W.; Buttery, J.P.; Lyon, M.; Bishop, R.F.; Kirkwood, C.D. Identification of Strains of Rotavirus Vaccine RotaTeq(R) in Infants with Gastroenteritis Following Routine Vaccination. J. Infect. Dis. 2012, 206, 377–383. [Google Scholar] [CrossRef]
- Hallowell, B.D.; Tate, J.; Parashar, U. An overview of rotavirus vaccination programs in developing countries. Expert Rev Vaccines 2020, 19, 529–537. [Google Scholar] [CrossRef]
- Yen, C.; Healy, K.; Tate, J.E.; Parashar, U.D.; Bines, J.; Neuzil, K.; Santosham, M.; Steele, A.D. Rotavirus vaccination and intussusception—Science, surveillance, and safety: A review of evidence and recommendations for future research priorities in low and middle income countries. Hum. Vaccines Immunother. 2016, 12, 2580–2589. [Google Scholar] [CrossRef]
- Mohammed, A.; Immergluck, L.; Parker, T.C.; Jain, S.; Leong, T.; Anderson, E.J.; Jerris, R.C. Association between mixed rotavirus vaccination types of infants and rotavirus acute gastroenteritis. Vaccine 2015, 33, 5670–5677. [Google Scholar] [CrossRef]
- Jeong, S.; Than, V.T.; Lim, I.; Kim, W. Differentiation of RotaTeq((R)) vaccine strains from wild-type strains using NSP3 gene in reverse transcription polymerase chain reaction assay. J. Virol. Methods 2016, 237, 72–78. [Google Scholar] [CrossRef]
- Gautam, R.; Mijatovic-Rustempasic, S.; Esona, M.D.; Tam, K.I.; Quaye, O.; Bowen, M.D. One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains (Rotarix(R) and RotaTeq(R)) in stool samples. PeerJ 2016, 4, e1560. [Google Scholar] [CrossRef]
- Bucardo, F.; Rippinger, C.M.; Svensson, L.; Patton, J.T. Vaccine-derived NSP2 segment in rotaviruses from vaccinated children with gastroenteritis in Nicaragua. Infect. Genet. Evol. 2012, 12, 1282–1294. [Google Scholar] [CrossRef]
- Burke, R.M.; Tate, J.E.; Kirkwood, C.D.; Steele, A.D.; Parashar, U.D. Current and new rotavirus vaccines. Curr. Opin. Infect. Dis. 2019, 32, 435–444. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Ma, L.F.; Carey, M.E.; Steele, A.D. The rotavirus vaccine development pipeline. Vaccine 2017, 37, 7328–7335. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).