Temporal and Spatial Patterns and a Space–Time Cluster Analysis of Foot-and-Mouth Disease Outbreaks in Ethiopia from 2010 to 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Description of Data
2.2. Data Analysis
3. Results
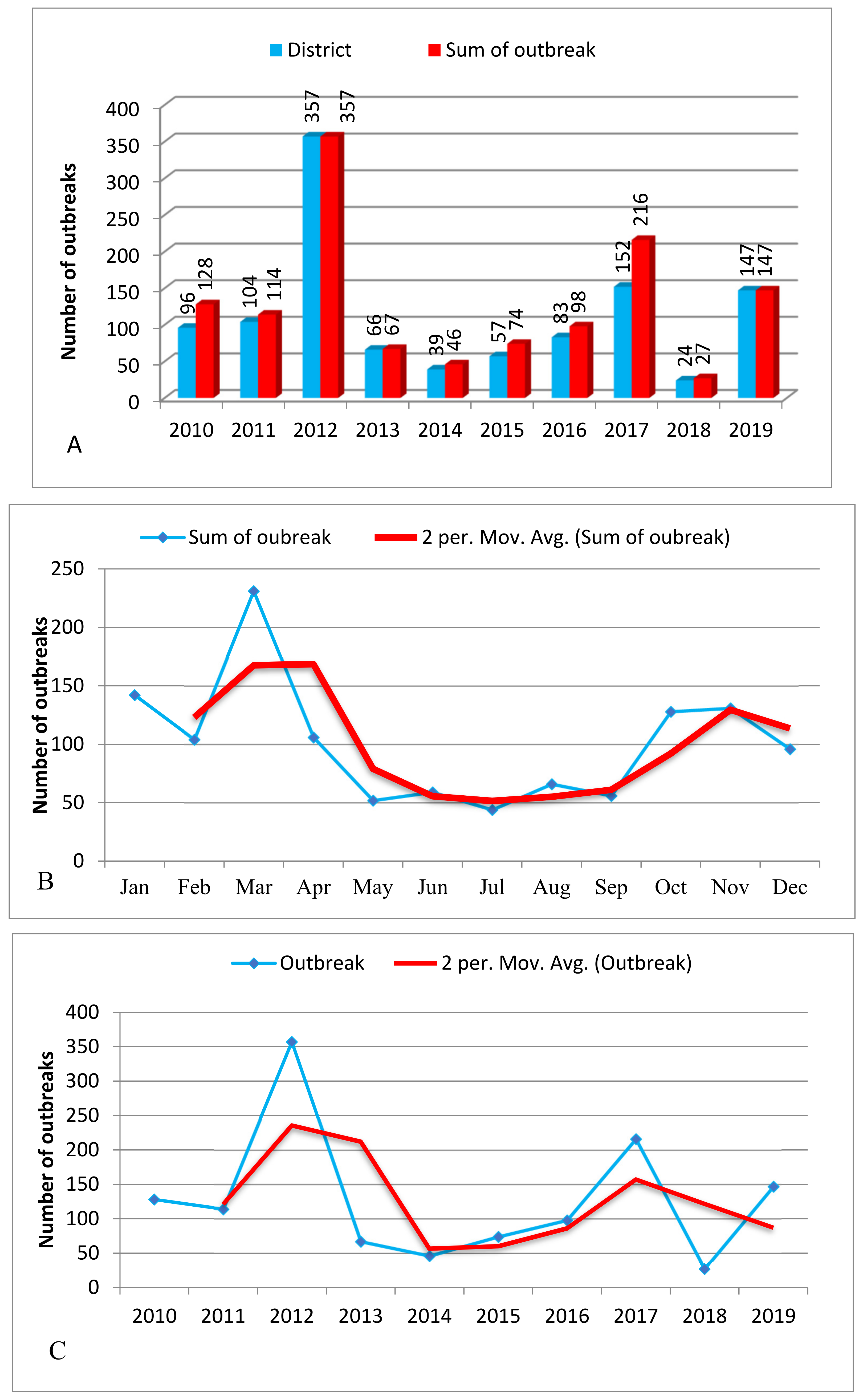
3.1. Case Distribution of FMD Infections by Year
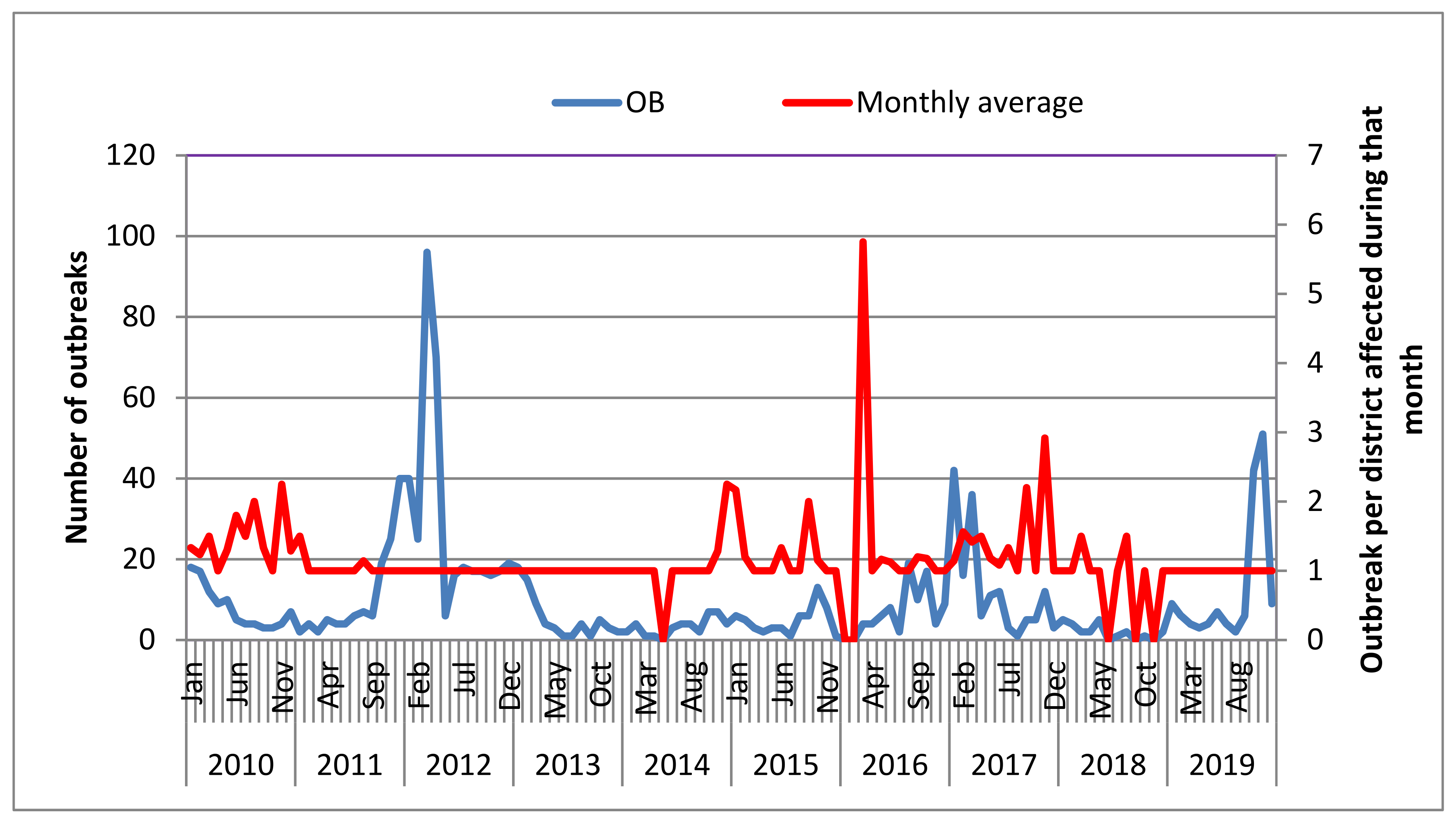
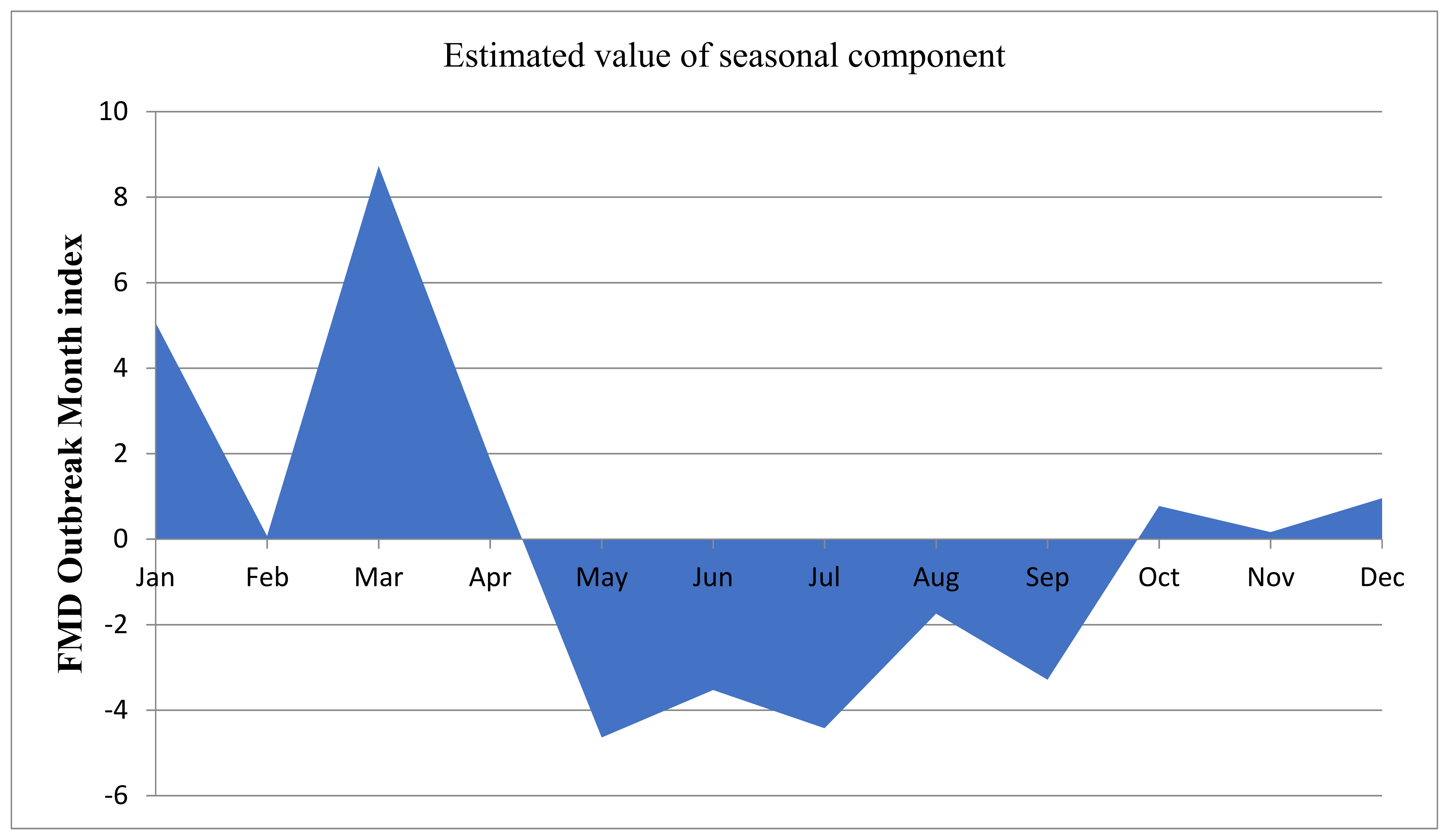
3.2. Temporal Distributions of FMD Outbreak by Year
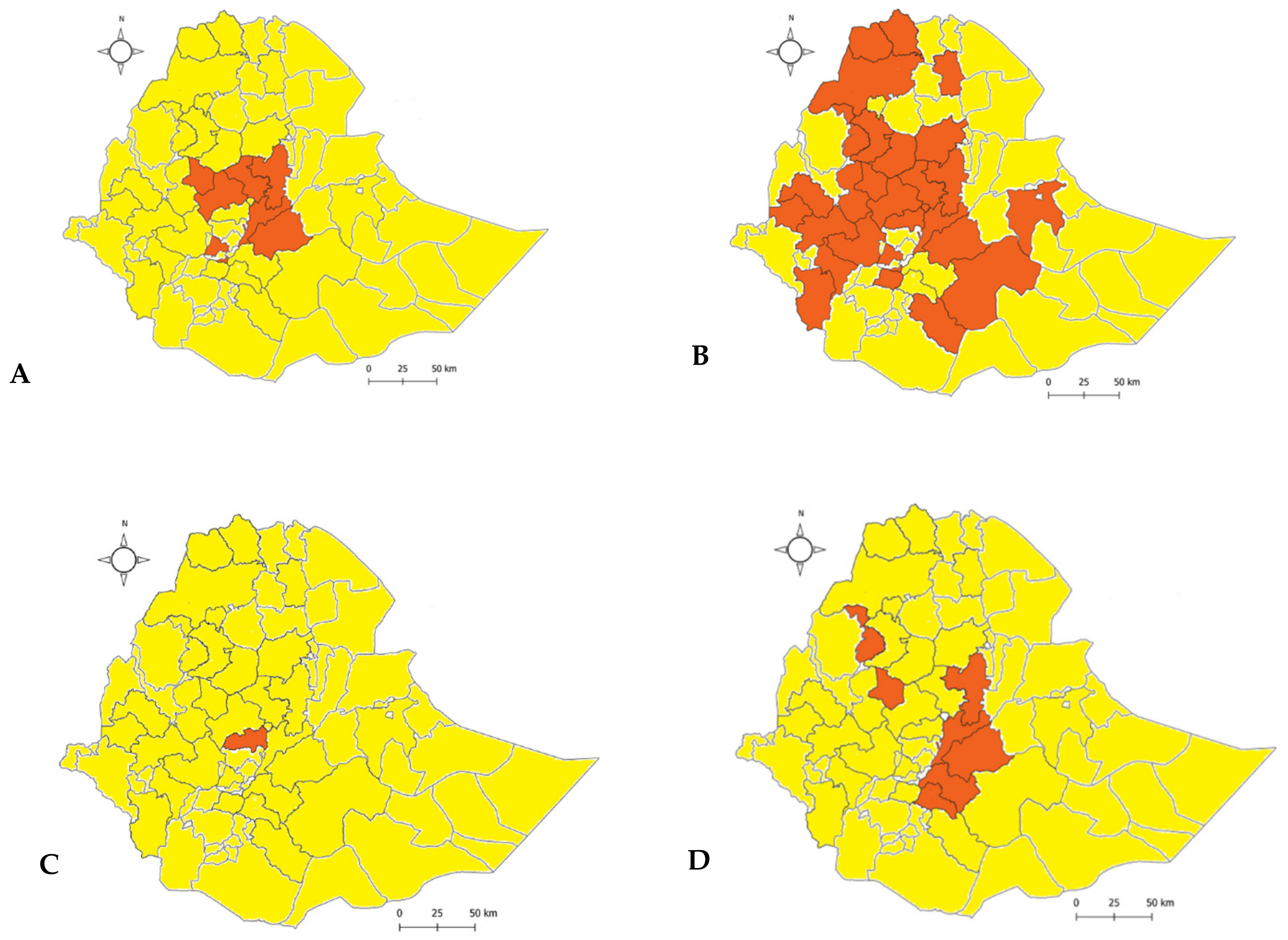
3.3. Outbreak and Serotype Distribution
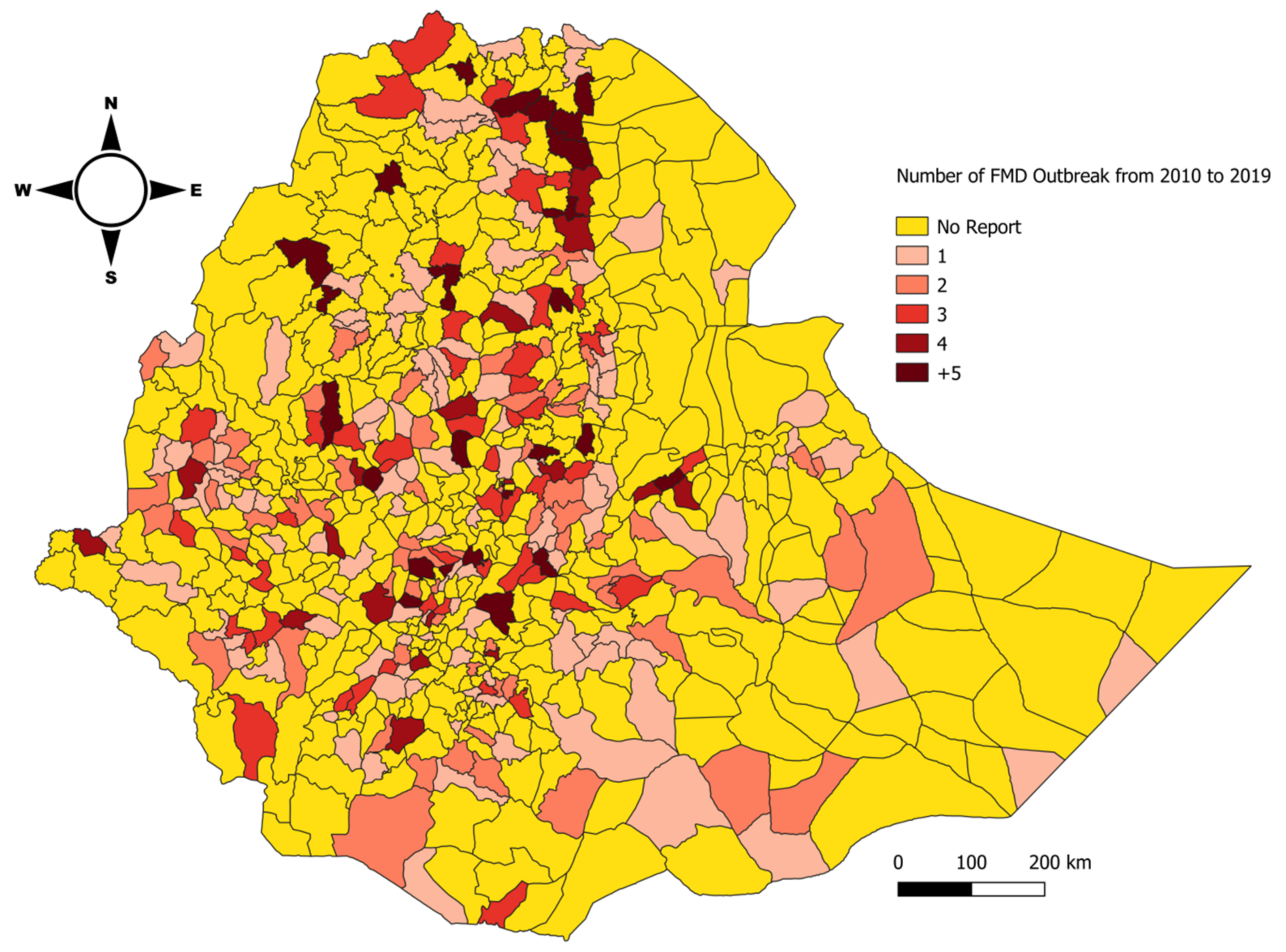
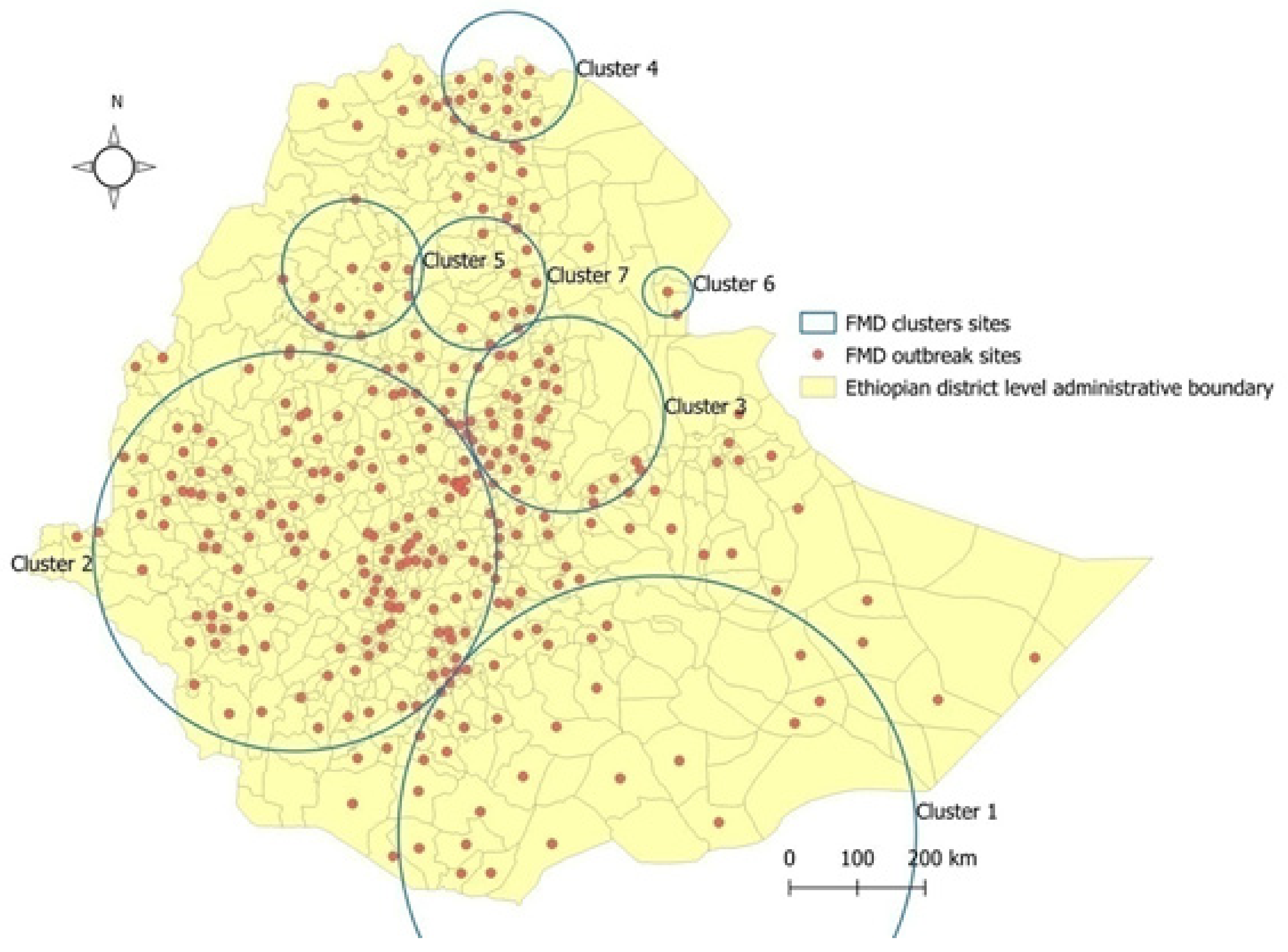
3.4. Space–Time Cluster Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grubman, M.J.; Baxt, B. Foot-and-Mouth Disease. Clin Microbiol Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, S.; Zhang, Z.; Donaldson, A.I.; Garland, A.J.M. The Pathogenesis and Diagnosis of Foot-and-Mouth Disease. J. Comp. Pathol. 2003, 129, 1–36. [Google Scholar] [CrossRef]
- Knowles, N.J.; Samuel, A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003, 91, 65–80. [Google Scholar] [CrossRef]
- Sahle, M.; Venter, E.H.; Dwarka, R.M.; Vosloo, W. Molecular epidemiology of serotype O foot-and-mouth disease virus isolated from cattle in Ethiopia between 1979–2001. Onderstepoort J. Veter Res. 2004, 71, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Tulman, E.R.; Delhon, G.; Lu, Z.; Carreno, A.; Vagnozzi, A.; Kutish, G.F.; Rock, D.L. Comparative Genomics of Foot-and-Mouth Disease Virus. J. Virol. 2005, 79, 6487–6504. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.G. Subtyping of foot-and-mouth disease virus. Dev. Biol. Stand. 1976, 35, 167–174. [Google Scholar] [PubMed]
- Klein, J. Understanding the molecular epidemiology of foot-and-mouth-disease virus. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2009, 9, 153–161. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Collect, Submiss Storage Diagnostic Specimens; OIE: Paris, France, 2009. [Google Scholar]
- Logan, G.; Newman, J.; Wright, C.F.; Lasecka-Dykes, L.; Haydon, D.T.; Cottam, E.M.; Tuthill, T.J. Deep Sequencing of Foot-and-Mouth Disease Virus Reveals RNA Sequences Involved in Genome Packaging. J. Virol. 2017, 92, e01159-17. [Google Scholar] [CrossRef]
- Martel, J.L. Foot-and-mouth disease in Ethiopia. Distribution of serotypes of foot-and-mouth disease virus. Rev. Elev. Med. Vet. Pays Trop. 1974, 27, 169–175. [Google Scholar] [CrossRef][Green Version]
- Mekonen, H.; Beyene, D.; Rufael, T.; Abunna, A. Study on the prevalence of Foot and Mouth Disease in Borana and Guji Zones, Southern Ethiopia. Study on the prevalence of Foot and Mouth Disease in Borana. Veter. World 2011, 4, 293–296. [Google Scholar] [CrossRef]
- Tesfaye, A.; Sehale, M.; Abebe, A.; Muluneh, A.; Gizaw, D. Sero-prevalence of foot and mouth disease in cattle in Borena Zone, Oromia regional state, Ethiopia. Ethiop. Veter. J. 2016, 20, 55. [Google Scholar] [CrossRef]
- Wagari, A. Seroprevalence of foot and mouth disease in bulls of Borana origin quarantined in Adama. Inl. J. Biochem. Biophys. Mol. Biol. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Ayelet, G.; Gelaye, E.; Negussie, H.; Asmare, K. Study on the epidemiology of foot and mouth disease in Ethiopia. Rev. Sci. Tech. 2012, 31, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Sulayeman, M.; Dawo, F.; Mammo, B.; Gizaw, D.; Shegu, D. Isolation, molecular characterization and sero-prevalence study of foot-and-mouth disease virus circulating in central Ethiopia. BMC Veter-Res. 2018, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Ayelet, G.; Mahapatra, M.; Gelaye, E.; Egziabher, B.G.; Rufeal, T.; Sahle, M.; Ferris, N.P.; Wadsworth, J.; Hutchings, G.H.; Knowles, N.J. Genetic Characterization of Foot-and-Mouth Disease Viruses, Ethiopia, 1981–2007. Emerg. Infect. Dis. 2009, 15, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Rweyemamu, M.; Roeder, P.; Mackay, D.; Sumption, K.; Brownlie, J.; Leforban, Y.; Valarcher, J.F.; Knowles, N.J.; Saraiva, V. Epidemiological Patterns of Foot-and-Mouth Disease Worldwide. Transbound. Emerg. Dis. 2008, 55, 57–72. [Google Scholar] [CrossRef]
- Sangula, A.K.; Siegismund, H.R.; Belsham, G.J.; Balinda, S.N.; Masembe, C.; Muwanika, V.B. Low diversity of foot-and-mouth disease serotype C virus in Kenya: Evidence for probable vaccine strain re-introductions in the field. Epidemiol. Infect. 2011, 139, 189–196. [Google Scholar] [CrossRef]
- Rufael, T.; Catley, A.; Bogale, A.; Sahle, M.; Shiferaw, Y. Foot and mouth disease in the Borana pastoral system, southern Ethiopia and implications for livelihoods and international trade. Trop. Anim. Heal. Prod. 2008, 40, 29–38. [Google Scholar] [CrossRef]
- Aman, E.; Molla, W.; Gebreegizabher, Z.; Jemberu, W.T. Spatial and temporal distribution of foot and mouth disease outbreaks in Amhara region of Ethiopia in the period 1999 to 2016. BMC Veter Res. 2020, 16, 185. [Google Scholar] [CrossRef]
- Behnke, R. The Economics of Pastoral Livestock Production in Sudan. 2011, p. 9. Available online: http://fic.tufts.edu/publication-item/briefing-paper-the-economics-of-pastoral-livestock-production-in-sudan/ (accessed on 20 August 2021).
- Funk, C.; Rowland, J.; Eilerts, G.; Kebebe, E.; Biru, N.; White, L.; Galu, G. A Climate Trend Analysis of Ethiopia. Climate Change Adaptation Series; Fact Sheet; US Geological Survey Famine Early Warning Systems Network-Informing (FEWSNET): Addis Abeba, Ethiopia, 2012. [Google Scholar]
- CSA. The Federal Democratic Republic Of Ethiopia Central Statistical Agency Volume Vii Report On Crop And Livestock Product Utilization (Private Peasant Holdings, Meher Season). Stat Bull. 2012; E VII: 577. Available online: https://www.statsethiopia.gov.et/wp-content/uploads/2019/06/Agricultural-Sample-Survey-Product-Utilization-Meher-Season-2012.pdf (accessed on 15 September 2021).
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. 2015. Available online: https://www.google.com/search? (accessed on 2 September 2021).
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2005. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Corder, G.W.; Foreman, D.I. Nonparametric Statistics: A Step-by-Step Approach; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Thrusfield, M.; Christley, R.; Brown, H.; Diggle, P.J.; French, N.; Howe, K.; Kelly, L.; O’Connor, A.; Sargeant, J.; Wood, H. Patterns of disease. In Veterinary Epidemilogyogy, 4th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 168–188. [Google Scholar] [CrossRef]
- QGIS Developement Team. QGIS Geographic Information System. Open Source Geospatial Foundation. 2009. Available online: http://qgis.osgeo.org (accessed on 5 August 2021).
- Kulldorff, M. Prospective time periodic geographical disease surveillance using a scan statistic. J. R. Stat. Soc. Ser. A Stat. Soc. 2001, 164, 61–72. [Google Scholar] [CrossRef]
- Mostashari, F.; Kulldorff, M.; Hartman, J.J.; Miller, J.R.; Kulasekera, V. Dead Bird Clusters as an Early Warning System for West Nile Virus Activity. Emerg. Infect. Dis. 2003, 9, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assunção, R.; Mostashari, F. A Space–Time Permutation Scan Statistic for Disease Outbreak Detection. PLoS Med. 2005, 2, e59. [Google Scholar] [CrossRef] [PubMed]
- Wubshet, A.K.; Dai, J.; Li, Q.; Zhang, J. Review on Outbreak Dynamics, the Endemic Serotypes, and Diversified Topotypic Profiles of Foot and Mouth Disease Virus Isolates in Ethiopia from 2008 to 2018. Viruses 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Jemberu, W.T.; Mourits, M.C.M.; Sahle, M.; Siraw, B.; Vernooij, J.C.M.; Hogeveen, H. Epidemiology of Foot and Mouth Disease in Ethiopia: A Retrospective Analysis of District Level Outbreaks, 2007–2012. Transbound. Emerg. Dis. 2016, 63, e246–e259. [Google Scholar] [CrossRef]
- Asfaw, W.; Sintaro, T. The status of FMD in Ethiopia, A growing concern. Ethiop. Vet. Epidemiol. 2000, 1, 15–35. [Google Scholar]
- Belayneh, N.; Molla, W.; Mesfine, M.; Jemberu, W.T. Modeling the transmission dynamics of foot and mouth disease in Amhara region, Ethiopia. Prev. Veter. Med. 2020, 181, 104673. [Google Scholar] [CrossRef]
- Gallego, M.L.; Perez, A.M.; Thurmond, M.C. Temporal and Spatial Distributions of Foot-and-Mouth Disease Under Three Different Strategies of Control and Eradication in Colombia (1982–2003). Veter. Res. Commun. 2007, 31, 819–834. [Google Scholar] [CrossRef]
- Peralta, E.A.; Carpenter, T.E.; Farver, T. The application of time series analysis to determine the pattern of foot and mouth disease in cattle in Paraguay. Prev. Vet. Med. 1982, 1, 27–36. [Google Scholar] [CrossRef]
- Rosenberg, F.J.; Astudillo, V. Evaluation of alternative strategies for foot and mouth disease control in Paraguay. In New Techniques in Veterinary Epidemiology and Economics. In Proceedings of the International Symposium on Information Theory, 21–24 June 1976; pp. 181–194. [Google Scholar]
- Sharma, S.K.; Singh, G.R. Cyclic behaviour of foot-and-mouth disease in India. Veter. Rec. 1993, 133, 448–450. [Google Scholar] [CrossRef]
- Lee, H.S.; Pham, T.L.; Wieland, B. Temporal patterns and space-time cluster analysis of foot-and-mouth disease (FMD) cases from 2007 to 2017 in Vietnam. Transbound. Emerg. Dis. 2020, 67, 584–591. [Google Scholar] [CrossRef]
- Leta, S.; Mesele, F. Spatial analysis of cattle and shoat population in Ethiopia: Growth trend, distribution and market access. SpringerPlus 2014, 3, 310. Available online: https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-310 (accessed on 5 September 2021). [CrossRef] [PubMed]
- Duong, M.C.; Alenius, S.; Huong, L.T.T.; Björkman, C. Prevalence of Neospora caninum and bovine viral diarrhoea virus in dairy cows in Southern Vietnam. Veter. J. 2008, 175, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Q.; Huang, Y.; Wang, N.; Shan, H. A 5-Year Review of Senecavirus A in China since Its Emergence in 2015. Front. Vet. Sci. 2020, 7, 567792. [Google Scholar] [CrossRef]
- Reid, S.M.; Ferris, N.P.; Hutchings, G.H.; King, D.P.; Alexandersen, S. Evaluation of real-time reverse transcription polymerase chain reaction assays for the detection of swine vesicular disease virus. J. Virol. Methods 2003, 116, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.T.; Long, N.T.; Brito, B.; Stenfeldt, C.; Phuong, N.T.; Hoang, B.H.; Pauszek, S.J.; Hartwig, E.J.; Smoliga, G.R.; Vu, P.P.; et al. First detection of foot-and-mouth disease virus O/Ind-2001d in Vietnam. PLoS ONE 2017, 12, e0177361. [Google Scholar] [CrossRef] [PubMed]

| Region | Cattle | Goat | Sheep | Region Share in % |
|---|---|---|---|---|
| Tigray | 3119.40 | 3005.50 | 1388.10 | 7.4 |
| Afar * | 1627.20 | 3398.20 | 1799.00 | 6.8 |
| Amhara | 11,757.30 | 5468.60 | 9469.70 | 26.4 |
| Oromiya | 21,375.70 | 7678.40 | 9391.10 | 38.1 |
| Somali * | 675.6 | 1710.40 | 1316.80 | 3.7 |
| Benishangul Gumuz | 363.6 | 371.5 | 85.3 | 0.8 |
| SNNP | 9574.70 | 2624.60 | 4000.10 | 16 |
| Gambella | 212.6 | 54.6 | 48.1 | 0.3 |
| Harari | 40.8 | 41.2 | 5 | 0.1 |
| Addis Ababa | 89.5 | 19.1 | 21.8 | 0.1 |
| Dire Dawa | 49.8 | 154.7 | 59.6 | 0.3 |
| Ethiopia | 48,886.20 | 24,526.90 | 27,584.60 | 100 |
| Year | Species | Total | |
|---|---|---|---|
| Cattle | Sheep and Goats | ||
| 2010 | 22,440 | 20 | 22,460 |
| 2011 | 114,444 | 67,889 | 182,333 |
| 2012 | 94,344 | 736 | 95,080 |
| 2013 | 6722 | 194 | 6916 |
| 2014 | 4710 | NR | 4710 |
| 2015 | 25,578 | NR | 25,578 |
| 2016 | 7257 | 3 | 7260 |
| 2017 | 22,904 | 700 | 23,604 |
| 2018 | 1012 | NR | 1012 |
| 2019 | 7808 | 1 | 7809 |
| Total | 307,219 | 69,543 | 376,762 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woldemariyam, F.T.; Leta, S.; Assefa, Z.; Tekeba, E.; Gebrewold, D.S.; Paeshuyse, J. Temporal and Spatial Patterns and a Space–Time Cluster Analysis of Foot-and-Mouth Disease Outbreaks in Ethiopia from 2010 to 2019. Viruses 2022, 14, 1558. https://doi.org/10.3390/v14071558
Woldemariyam FT, Leta S, Assefa Z, Tekeba E, Gebrewold DS, Paeshuyse J. Temporal and Spatial Patterns and a Space–Time Cluster Analysis of Foot-and-Mouth Disease Outbreaks in Ethiopia from 2010 to 2019. Viruses. 2022; 14(7):1558. https://doi.org/10.3390/v14071558
Chicago/Turabian StyleWoldemariyam, Fanos Tadesse, Samson Leta, Zerihun Assefa, Etsegent Tekeba, Dereje Shegu Gebrewold, and Jan Paeshuyse. 2022. "Temporal and Spatial Patterns and a Space–Time Cluster Analysis of Foot-and-Mouth Disease Outbreaks in Ethiopia from 2010 to 2019" Viruses 14, no. 7: 1558. https://doi.org/10.3390/v14071558
APA StyleWoldemariyam, F. T., Leta, S., Assefa, Z., Tekeba, E., Gebrewold, D. S., & Paeshuyse, J. (2022). Temporal and Spatial Patterns and a Space–Time Cluster Analysis of Foot-and-Mouth Disease Outbreaks in Ethiopia from 2010 to 2019. Viruses, 14(7), 1558. https://doi.org/10.3390/v14071558






