Independent Cis-Regulatory Modules within the Herpes Simplex Virus 1 Infected Cell Protein 0 (ICP0) Promoter Are Transactivated by Krüppel-like Factor 15 and Glucocorticoid Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Dual Luciferase Assay
2.2.1. Plasmids
2.2.2. Statistical Analysis
3. Results
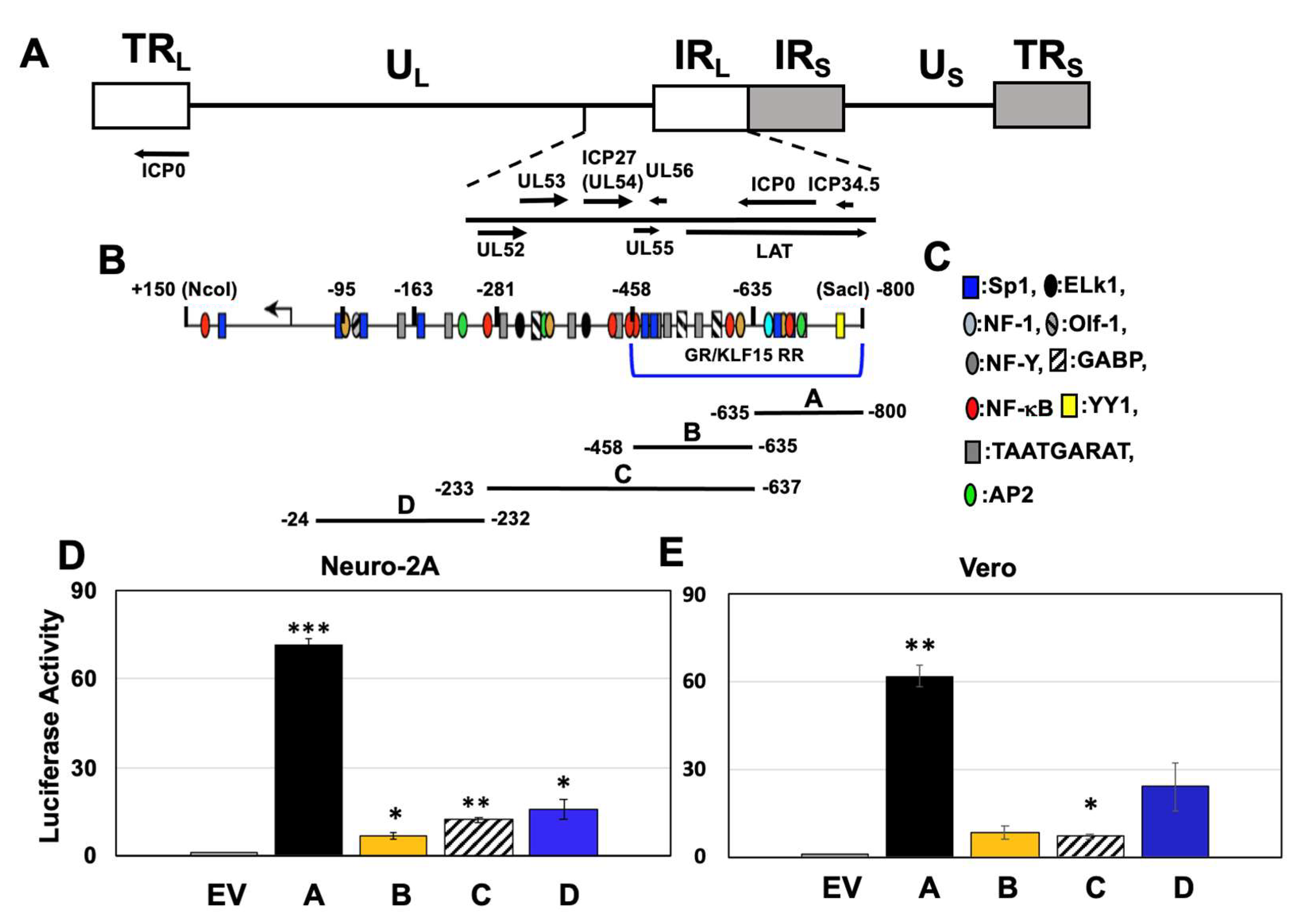
3.1. GR and KLF15 Transactivate Specific ICP0 Promoter CRM Fragments
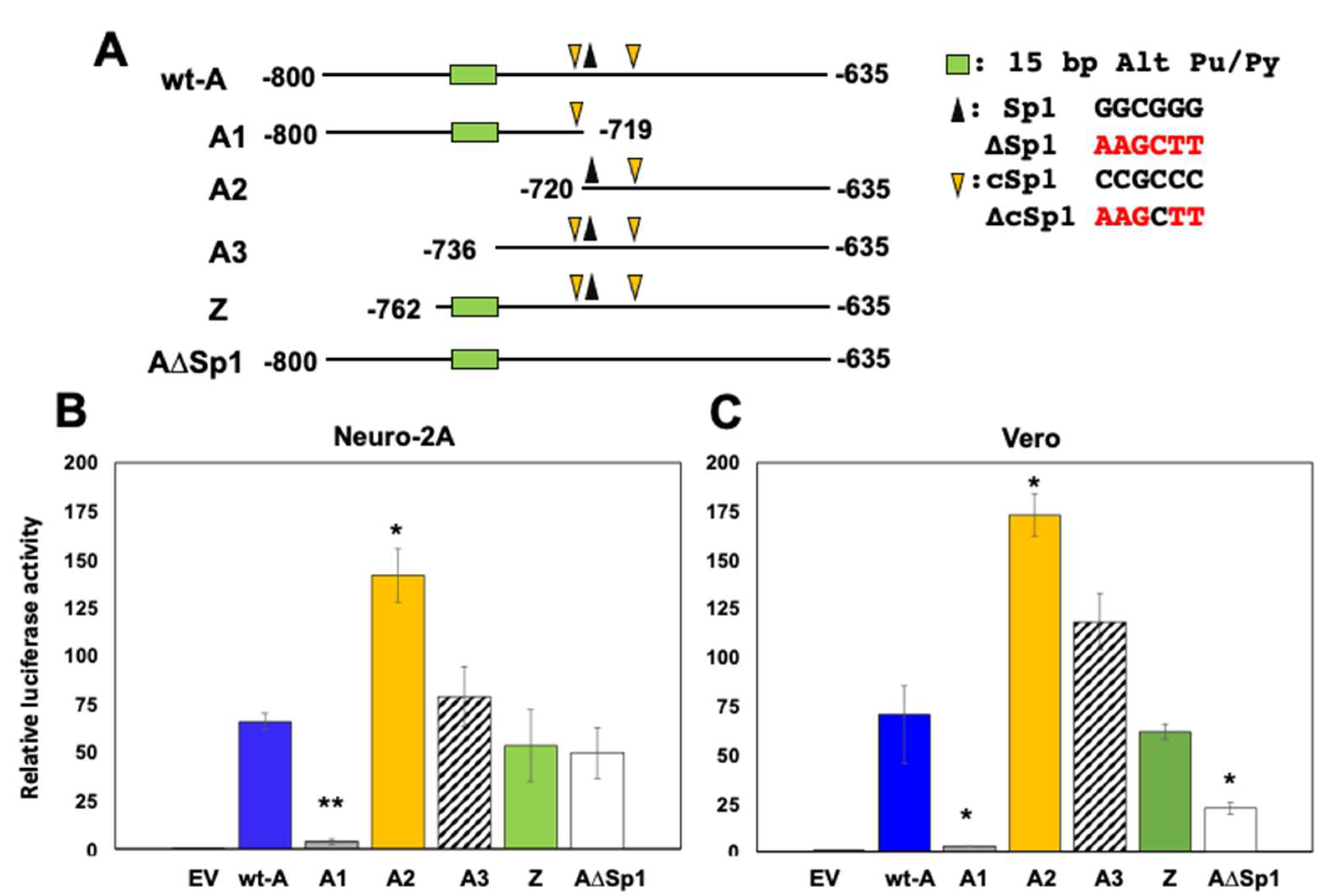
3.2. Localization of Fragment A CRM Activity
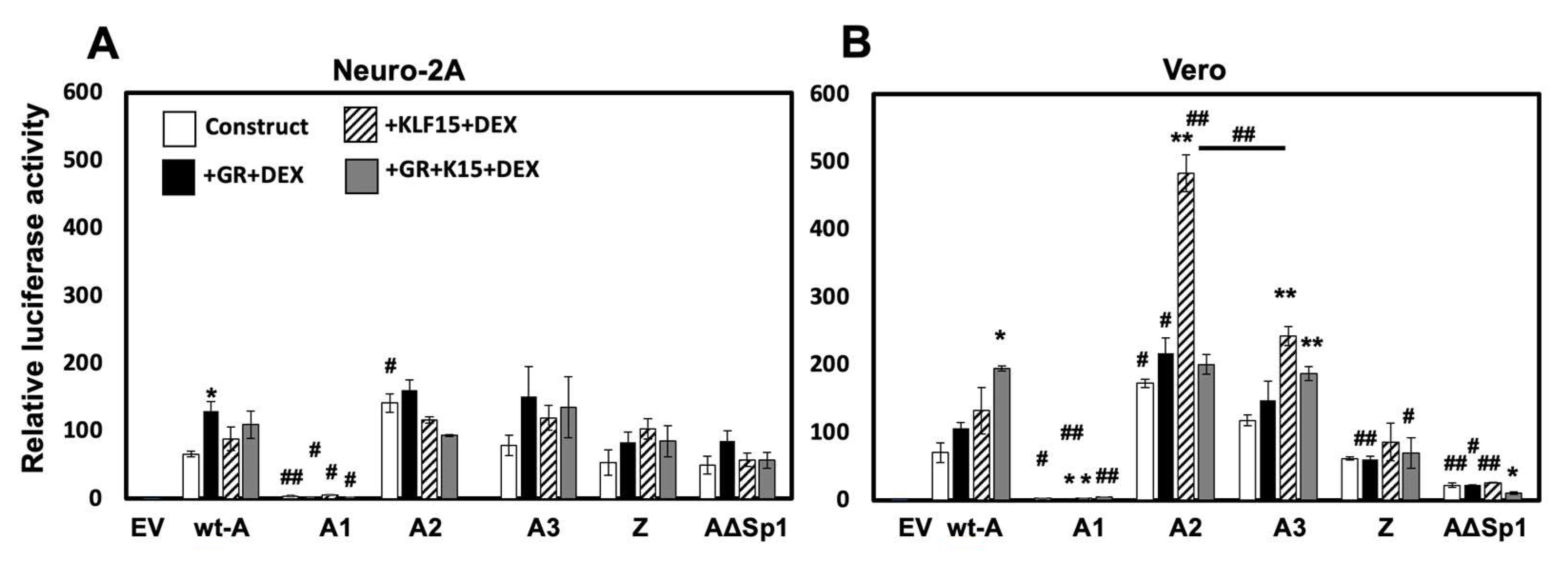
3.3. Localization of Fragment A Sequences That Mediate Transactivation by GR, KLF15, and/or DEX
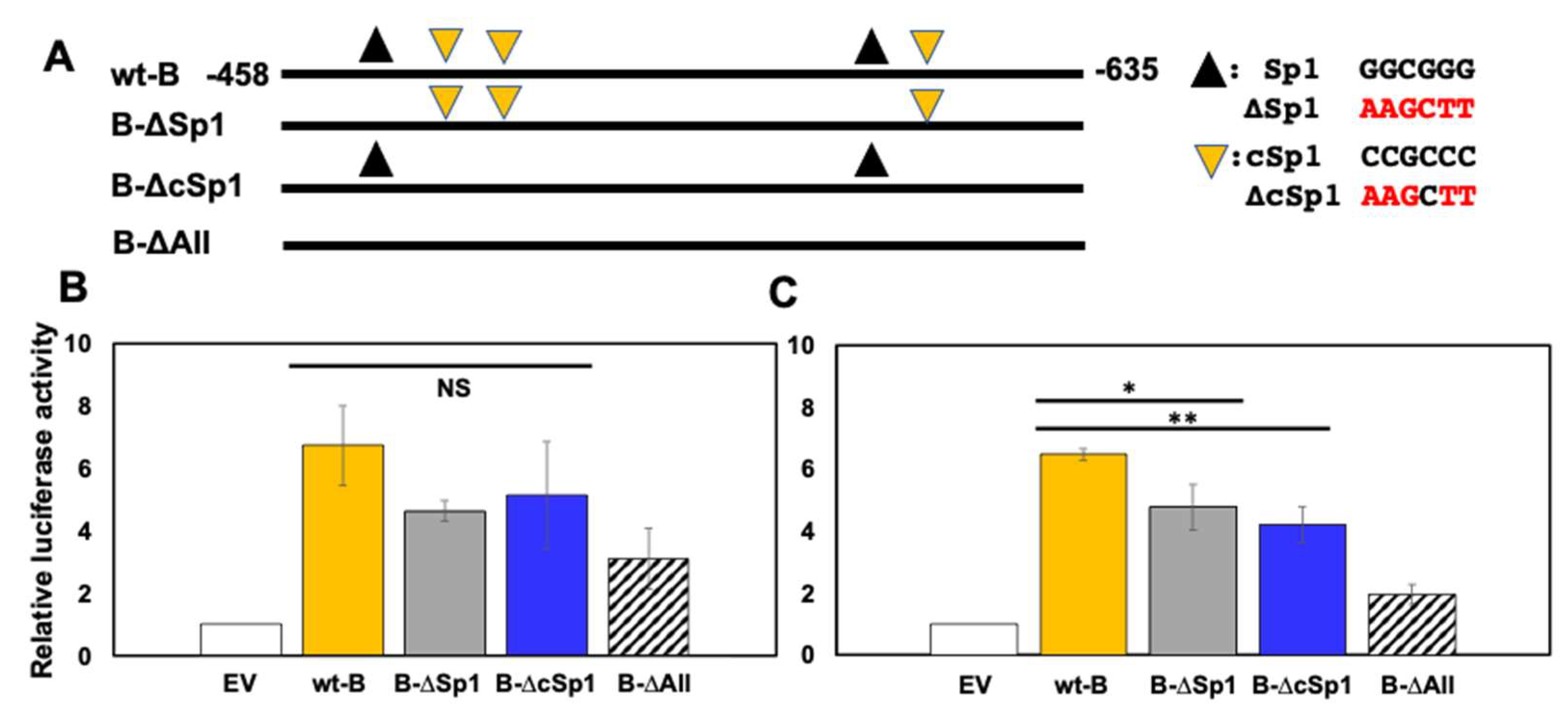
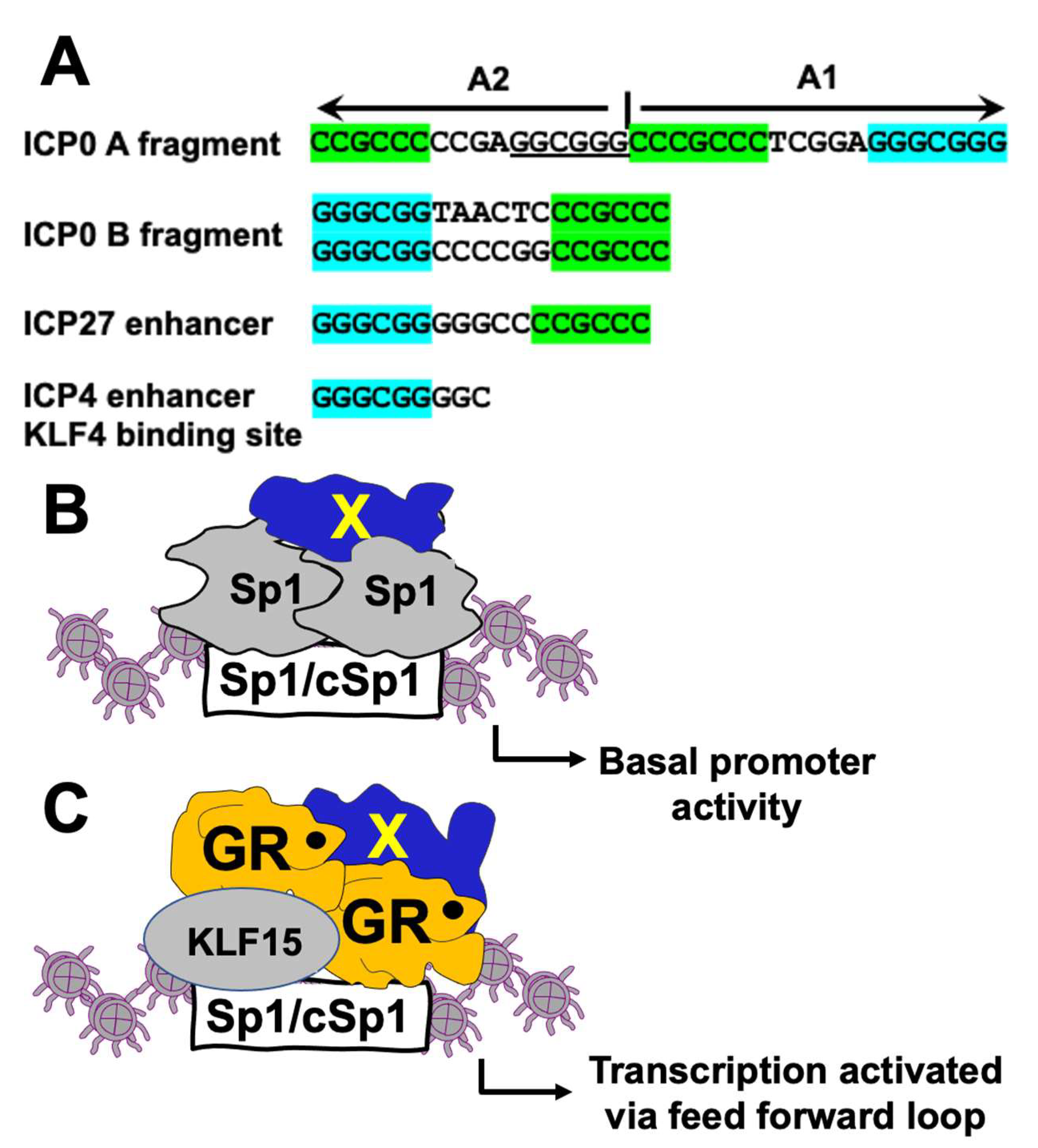
3.4. Sp1 and cSp1 Binding Sites in the B Fragment Mediate CRM Activity
3.5. Sp1 Binding Sites Mediate Transactivation of B Fragment by GR, KLF15, and DEX In Vero Cells
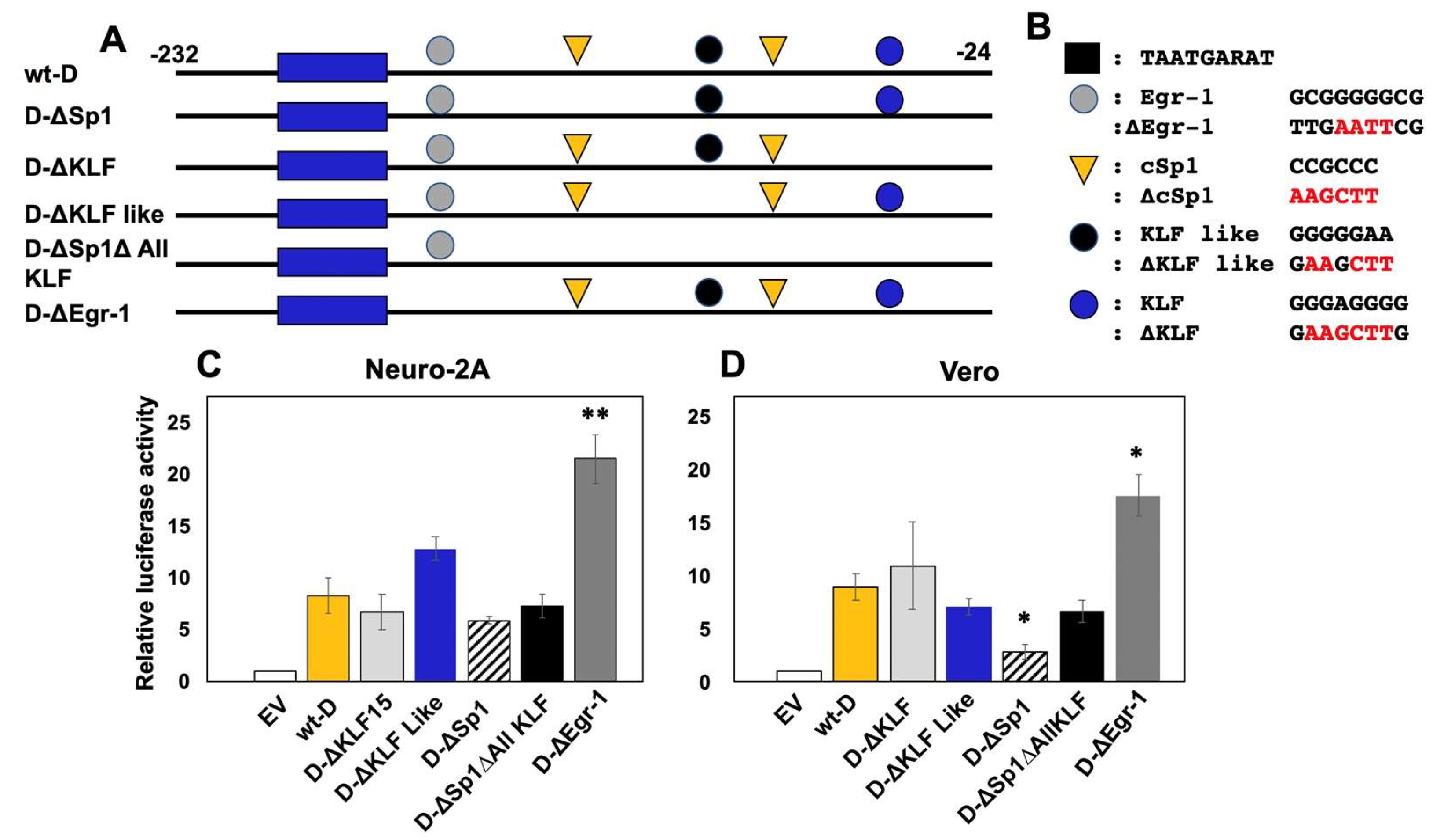
3.6. Sp1 Binding Sites in Fragment D are Necessary for Stimulating Transcription In Vero Cells
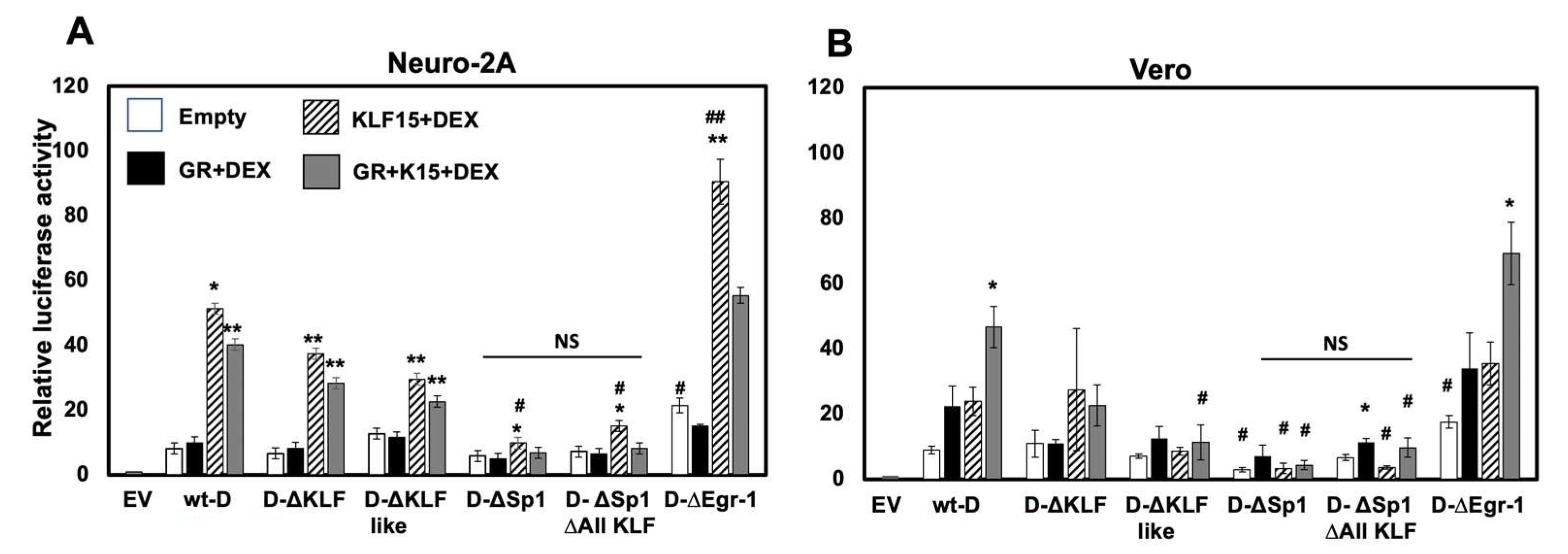
3.7. Cell-Type Dependent Transactivation of Fragment D by GR, KLF15, and DEX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perng, G.-C.; Jones, C. Towards an Understanding of the Herpes Simplex Virus Type 1 Latency-Reactivation Cycle. Interdiscip. Perspect. Infect. Dis. 2010, 2010, 262415. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, L.J.; Clerkin, P.P.; Clement, C.; McFerrin, H.; Bhattacharjee, P.S.; Varnell, E.D.; Kaufman, H.; Hill, J.M. Ocular herpes simplex virus: How are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiol. 2011, 6, 877–907. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, T.; Openshaw, H. Encephalitis resulting from reactivation of latent herpes simplex virus in mice. J. Virol. 1984, 50, 263–266. [Google Scholar] [CrossRef]
- Honess, R.W.; Roizman, B. Regulation of herpes virus macromalecular synthesis: Cascade regulation of three groups of viral proteins. J. Virol. 1974, 14, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kristie, T.M. Early pre-initiation of alphaherpesvirus viral gene expression. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, NY, USA, 2007; Volume 1, pp. 112–127. [Google Scholar]
- Kristie, T.M. Dynamic modulation of HSV chromatin drives initiation of infection and provides targets for epigenetic therapies. Virology 2015, 479-480, 555–561. [Google Scholar] [CrossRef]
- Boutell, C.; Everett, R.D. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J. Gen. Virol. 2013, 94, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 2000, 22, 761–770. [Google Scholar] [CrossRef]
- Cliffe, A.; Knipe, D. Herpes Simplex Virus ICP0 Promotes both Histone Removal and Acetylation on Viral DNA during Lytic Infection. J. Virol. 2008, 82, 12030–12038. [Google Scholar] [CrossRef]
- Halford, W.P.; Kemp, C.D.; Isler, J.A.; Davido, D.J.; Schaffer, P.A. ICP0, ICP4, or VP16 Expressed from Adenovirus Vectors Induces Reactivation of Latent Herpes Simplex Virus Type 1 in Primary Cultures of Latently Infected Trigeminal Ganglion Cells. J. Virol. 2001, 75, 6143–6153. [Google Scholar] [CrossRef]
- Halford, W.P.; Schaffer, P.A. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 2001, 75, 3240–3249. [Google Scholar] [CrossRef]
- Linderman, J.A.; Kobayashi, M.; Rayannavar, V.; Fak, J.J.; Darnell, R.B.; Chao, M.V.; Wilson, A.C.; Mohr, I. Immune Escape via a Transient Gene Expression Program Enables Productive Replication of a Latent Pathogen. Cell Rep. 2017, 18, 1312–1323. [Google Scholar] [CrossRef]
- Meadows, C.L.J.; Catalan, J.; Barton, S. Are stress and coping style associated with frequent recurrence of genital hereps? Genitourin. Med. 1997, 73, 263–266. [Google Scholar]
- Glaser, R.; Kiecolt-Glaser, J.K.; Speicher, C.E.; Holliday, J.E. Stress, loneliness, and changes in herpesvirus latency. J. Behav. Med. 1985, 8, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Padgett, D.A.; Sheridan, J.F.; Dorne, J.; Berntson, G.G.; Candelora, J.; Glaser, R. Social stress and the reactivation of latent herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 1998, 95, 7231–7235. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.F.; Straus, S.E.; Mannix, M.L.; Wohlenberg, C.R.; Banks, S.; Jagannath, S.; Brauer, J.E.; Notkins, A.L. UV Light-Induced Reactivation of Herpes Simplex Virus Type 2 and Prevention by Acyclovir. J. Infect. Dis. 1992, 166, 500–506. [Google Scholar] [CrossRef]
- Jones, C. Alphaherpesvirus Latency: Its Role in Disease and Survival of the Virus in Nature. Adv. Virus Res. 1998, 51, 81–133. [Google Scholar] [CrossRef]
- Jones, C. Herpes Simplex Virus Type 1 and Bovine Herpesvirus 1 Latency. Clin. Microbiol. Rev. 2003, 16, 79–95. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy. Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Mangan, S.; Alon, U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA 2003, 100, 11980–11985. [Google Scholar] [CrossRef]
- Sasse, S.K.; Zuo, Z.; Kadiyala, V.; Zhang, L.; Pufall, M.A.; Jain, M.K.; Phang, T.L.; Stormo, G.D.; Gerber, A.N. Response Element Composition Governs Correlations between Binding Site Affinity and Transcription in Glucocorticoid Receptor Feed-forward Loops. J. Biol. Chem. 2015, 290, 19756–19769. [Google Scholar] [CrossRef]
- Mailoux, C.M.; Barczak, A.J.; Wang, Q.; Altonsy, M.O.; Jain, M.K.; Haldar, S.M.; Gerber, A.N. The glucocorticoid receptor and KLF15 regulate gene expression dynamics and integrate signals through feed-forward circuitry. Mol. Cell Biol. 2013, 33, 2104–2115. [Google Scholar]
- Erlandsson, A.C.; Bladh, L.-G.; Stierna, P.; Yucel-Lindberg, T.; Hammarsten, O.; Modéer, T.; Harmenberg, J.; Wikström, A.-C. Herpes simplex virus type 1 infection and glucocorticoid treatment regulate viral yield, glucocorticoid receptor and NF-kappaB levels. J. Endocrinol. 2002, 175, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.S.; Schroeder, K.; Thunuguntla, P.; Jones, C. The glucocorticoid receptor (GR) stimulates Herpes Simplex Virus 1 productive infection, in part because the infected cell protein 0 (ICP0) promoter is cooperatively transactivated by the GR and Krüppel-like transcription factor 15. J. Virol. 2019, 93, e02018–e02063. [Google Scholar]
- Du, T.; Zhou, G.; Roizman, B. Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc. Natl. Acad. Sci. USA 2012, 109, 14616–14621. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhou, G.; Roizman, B. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 18820–18824. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Thunuguntla, P.; Jones, C. Antagonizing the glucocorticoid receptor impairs explant-induced reactivation in mice latently infected with herpes simplex virus 1. J. Virol. 2019, 93, e00418–e00419. [Google Scholar]
- El-mayet, F.S.; Harrison, K.S.; Jones, C. Regulation of Krüppel-Like Factor 15 Expression by Herpes Simplex Virus Type 1 or Bovine Herpesvirus 1 Productive Infection. Viruses 2021, 13, 1148. [Google Scholar] [CrossRef]
- Ostler, J.B.; Jones, C. Stress induced transcription factors transactivate the herpes simplex virus 1 infected cell protein 27 (ICP27) transcriptional enhancer. Viruses 2021, 13, 2296. [Google Scholar] [CrossRef]
- Thunguntla, P.; Hendrickson, B.Y.; Jones, C. Transactivation of HSV-1 infected cell protein 4 (ICP4) enhancer by glucocorticoid receptor and stress-induced transcription factors requires overlapping Krüppel like transcription factor 4/Sp1 binding sites. J. Virol. 2021, 95, e01720–e01776. [Google Scholar]
- Black, A.R.; Black, J.D.; Azizkhan-Clifford, J. Sp1 and Kruppel-like transcription factor family of transcription factors in cell growth and cancer. J. Cell. Physiol. 2001, 188, 143–160. [Google Scholar] [CrossRef]
- Kaczynski, J.; Cook, T.; Urrutia, R. Sp1- and Krüppel-like transcription factors. Genome Biol. 2003, 4, 206.201–206.208. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.C.; Giordani, N.V.; Kwiatkowski, D.L. Epigenetic regulation of latent HSV-1 gene expression. Biochim. Biophys. Acta 2010, 1799, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.M.; Cliffe, A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008, 6, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, A.S.; Davido, D.J.; Schaffer, P.A. Role of Nuclear Factor Y in Stress-Induced Activation of the Herpes Simplex Virus Type 1 ICP0 Promoter. J. Virol. 2010, 84, 188–200. [Google Scholar] [CrossRef]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef]
- El-Mayet, F.S.; Sawant, L.; Thungunutla, P.; Jones, C. Combinatorial effects of the glucocorticoid receptor and Krüppel-like transcription factor 15 on bovine herpesvirus 1 transcription and productive infection. J. Virol. 2017, 91, e00904–e00917. [Google Scholar] [CrossRef]
- Liu, Z.; Cidlowski, J.A. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006, 16, 301–307. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.J.; Cidlowski, J.A. Emerging Roles of Glucocorticoid Receptor Phosphorylation in Modulating Glucocorticoid Hormone Action in Health and Disease. IUBMB Life 2009, 6, 979–986. [Google Scholar] [CrossRef]
- Rothenburg, S.; Koch-Nolte, F.; Rich, A.; Haag, F. A polymorphic dinucleotide repeat in the rat nucleolin gene forms Z-DNA and inhibits promoter activity. Proc. Natl. Acad. Sci. USA 2001, 98, 8985–8990. [Google Scholar] [CrossRef]
- Cao, X.; Mahendran, R.; Guy, G.; Tan, Y. Detection and characterization of cellular EGR-1 binding to its recognition site. J. Biol. Chem. 1993, 268, 16949–16957. [Google Scholar] [CrossRef]
- Thompson, R.L.; Sawtell, N.M. Targeted promoter replacement reveals that herpes simplex virus type-1 and 2 specific VP16 promoters direct distinct rates of entry into the lytic program in snesory neurons in vivo. Front. Microbiol. 2019, 10, 1624. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Peng, H.; Osaki, M.; Choy, B.K.; Auron, P.E.; Sandell, L.J.; Goldring, M.B. Egr-1 Mediates Transcriptional Repression of COL2A1Promoter Activity by Interleukin-1β. J. Biol. Chem. 2003, 278, 17688–17700. [Google Scholar] [CrossRef] [PubMed]
- Galliher-Beckley, A.J.; Williams, J.G.; Cidlowski, J. Ligand-Independent Phosphorylation of the Glucocorticoid Receptor Integrates Cellular Stress Pathways with Nuclear Receptor Signaling. Mol. Cell. Biol. 2011, 31, 4663–4675. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Karthikeyan, N.; Lynch, J.T.; Sial, E.-A.; Gkourtsa, A.; Demonacos, C.; Krstic-Demonacos, M. Cross Talk of Signaling Pathways in the Regulation of the Glucocorticoid Receptor Function. Mol. Endocrinol. 2008, 22, 1331–1344. [Google Scholar] [CrossRef]
- Skobowiat, C.; Sayre, R.M.; Dowdy, J.C.; Slominski, A.T. Ultraviolet radiation regulates cortisol activity in a waveband dependent manner in human skin ex-vivo. Br. J. Derm. 2013, 168, 595–601. [Google Scholar] [CrossRef]
- Perna, J.J.; Mannix, M.L.; Rooney, J.F.; Notkins, A.L.; Straus, S.E. Reactivation of latent herpes simplex virus infection by ultraviolet light: A human model. J. Am. Acad. Dermatol. 1987, 17, 473–478. [Google Scholar] [CrossRef][Green Version]
- Spruance, S.L. Pathogenesis of herpes simplex labialis: Experimental induction of lesions with UV light. J. Clin. Microbiol. 1985, 22, 366–368. [Google Scholar] [CrossRef]
- Ritter, H.D.; Antonova, L.; Mueller, C.R. The Unliganded Glucocorticoid Receptor Positively Regulates the Tumor Suppressor Gene BRCA1 through GABP Beta. Mol. Cancer Res. 2012, 10, 558–569. [Google Scholar] [CrossRef]
- Li, Z.J.; Choi, D.-Y.; Sohn, K.-C.; Lim, E.-H.; Lee, Y.H.; Kim, S.; Im, M.; Lee, Y.; Seo, Y.-J.; Lee, J.-H.; et al. Glucocorticoid receptor enhances involucrin expression of keratinocye in a ligand-independent manner. Mol. Cell Biochem. 2014, 390, 289–295. [Google Scholar]
- Ng, H.P.; Jennings, S.; Wang, J.; Molina, P.E.; Nelson, S.; Wang, G. Non-canonical Glucocorticoid Receptor Transactivation of gilz by Alcohol Suppresses Cell Inflammatory Response. Front. Immunol. 2017, 8, 661. [Google Scholar] [CrossRef]
- Sawant, L.; Ostler, J.B.; Jones, C. A Pioneer Transcription Factor and Type I Nuclear Hormone Receptors Synergistically Activate the Bovine Herpesvirus 1 Infected Cell Protein 0 (ICP0) Early Promoter. J. Virol. 2021, 95, JVI0076821. [Google Scholar] [CrossRef] [PubMed]
- Elmayet, F.; Sawant, L.; Thunuguntla, P.; Zhao, J.; Jones, C. Two Pioneer Transcription Factors, Krüppel-Like Transcription Factor 4 and Glucocorticoid Receptor, Cooperatively Transactivate the Bovine Herpesvirus 1 ICP0 Early Promoter and Stimulate Productive Infection. J. Virol. 2020, 94, e01670-19. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesekera, N.; Hazell, N.; Jones, C. Independent Cis-Regulatory Modules within the Herpes Simplex Virus 1 Infected Cell Protein 0 (ICP0) Promoter Are Transactivated by Krüppel-like Factor 15 and Glucocorticoid Receptor. Viruses 2022, 14, 1284. https://doi.org/10.3390/v14061284
Wijesekera N, Hazell N, Jones C. Independent Cis-Regulatory Modules within the Herpes Simplex Virus 1 Infected Cell Protein 0 (ICP0) Promoter Are Transactivated by Krüppel-like Factor 15 and Glucocorticoid Receptor. Viruses. 2022; 14(6):1284. https://doi.org/10.3390/v14061284
Chicago/Turabian StyleWijesekera, Nishani, Nicholas Hazell, and Clinton Jones. 2022. "Independent Cis-Regulatory Modules within the Herpes Simplex Virus 1 Infected Cell Protein 0 (ICP0) Promoter Are Transactivated by Krüppel-like Factor 15 and Glucocorticoid Receptor" Viruses 14, no. 6: 1284. https://doi.org/10.3390/v14061284
APA StyleWijesekera, N., Hazell, N., & Jones, C. (2022). Independent Cis-Regulatory Modules within the Herpes Simplex Virus 1 Infected Cell Protein 0 (ICP0) Promoter Are Transactivated by Krüppel-like Factor 15 and Glucocorticoid Receptor. Viruses, 14(6), 1284. https://doi.org/10.3390/v14061284






