Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Reagents
2.2. In Vitro Antiviral Assay
2.3. Time-of-Drug-Addition Assay
2.4. ZIKV Replicon Inhibition Assay
2.5. Quantitative Real-Time PCR (qRT-PCR)
- ZIKV forward primer—GGTCAGCGTCCTCTCTAATAAACG;
- Reverse primer—GCACCCTAGTGTCCACTTTTTCC;
- Probe—AGCCATGACCGACACCACACCGT;
- DENV forward primer—AGGTCGGATTAAGCCATAGTACG;
- Reverse primer—TGGCCTGACTTCTTTTAACGTC;
- Probe—AAAAACTATGCTACCTGTGAGCCCCGTCC.
2.6. RdRp Enzymatic Inhibition Assay
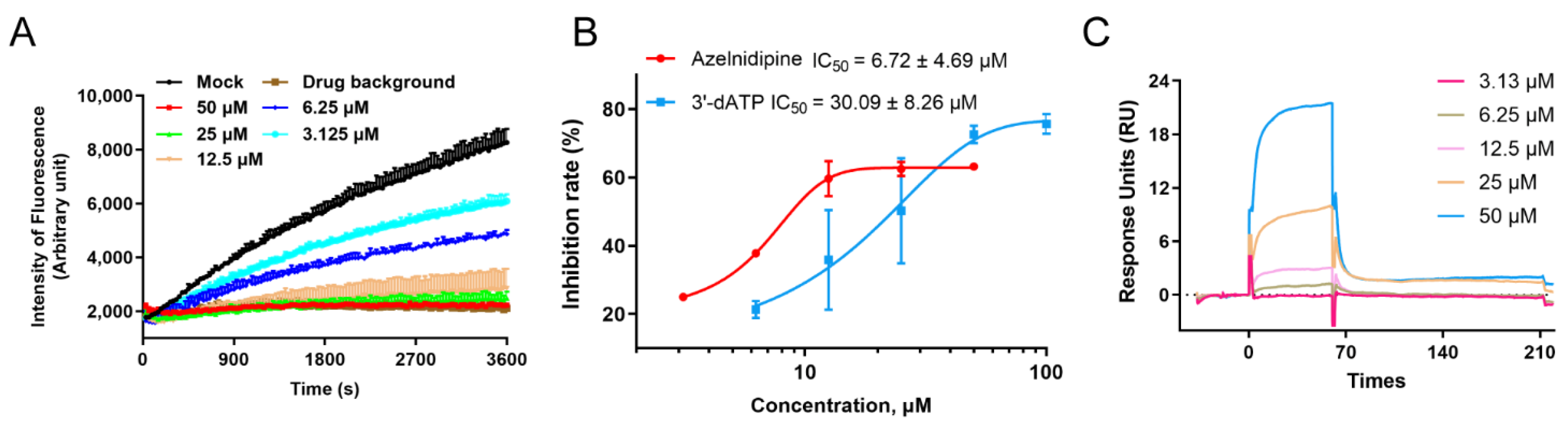
2.7. Surface Plasmon Resonance (SPR) Assay
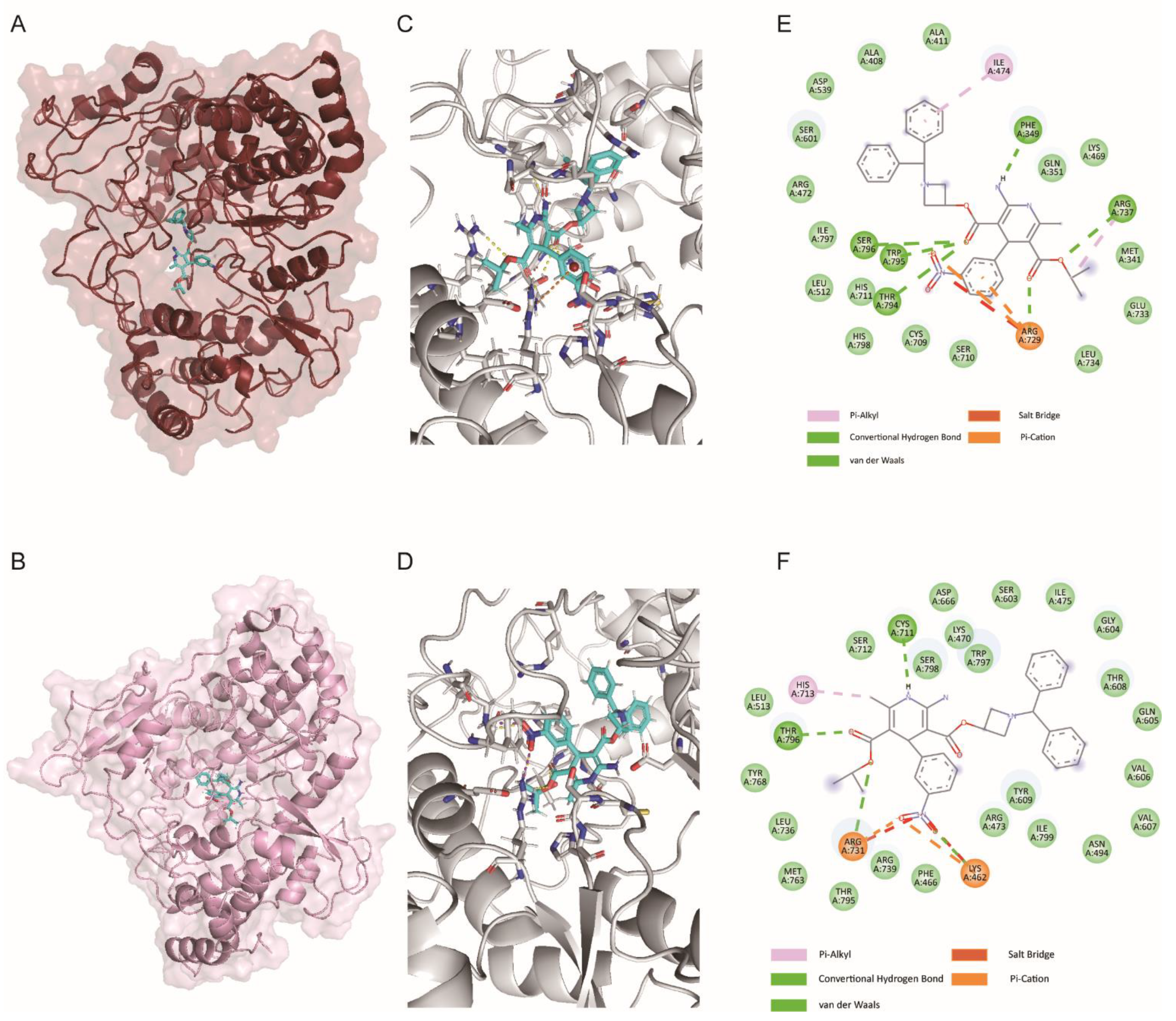
2.8. Molecular Docking Simulations
2.9. In Vivo Antiviral Efficacy
2.10. Statistical Analyses
3. Results
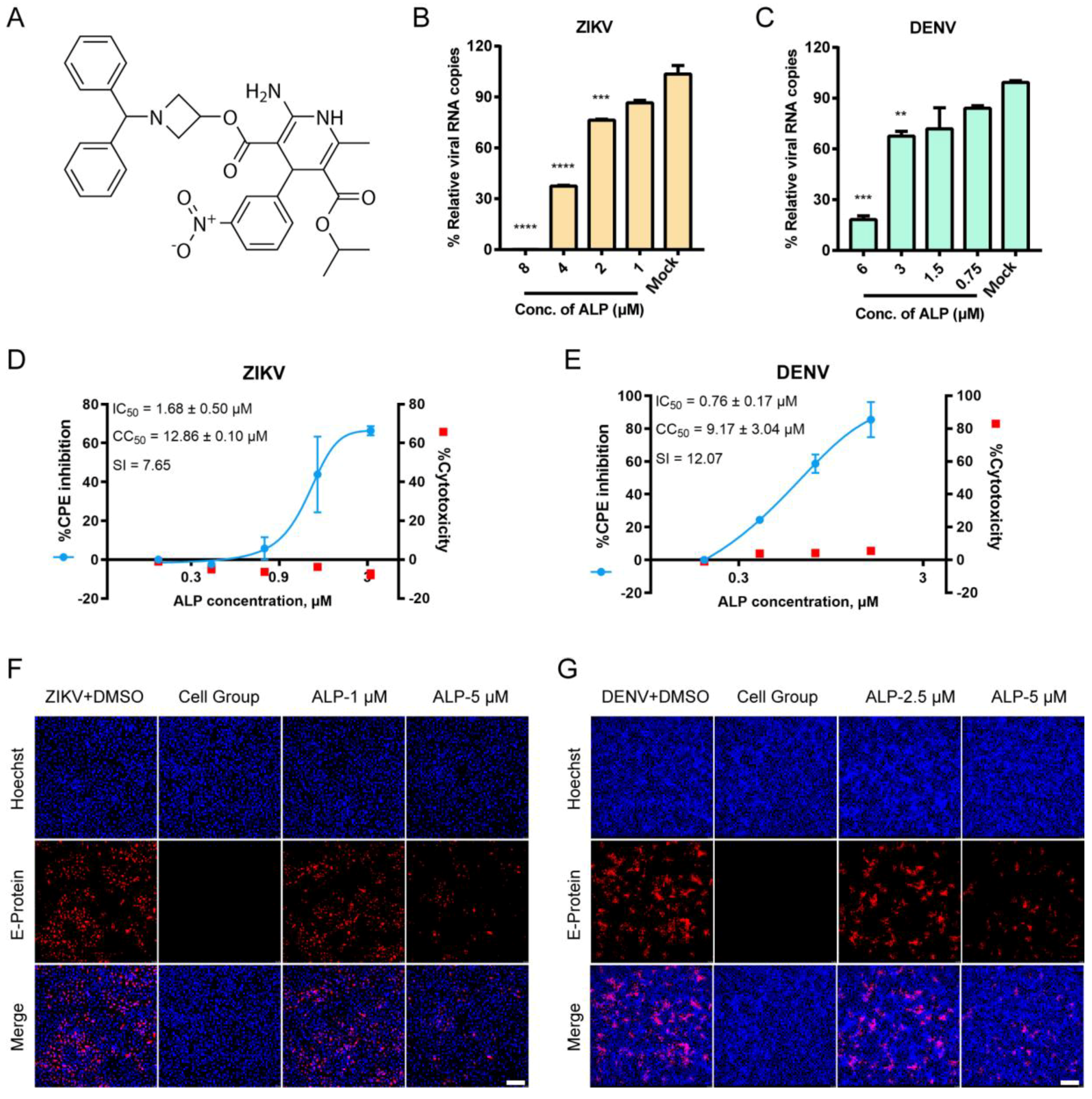
3.1. ALP Exhibits Favorable Antiviral Effects against ZIKV and DENV Infections In Vitro
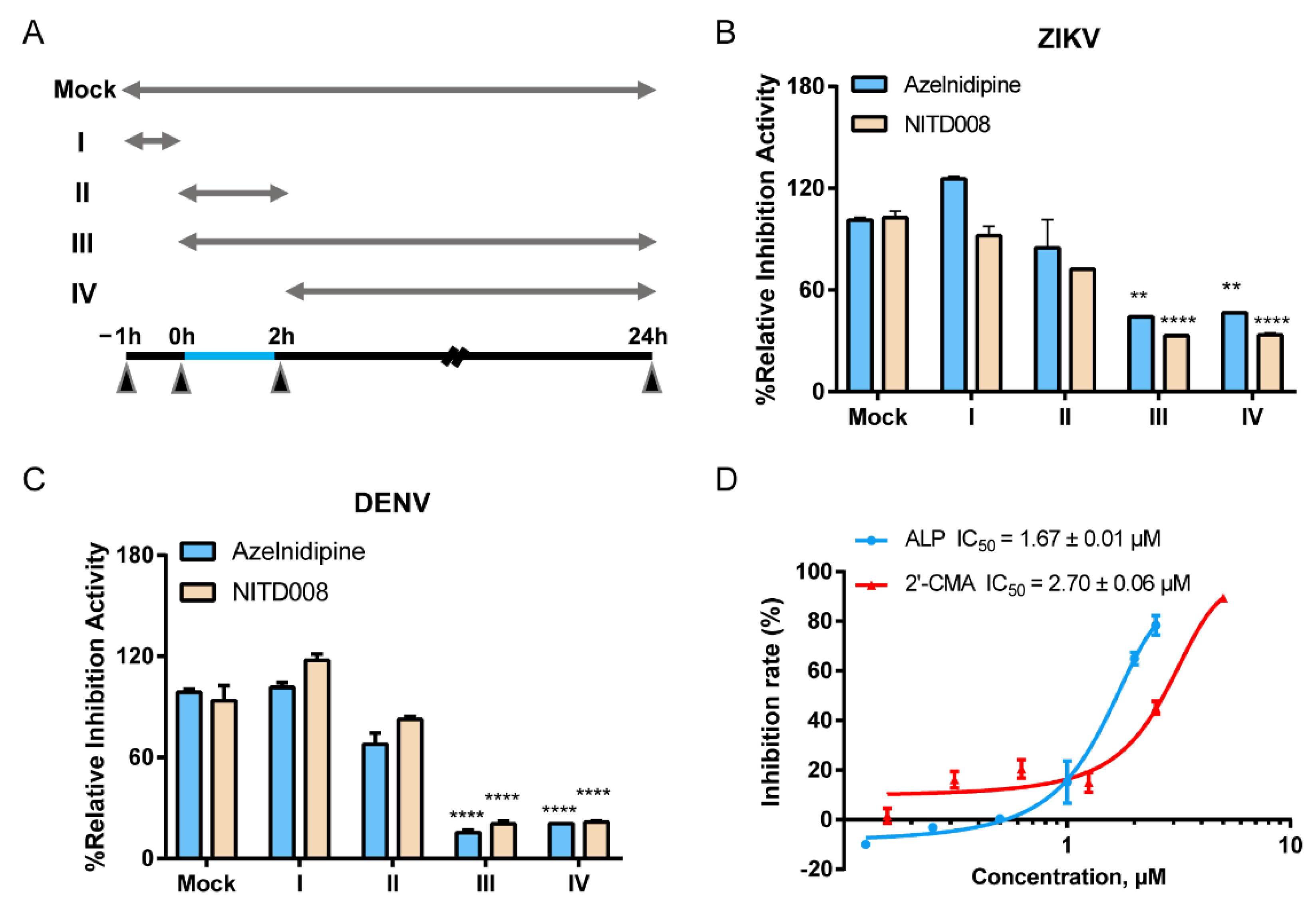
3.2. ALP Mainly Acts at the Post-Entry Stage of Flavivirus Infections
3.3. ALP Exerts Antiviral Effects by Inhibiting the Viral RNA-Dependent RNA Polymerase
3.4. ALP Binds to the N Pocket of RdRp Protein In Silico
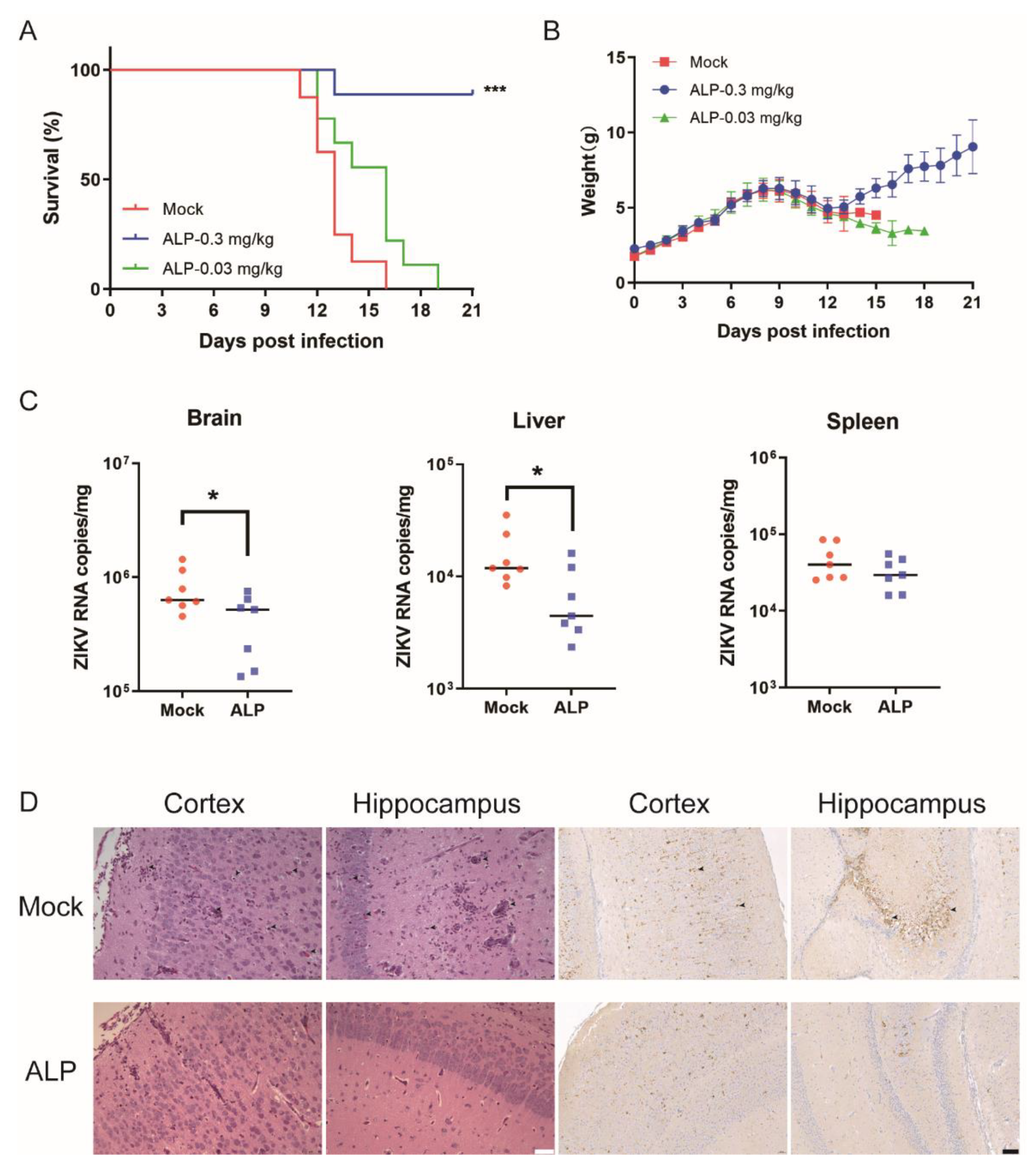
3.5. ALP Exerts Antiviral Efficacy against ZIKV Infection In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology, biology, and disease aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Yoon, I.K. A review of Dengvaxia®: Development to deployment. Hum. Vaccines Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef]
- Iani, F.C.M.; Giovanetti, M.; Fonseca, V.; Souza, W.M.; Adelino, T.E.R.; Xavier, J.; Jesus, J.G.; Pereira, M.A.; Silva, M.V.F.; Costa, A.V.B.; et al. Epidemiology and evolution of Zika virus in Minas Gerais, Southeast Brazil. Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 91, 104785. [Google Scholar] [CrossRef]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Góis, A.L.; Belfort, R., Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef] [Green Version]
- Asadi-Pooya, A.A. Zika virus-associated seizures. Seizure 2016, 43, 13. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2019, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Madrid, P.B.; Chopra, S.; Manger, I.D.; Gilfillan, L.; Keepers, T.R.; Shurtleff, A.C.; Green, C.E.; Iyer, L.V.; Dilks, H.H.; Davey, R.A.; et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS ONE 2013, 8, e60579. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, L.K.; Li, S.F.; Zhang, S.F.; Wan, W.W.; Zhang, Y.L.; Xin, Q.L.; Dai, K.; Hu, Y.Y.; Wang, Z.B.; et al. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019, 29, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Guo, J.; Wang, P.; Zhang, L.; Xiao, G.; Wang, W. Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection. J. Virol. 2017, 91, e01055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellington, K.; Scott, L.J. Azelnidipine. Drugs 2003, 63, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Zhang, Y.Z.; Luo, J.Q.; Zhang, W. Clinical use of azelnidipine in the treatment of hypertension in Chinese patients. Ther. Clin. Risk Manag. 2015, 11, 309–318. [Google Scholar] [PubMed] [Green Version]
- Teng, T.; Ridgley, D.M.; Tsoy, A.; Sun, G.Y.; Askarova, S.; Lee, J.C. Azelnidipine Attenuates the Oxidative and NFκB Pathways in Amyloid-β-Stimulated Cerebral Endothelial Cells. ACS Chem. Neurosci. 2019, 10, 209–215. [Google Scholar] [CrossRef]
- Zhou, X.; Jiao, L.; Qian, Y.; Dong, Q.; Sun, Y.; Zheng, W.V.; Zhao, W.; Zhai, W.; Qiu, L.; Wu, Y.; et al. Repositioning Azelnidipine as a Dual Inhibitor Targeting CD47/SIRPα and TIGIT/PVR Pathways for Cancer Immuno-Therapy. Biomolecules 2021, 11, 706. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, Y.; Dai, Q.; Li, X.; Xu, K.; Zou, G.; Yang, K.; Li, W.; Guo, X.; Yang, J.; et al. Development of Novel Anti-influenza Thiazolides with Relatively Broad-Spectrum Antiviral Potentials. Antimicrob. Agents Chemother. 2020, 64, e00222. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Yan, Y.; Li, W.; Zhao, L.; Dai, Q.; Li, Y.; Li, S.; Zhong, J.; Cao, R.; et al. Small Molecule Inhibitor of ATPase Activity of HSP70 as a Broad-Spectrum Inhibitor against Flavivirus Infections. ACS Infect. Dis. 2020, 6, 832–843. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Xiao, D.; Yin, J.; Song, M.; Xu, Y.; Zhao, L.; Dai, Q.; Li, Y.; Wang, C.; et al. Nafamostat mesylate as a broad-spectrum candidate for the treatment of flavivirus infections by targeting envelope proteins. Antivir. Res. 2022, 202, 105325. [Google Scholar] [CrossRef]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting flavivirus RNA dependent RNA polymerase through a pyridobenzothiazole inhibitor. Antivir. Res. 2016, 134, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Sáez-Álvarez, Y.; Arias, A.; Del Águila, C.; Agudo, R. Development of a fluorescence-based method for the rapid determination of Zika virus polymerase activity and the screening of antiviral drugs. Sci. Rep. 2019, 9, 5397. [Google Scholar] [CrossRef] [PubMed]
- Zmurko, J.; Marques, R.E.; Schols, D.; Verbeken, E.; Kaptein, S.J.; Neyts, J. The Viral Polymerase Inhibitor 7-Deaza-2’-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl. Trop. Dis. 2016, 10, e0004695. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Zhang, N.N.; Li, C.F.; Tian, M.; Hao, J.N.; Xie, X.P.; Shi, P.Y.; Qin, C.F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Álvarez, Y.; Jiménez de Oya, N.; Del Águila, C.; Saiz, J.C.; Arias, A.; Agudo, R.; Martín-Acebes, M.A. Novel Nonnucleoside Inhibitors of Zika Virus Polymerase Identified through the Screening of an Open Library of Antikinetoplastid Compounds. Antimicrob. Agents Chemother. 2021, 65, e0089421. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.; Lescar, J.; Arora, R.; Benson, T.; Nilar, S.; et al. Potent Allosteric Dengue Virus NS5 Polymerase Inhibitors: Mechanism of Action and Resistance Profiling. PLoS Pathog. 2016, 12, e1005737. [Google Scholar] [CrossRef] [Green Version]
- Gharbi-Ayachi, A.; Santhanakrishnan, S.; Wong, Y.H.; Chan, K.W.K.; Tan, S.T.; Bates, R.W.; Vasudevan, S.G.; El Sahili, A.; Lescar, J. Non-nucleoside Inhibitors of Zika Virus RNA-Dependent RNA Polymerase. J. Virol. 2020, 94, e00794. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Eisenstein, E.M. Targeting host store-operated Ca(2+) release to attenuate viral infections. Curr. Top. Med. Chem. 2013, 13, 1916–1932. [Google Scholar] [CrossRef]
- Scherbik, S.V.; Brinton, M.A. Virus-induced Ca2+ influx extends survival of west nile virus-infected cells. J. Virol. 2010, 84, 8721–8731. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef] [Green Version]
- Bezemer, B.; van Cleef, K.W.R.; Overheul, G.J.; Miesen, P.; van Rij, R.P. The calcium channel inhibitor lacidipine inhibits Zika virus replication in neural progenitor cells. Antivir. Res. 2022, 202, 105313. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yan, Y.; Dai, Q.; Xu, Y.; Yin, J.; Li, W.; Li, Y.; Yang, X.; Guo, X.; Liu, M.; et al. Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp. Viruses 2022, 14, 1228. https://doi.org/10.3390/v14061228
Wang Z, Yan Y, Dai Q, Xu Y, Yin J, Li W, Li Y, Yang X, Guo X, Liu M, et al. Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp. Viruses. 2022; 14(6):1228. https://doi.org/10.3390/v14061228
Chicago/Turabian StyleWang, Zhuang, Yunzheng Yan, Qingsong Dai, Yijie Xu, Jiye Yin, Wei Li, Yuexiang Li, Xiaotong Yang, Xiaojia Guo, Miaomiao Liu, and et al. 2022. "Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp" Viruses 14, no. 6: 1228. https://doi.org/10.3390/v14061228
APA StyleWang, Z., Yan, Y., Dai, Q., Xu, Y., Yin, J., Li, W., Li, Y., Yang, X., Guo, X., Liu, M., Chen, X., Cao, R., & Zhong, W. (2022). Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp. Viruses, 14(6), 1228. https://doi.org/10.3390/v14061228





