Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County—Results from a Population-Based Longitudinal Study in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort Design, Inclusion Criteria, and Study Program
2.2. Data on Registered COVID-19 Related Deaths, Registered Infected, and Tirschenreuth County Inhabitants
2.3. Observation Periods
2.4. Assessment of Educational Status, Comorbidities, Self-Reported Previous Infections, and Vaccination Status
2.5. Blood Sampling, Transport and Antibody Measurements
2.6. Statistical Analysis
2.7. Standardization
2.8. Confidence and Credibility Intervals
2.9. Vaccination
3. Results
3.1. Participant Characteristics and Dropout Analysis
3.2. Crude N-Antibody Seropositivity over Time
3.3. Serology vs. Positive PCR (Self-Reported and Confirmed by Health Authorities) across the Observation Periods
3.4. Dynamics of Standardized N-Antibody Seropositivity across the Observation Periods
3.5. Development of SDR and IFR across the Observation Periods
3.6. Contribution of Senior Care Homes to Overall N-Antibody Seropositivity, SDR and IFR across the Observation Periods
3.7. Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.; Anderson, E.; Campbell, T.; Clark, J.; Jackson, L.; Fichtenbaum, C.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Giles, B.; Meredith, P.; Robson, S.; Smith, G.; Chauhan, A. The SARS-CoV-2 B.1.1.7 variant and increased clinical severity—The jury is out. Lancet Infect. Dis. 2021, 21, 1213–1214. [Google Scholar] [CrossRef]
- Weekly Epidemiological Update on COVID-19-11 May 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---11-may-2021 (accessed on 11 March 2022).
- GISAID-hCov19 Variants. Available online: https://www.gisaid.org/hcov19-variants/ (accessed on 11 March 2022).
- Update on Omicron. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron (accessed on 11 March 2022).
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Peterhoff, D.; Beileke, S.; Günther, F.; Berr, M.; Einhauser, S.; Schütz, A.; Niller, H.H.; Steininger, P.; Knöll, A.; et al. Estimates and Determinants of SARS-Cov-2 Seroprevalence and Infection Fatality Ratio Using Latent Class Analysis: The Population-Based Tirschenreuth Study in the Hardest-Hit German County in Spring 2020. Viruses 2021, 13, 1118. [Google Scholar] [CrossRef]
- Espenhain, L.; Tribler, S.; Jørgensen, C.; Hansen, H.; Sönksen, U.; Ethelberg, S. Prevalence of SARS-CoV-2 antibodies in Denmark: Nationwide, population-based seroepidemiological study. Eur. J. Epidemiol. 2021, 36, 715–725. [Google Scholar] [CrossRef]
- Tunheim, G.; Rø, G.I.; Tran, T.; Kran, A.B.; Andersen, J.T.; Vaage, E.B.; Kolderup, A.; Vaage, J.T.; Lund-Johansen, F.; Hungnes, O. Trends in seroprevalence of SARS-CoV-2 and infection fatality rate in the Norwegian population through the first year of the COVID-19 pandemic. Influenza Other Respi. Viruses 2022, 16, 204–212. [Google Scholar] [CrossRef]
- Cantoe, C.L.; Gomes, A.; Serrano, M.; Pereira, A.; Ribeiro, R.; Napoleão, P.; Domingues, I.; Silva, C.; Fanczal, J.; Afonso, A.; et al. Longitudinal SARS-CoV-2 seroprevalence in Portugal and antibody maintenance 12 months after infection. Eur. J. Immunol. 2022, 52, 149–160. [Google Scholar] [CrossRef]
- Gornyk, D.; Harries, M.; Glöckner, S.; Strengert, M.; Kerrinnes, T.; Heise, J.-K.; Maaß, H.; Ortmann, J.; Kessel, B.; Kemmling, Y.; et al. SARS-CoV-2 seroprevalence in Germany. Dtsch. Arztebl. Int. 2021, 188, 824–831. [Google Scholar] [CrossRef]
- Dorigatti, I.; Lavezzo, E.; Manuto, L.; Ciavello, C.; Panceti, M.; Boldrin, C.; Cattai, M.; Saluzzo, F.; Franchin, E.; Del Vechio, C.; et al. SARS-CoV-2 antibody dynamics and transmission from community-wide serological testing in the Italian municipality of Vo’. Nat. Commun. 2021, 12, 4383. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.; Corman, V.; Echterhoff, A.; Müller, M.; Richter, A.; Schmandke, A.; Schmidt, M.; Schmidt, T.; de Vries, F.; Drosten, C.; et al. Seroprevalence and correlates of SARS-CoV-2 neutralizing antibodies from a population-based study in Bonn, Germany. Nat. Commun. 2021, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Saathoff, E.; Pritsch, M.; Noller, J.; Kroidl, I.; Olbrich, L.; Thiel, V.; Diefenbach, M.; Riess, F.; Forster, F.; et al. Protocol of a population-based prospective COVID-19 cohort study Munich, Germany (KoCo19). BMC Public Health 2020, 20, 1036. [Google Scholar]
- RKI-Corona-Monitoring, Lokal-Corona-Monitoring. Available online: https://www.rki.de/DE/Content/Gesundheitsmonitoring/Studien/cml-studie/Factsheet_Berlin-Mitte.html (accessed on 11 March 2022).
- Streeck, H.; Schulte, B.; Kümmerer, B.; Richter, E.; Höller, T.; Fuhrmann, C.; Bartok, E.; Dolscheid-Pommerich, R.; Berger, M.; Wessendorf, L.; et al. Infection fatality rate of SARS-CoV2 in a super-spreading event in Germany. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Wachtler, B.; Neuhauser, H.; Haller, S.; Grabka, M.M.; Zinn, S.; Schaade, L.; Hövener, C.; Hoebel, J. The risk of infection with SARS-CoV-2 among healthcare workers during the pandemic. Dtsch. Arztebl. Int. 2021, 118, 842–843. [Google Scholar] [CrossRef]
- Fischer, B.; Knabbe, C.; Vollmer, T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Eurosurveillance 2020, 25, 2001285. [Google Scholar] [CrossRef]
- Hippich, M.; Sifft, P.; Zapardiel-Gonzalo, J.; Böhmer, M.; Lampasona, V.; Bonifacio, E.; Ziegler, A. A public health antibody screening indicates a marked increase of SARS-CoV-2 exposure rate in children during the second wave. Med. N. Y. 2021, 2, 571–572. [Google Scholar] [CrossRef]
- Theuring, S.; Thielecke, M.; van Loon, W.; Hommes, F.; Hülso, C.; von der Haar, A.; Körner, J.; Schmidt, M.; Böhringer, F.; Mall, M.; et al. SARS-CoV-2 infection and transmission in school settings during the second COVID-19 wave: A cross-sectional study, Berlin, Germany, November 2020. Eurosurveillance 2021, 26, 2100184. [Google Scholar] [CrossRef]
- Lohse, S.; Sternjakob-Marthaler, A.; Lagemann, P.; Schöpe, J.; Rissland, J.; Seiwert, N.; Pfuhl, T.; Müllendorff, A.; Kiefer, L.; Vogelgesang, M.; et al. German federal-state-wide seroprevalence study of 1st SARS-CoV-2 pandemic wave shows importance of long-term antibody test performance. Commun. Med. 2022, 21, 52. [Google Scholar] [CrossRef]
- Peterhoff, D.; Einhauser, S.; Beileke, S.; Niller, H.; Günther, F.; Schachtner, M.; Asbach, B.; Steininger, P.; Tenbusch, M.; Peter, A.; et al. Comparative Immunogenicity of COVID-19 Vaccines in a Population-Based Cohort Study with SARS-CoV-2-Infected and Uninfected Participants. Vaccines 2022, 10, 324. [Google Scholar] [CrossRef]
- Brandl, M.; Selb, R.; Seidl-Pillmeier, S.; Marosevic, D.; Buchholz, U.; Rehmet, S. Mass gathering events and undetected transmission of SARS-CoV-2 in vulnerable populations leading to an outbreak with high case fatality ratio in the district of Tirschenreuth, Germany. Epidemiol. Infect. 2020, 148, e252. [Google Scholar] [CrossRef] [PubMed]
- Tirschenreuth, L. RKI-Ergebnis Tirschenreuth. 2020. Available online: https://www.kreis-tir.de/fileadmin/user_upload/rki_ergebnis.pdf (accessed on 11 March 2022).
- Kellam, P.; Barclay, W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J. Gen. Virol. 2020, 101, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Koch-Institut, R. Epidemiologisches Bulletin des Robert Koch-Instituts-Online vorab Ausgabe 17/2020. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2020/Ausgaben/17_20.pdf?__blob=publicationFile (accessed on 11 March 2022).
- Khalili, M.; Karamouzian, M.; Nasiri, N.; Javadi, S.; Mirzazadeh, A.; Sharifi, H. Epidemiological Characteristics of COVID-19: A Systematic Review and Meta-Analysis. Epidemiol. Infect. 2020, 148, e130. [Google Scholar] [CrossRef] [PubMed]
- Peterhoff, D.; Glück, V.; Vogel, M.; Schuster, P.; Schütz, A.; Neubert, P.; Albert, V.; Frisch, S.; Kiessling, M.; Pervan, P.; et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection 2020, 1, 75–82. [Google Scholar] [CrossRef]
- Einhauser, S.; Peterhofdd, D.; Niller, H.; Beileke, S.; Günther, F.; Steininger, P.; Burkhardt, R.; Heid, I.; Pfahlberg, A.; Überla, K.; et al. Spectrum Bias and Individual Strengths of SARS-CoV-2 Serological Tests—A Population-Based Evaluation. Diagnostics 2021, 11, 1843. [Google Scholar] [CrossRef]
- Sempos, C.T.; Tian, L. Adjusting Coronavirus Prevalence Estimates for Laboratory Test Kit Error. Am. J. Epidemiol. 2021, 190, 109–115. [Google Scholar] [CrossRef]
- Healy, B.; Khan, A.; Metezai, H.; Blyth, I.; Asad, H. The impact of false positive COVID-19 results in an area of low prevalence. Clin. Med. J. R. Coll. Phys. Lond. 2021, 21, E54–E56. [Google Scholar] [CrossRef]
- He, Z.; Ren, L.; Yang, J.; Guo, L.; Feng, L.; Ma, C.; Wang, X.; Leng, Z.; Tong, X.; Zhou, W.; et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: A longitudinal, population-level, cross-sectional study. Lancet 2021, 397, 1075–1084. [Google Scholar] [CrossRef]
- Pritsch, M.; Radon, K.; Bakuli, A.; Le Gleut, R.; Olbrich, L.; Noller, J.G.; Saathoff, E.; Castelletti, N.; Garí, M.; Pütz, P.; et al. Prevalence and Risk Factors of Infection in the Representative COVID-19 Cohort Munich. Int. J. Environ. Res. Public Health 2021, 18, 3572. [Google Scholar] [CrossRef]
- Neuhauser, H.; Rosario, A.; Butschalowsky, H.; Haller, S.; Hoebel, J.; Michel, J.; Nitsche, A.; Poethko-Müller, C.; Prütz, F.; Schlaud, M.; et al. Germany’s low SARS-CoV-2 seroprevalence confirms effective containment in 2020: Results of the nationwide RKI-SOEP study. medRxiv 2021. [Google Scholar] [CrossRef]
- Radon, K.; Bakuli, A.; Pütz, P.; Le Gleut, R.; Noller, J.; Olbrich, L.; Saathoff, E.; Garí, M.; Schälte, Y.; Frahnow, T.; et al. From first to second wave: Follow-up of the prospective COVID-19 cohort (KoCo19) in Munich (Germany). BMC Infect. Dis. 2021, 21, 925. [Google Scholar] [CrossRef] [PubMed]
- GEMEINSAM. GEGEN COVID: Prospektive COVID-19 Kohorte München (KoCo19). Available online: http://www.klinikum.uni-muenchen.de/Abteilung-fuer-Infektions-und-Tropenmedizin/de/COVID-19/KoCo19/index.html (accessed on 11 March 2022).
- Experience. Available online: https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4 (accessed on 11 March 2022).
- Fenwick, C.; Croxatto, A.; Coste, A.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Abbott, S.; Barnard, R.; Jarvis, C.; Kucharski, A.; Munday, J.; Pearson, C.; Russell, T.; Tully, D.; Washburne, A.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, 6538. [Google Scholar]
- Davies, N.G.; Jarvis, C.; CMMID COVID-19 Working Group; Edmunds, W.; Jewell, N.; Diaz-Ordaz, K.; Keogh, R. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar]
- COVID-19 Forecasting Team Variation in the COVID-19 infection–fatality ratio by age, time, and geography during the pre-vaccine era: A systematic analysis. Lancet 2022, 399, 1469–1488. [CrossRef]
- COVID-19 Vaccination Dashboard. Available online: https://impfdashboard.de/en/ (accessed on 20 May 2022).
- Peters, A.; Rospleszcz, S.; Greiser, K.H.; Dallavalle, M.; Berger, K. The Impact of the COVID-19 Pandemic on Self-Reported Health. Dtsch. Arztebl. Int. 2020, 117, 861–867. [Google Scholar] [CrossRef]
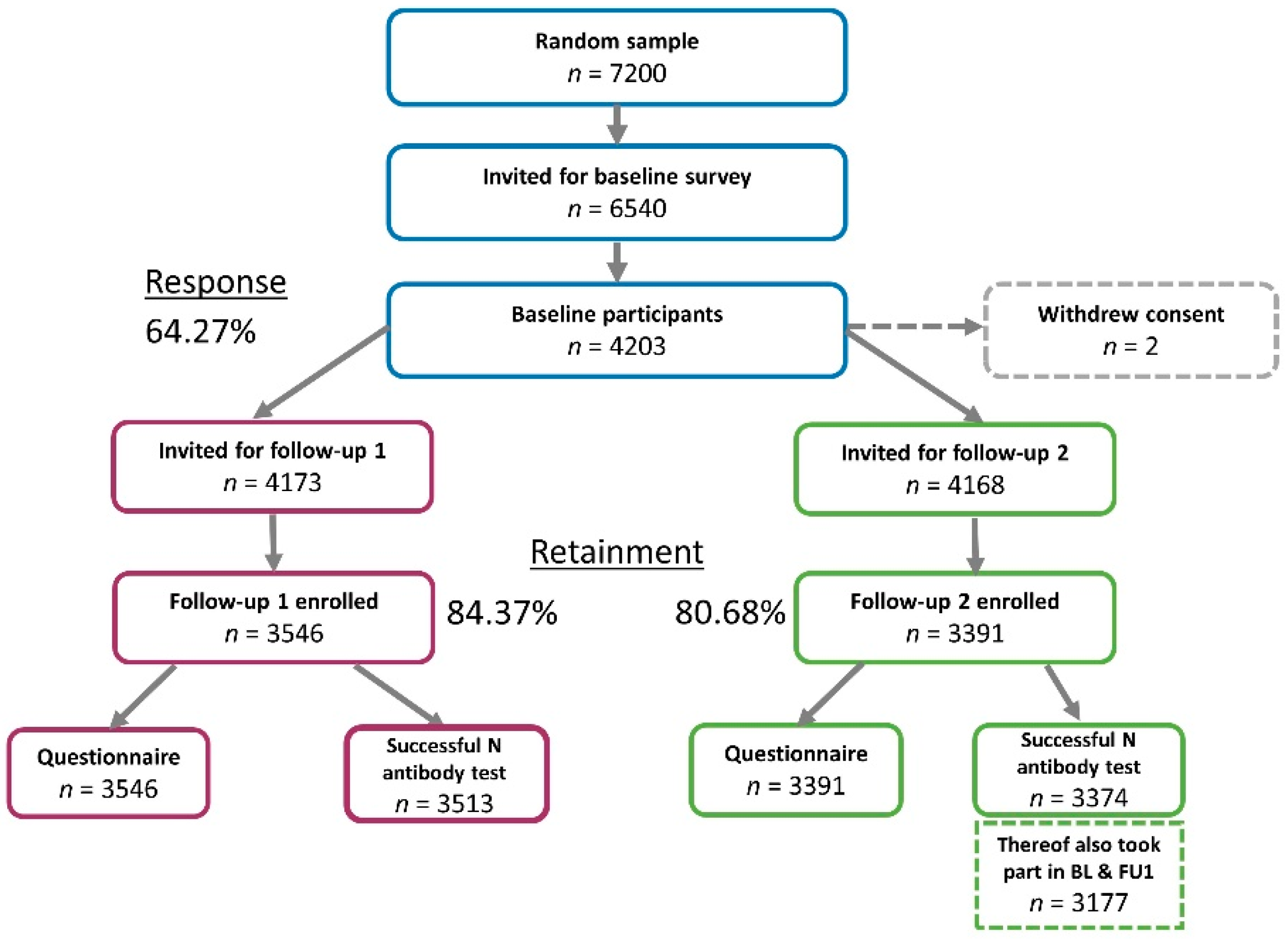
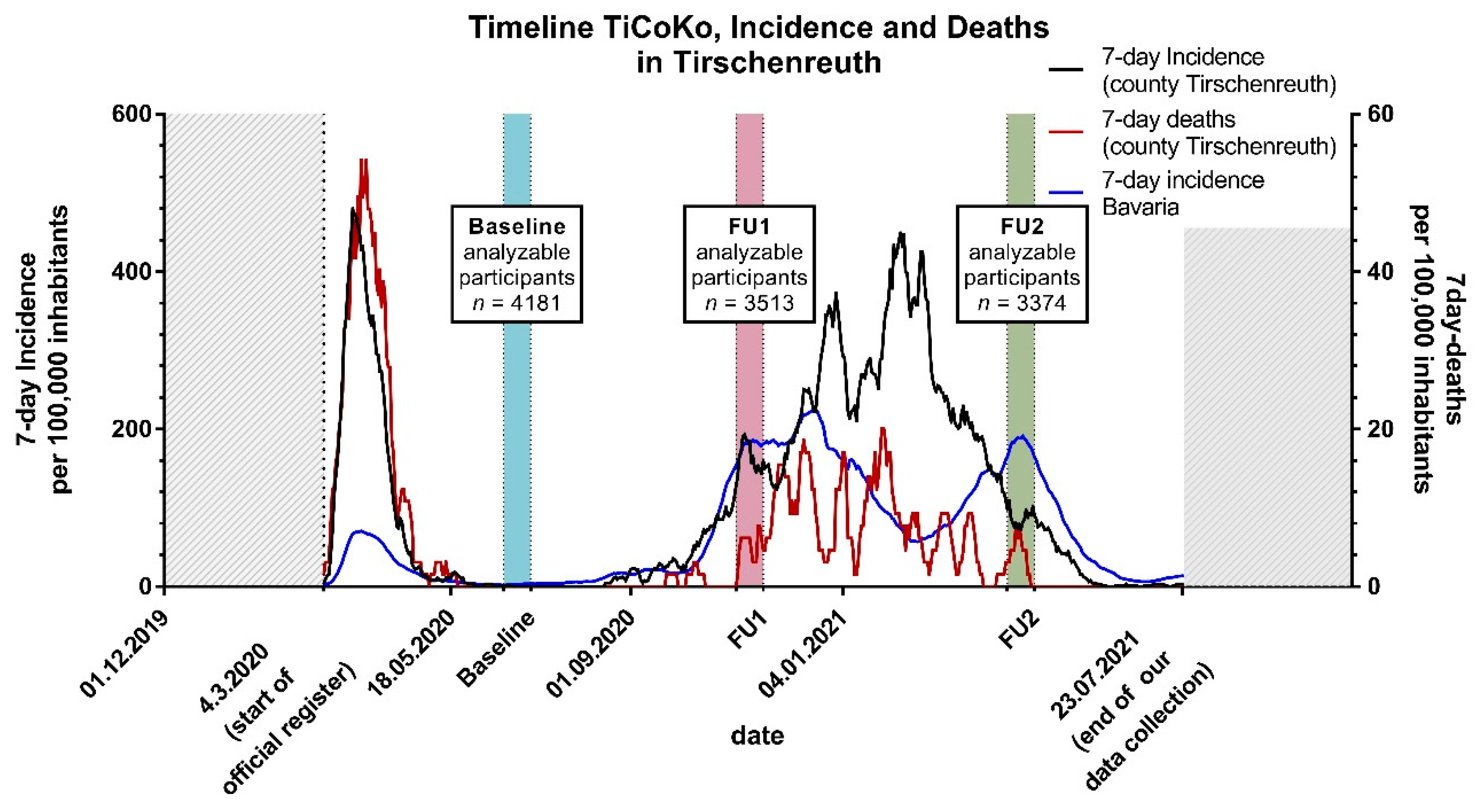

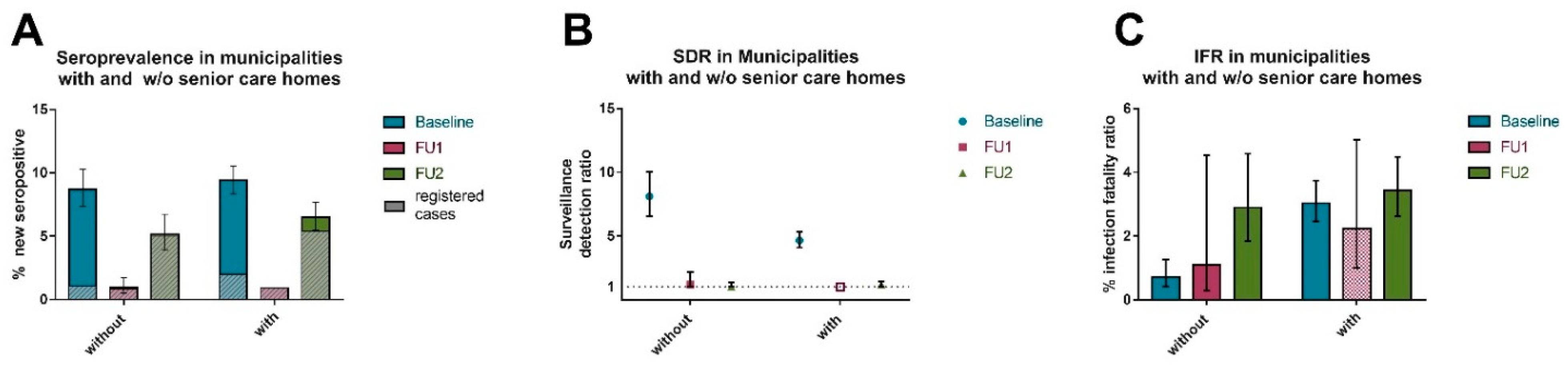
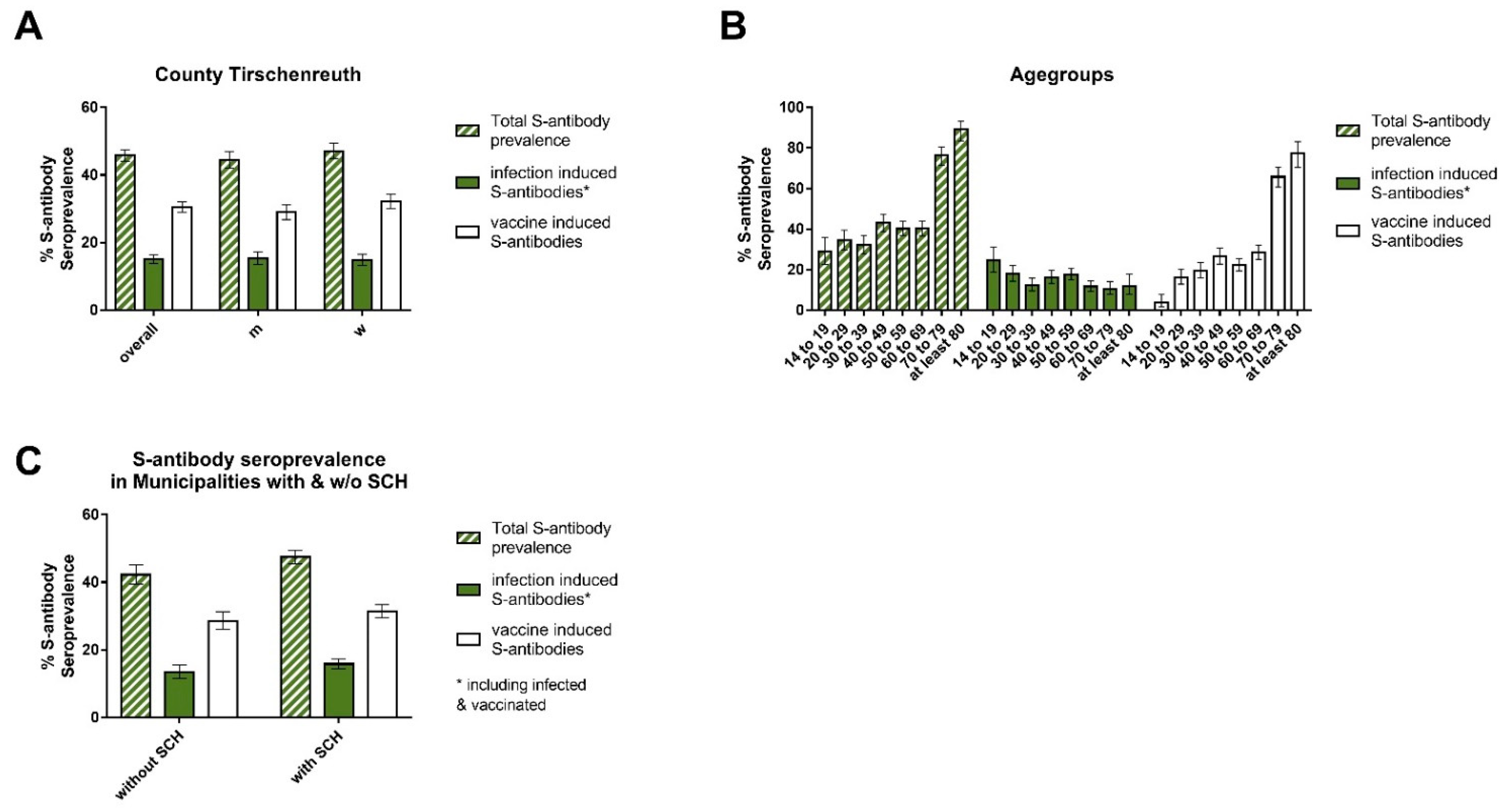
| Variable | BL [Participants] | FU_1 [Participants] | FU_2 [Participants] |
|---|---|---|---|
| Age median (min, max, IQR) | 52.0 (14.0, 102.0, 35.0–64.0) [n = 4181] | 53.0 (14.0, 102.0, 37.0–64.0) [n = 3513] | 53.0 (14.0, 102.0, 37.0–64.0) [n = 3374] |
| Age 14–20 % (n) | 5.4 (225) [n = 4181] | 5.0 (176) [n = 3513] | 5.2 (177) [n = 3374] |
| Age 20–49 % (n) | 40.8 (1707) [n = 4181] | 38.3 (1345) [n = 3513] | 38.1 (1284) [n = 3374] |
| Age 50–69 % (n) | 38.8 (1624) [n = 4181] | 41.2 (1449) [n = 3513] | 41.2 (1389) [n = 3374] |
| Age 70+ % (n) | 14.9 (625) [n = 4181] | 15.5 (543) [n = 3513] | 15.5 (524) [n = 3374] |
| Female % (n) | 51.6 (2158) [n = 4181] | 53.0 (1861) [n = 3513] | 53.7 (1813) [n = 3374] |
| BMI median (min, max, IQR) | 26.6 (13.9, 62.1, 23.7–30.4) [n = 4134] | 26.6 (13.9, 62.1, 23.7–30.3) [n = 3474] | 26.6 (13.9, 62.1, 23.7–30.4) [n = 3339] |
| Disease 1 | |||
| autoimmune % (n) | 7.1 (289) [n = 4081] | 7.3 (250) [n = 3435] | 7.4 (243) [n = 3300] |
| cancer % (n) | 4.9 (202) [n = 4081] | 5.2 (178) [n = 3435] | 5.0 (164) [n = 3300] |
| diabetes % (n) | 7.6 (312) [n = 4081] | 7.5 (259) [n = 3435] | 7.4 (245) [n = 3300] |
| cardiovascular % (n) | 9.9 (402) [n = 4081] | 9.6 (331) [n = 3435] | 9.5 (314) [n = 3300] |
| none 4 % (n) | 75.8 (3093) [n = 4081] | 75.6 (2596) [n = 3435] | 76.0 (2507) [n = 3300] |
| Education | |||
| Years 2 median (min, max, IQR) | 11.0 (6.0, 22.0, 10.0–14.0) [n = 4085] | 11.0 (6.0, 22.0, 10.0–13.0) [n = 3433] | 11.0 (6.0, 22.0, 10.0–14.0) [n = 3301] |
| High 3 % (n) | 30.0 (1225) [n = 4085] | 29.5 (1013) [n = 3433] | 29.8 (985) [n = 3301] |
| antibody status | |||
| N-antibody positive at BL % (n) | 8.9 (374) [n = 4181] | 10.0 (351) [n = 3513] | 10.3 (349) [n = 3374] |
| Time of Analysis Sex | AnalyzableParticipants 1 n | Total N Antibody Positive % (n) 2 | Analyzable Participants n Previously Pos/Neg | Newly N Antibody Positive % (n) 3 | Newly N Antibody Negative % (n) 4 | Ever Seropositive (%) 5 | Total N Antibody Positives (%) 5 |
|---|---|---|---|---|---|---|---|
| Baseline | 4181 | 8.95 (374) | 0/4181 | 8.95 (374) | (n/a) | ||
| women | 2158 | 9.08 (196) | 0/2158 | 9.08 (196) | (n/a) | ||
| men | 2023 | 8.80 (178) | 0/2023 | 8.8 (178) | (n/a) | ||
| FU1 | 3513 | 10.22 (359) | 351/3162 | 0.66 (21) | 3.70 (13) | 9.55 | 9.22 |
| women | 1861 | 10.26 (191) | 187/1674 | 0.66 (11) | 3.74 (7) | 9.54 | 9.21 |
| men | 1652 | 10.17 (168) | 164/1488 | 0.67 (10) | 3.66 (6) | 9.58 | 9.23 |
| FU2 | 3177 | 15.68 (498) | 349/2828 | 5.80 (164) | 4.30 (15) | 14.80 | 14.09 |
| women | 1710 | 15.56 (266) | 186/1524 | 5.91 (90) | 5.38 (10) | 14.89 | 14.08 |
| men | 1467 | 15.81 (232) | 163/1304 | 5.67 (74) | 3.07 (5) | 14.69 | 14.10 |
| Timepoint | # Confirmed Positive Registered PCR Test (# Total Self-Reported PCR Tests) | N-Antibody Negative # (%[Group]; %[All Tests]) | N-Antibody Positive # (%[Group]; %[All Tests]) |
|---|---|---|---|
| Until BL 12/2019–4/2020 | n = 66 (n = 501) | 4 (6.06; 0.80) | 62 (93.94; 12.38) |
| between BL and FU1 6/2020–11/2020 | n = 19 (n = 1064) | 6 (31.58; 0.56) | 13 (68.4; 1.22) |
| between FU1 and FU2 11/2020–4/2021 | n = 153 (n = 1568) | 8 (5.23; 0.51) | 145 (94.77; 9.25) |
| until FU2 12/2019–4/2021 | n = 238 (n = 3133) | 18 (7.56; 0.57) | 220 (92.44; 7.02) |
| Vaccination 1- and Serostatus | N + S | Only S | S | S |
|---|---|---|---|---|
| Antibody Positive | Antibody Positive | Seropositive | Antibody Negative | |
| % (#) | % (#) | % (#) | % (#) | |
| Not vaccinated, | 20.17 (375) | 1.61 (30) | 21.79 (405) | 78.21 (1454) |
| n = 1859 | ||||
| vaccinated (all), | 9.18 (137) | 63.47 (947) | 72.65 (1084) | 27.35 (408) |
| n = 1492 | ||||
| 1× vaccinated (<14 d), | 8.48 (38) | 14.73 (66) | 23.21 (104) | 76.79 (344) |
| n = 448 | ||||
| 1× vaccinated (>14 d), | 10.69 (76) | 81.43 (579) | 92.12 (655) | 7.88 (56) |
| n = 711 | ||||
| 2× vaccinated (<14 d), | 25 (2) | 75 (6) | 100 (8) | 0 (0) |
| n = 8 | ||||
| 2× vaccinated (>14 d), | 6.21 (19) | 93.46 (286) | 99.67 (305) | 0.33 (1) |
| n = 306 | ||||
| No of vaccinations unknown | 10.5 (2) | 52.6 (10) | 63.2 (12) | 36.8 (7) |
| n = 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einhauser, S.; Peterhoff, D.; Beileke, S.; Günther, F.; Niller, H.-H.; Steininger, P.; Knöll, A.; Korn, K.; Berr, M.; Schütz, A.; et al. Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County—Results from a Population-Based Longitudinal Study in Germany. Viruses 2022, 14, 1168. https://doi.org/10.3390/v14061168
Einhauser S, Peterhoff D, Beileke S, Günther F, Niller H-H, Steininger P, Knöll A, Korn K, Berr M, Schütz A, et al. Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County—Results from a Population-Based Longitudinal Study in Germany. Viruses. 2022; 14(6):1168. https://doi.org/10.3390/v14061168
Chicago/Turabian StyleEinhauser, Sebastian, David Peterhoff, Stephanie Beileke, Felix Günther, Hans-Helmut Niller, Philipp Steininger, Antje Knöll, Klaus Korn, Melanie Berr, Anja Schütz, and et al. 2022. "Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County—Results from a Population-Based Longitudinal Study in Germany" Viruses 14, no. 6: 1168. https://doi.org/10.3390/v14061168
APA StyleEinhauser, S., Peterhoff, D., Beileke, S., Günther, F., Niller, H.-H., Steininger, P., Knöll, A., Korn, K., Berr, M., Schütz, A., Wiegrebe, S., Stark, K. J., Gessner, A., Burkhardt, R., Kabesch, M., Schedl, H., Küchenhoff, H., Pfahlberg, A. B., Heid, I. M., ... Wagner, R. (2022). Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County—Results from a Population-Based Longitudinal Study in Germany. Viruses, 14(6), 1168. https://doi.org/10.3390/v14061168






