Molnupiravir and Its Active Form, EIDD-1931, Show Potent Antiviral Activity against Enterovirus Infections In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Reagents
2.2. Antiviral Activity Assay
2.3. Immunofluorescence Assay
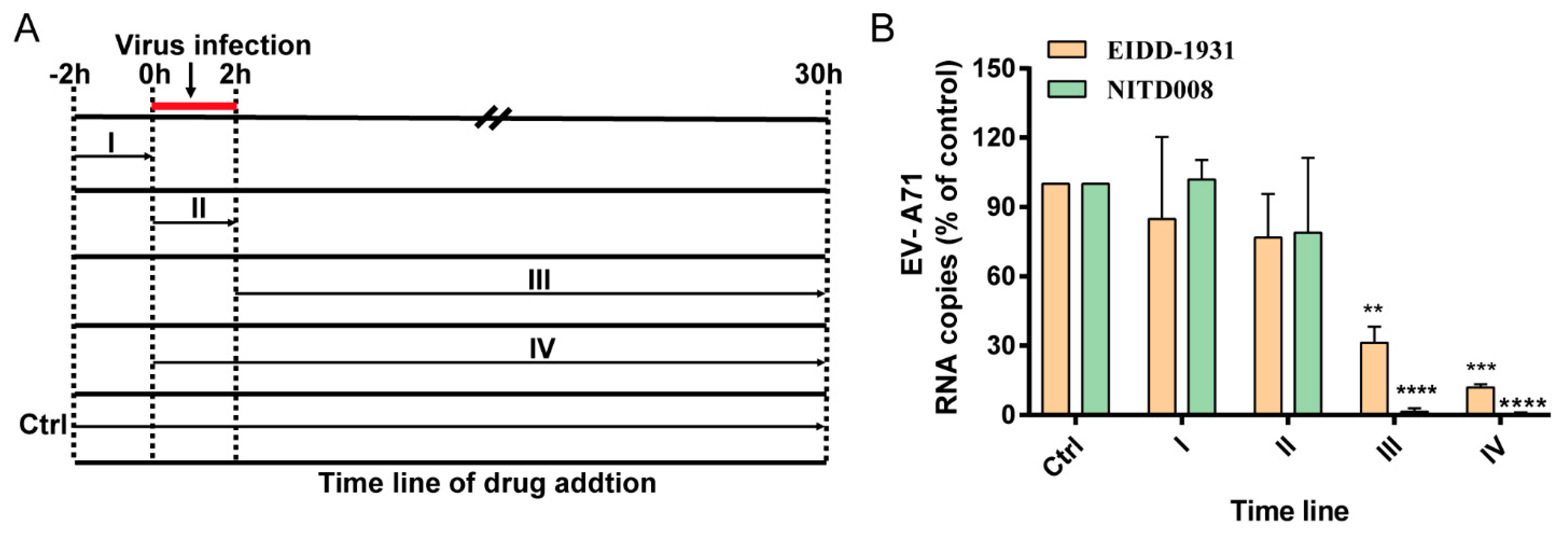
2.4. Time-of-Drug-Addition Assay
2.5. Quantitative Real-Time PCR
2.6. Western Blot Assay
2.7. EV-A71 Infection in Mice
2.8. Ethics Statement
2.9. Statistical Analysis
3. Results
3.1. EIDD-1931 and EIDD-2801 Inhibit EV-A71 Infection In Vitro
3.2. EIDD-1931 and EIDD-2801 Reduce the Production of EV-A71 Virus Protein
3.3. EIDD-1931 Acts at the Post-Entry Stage of EV-A71 infection
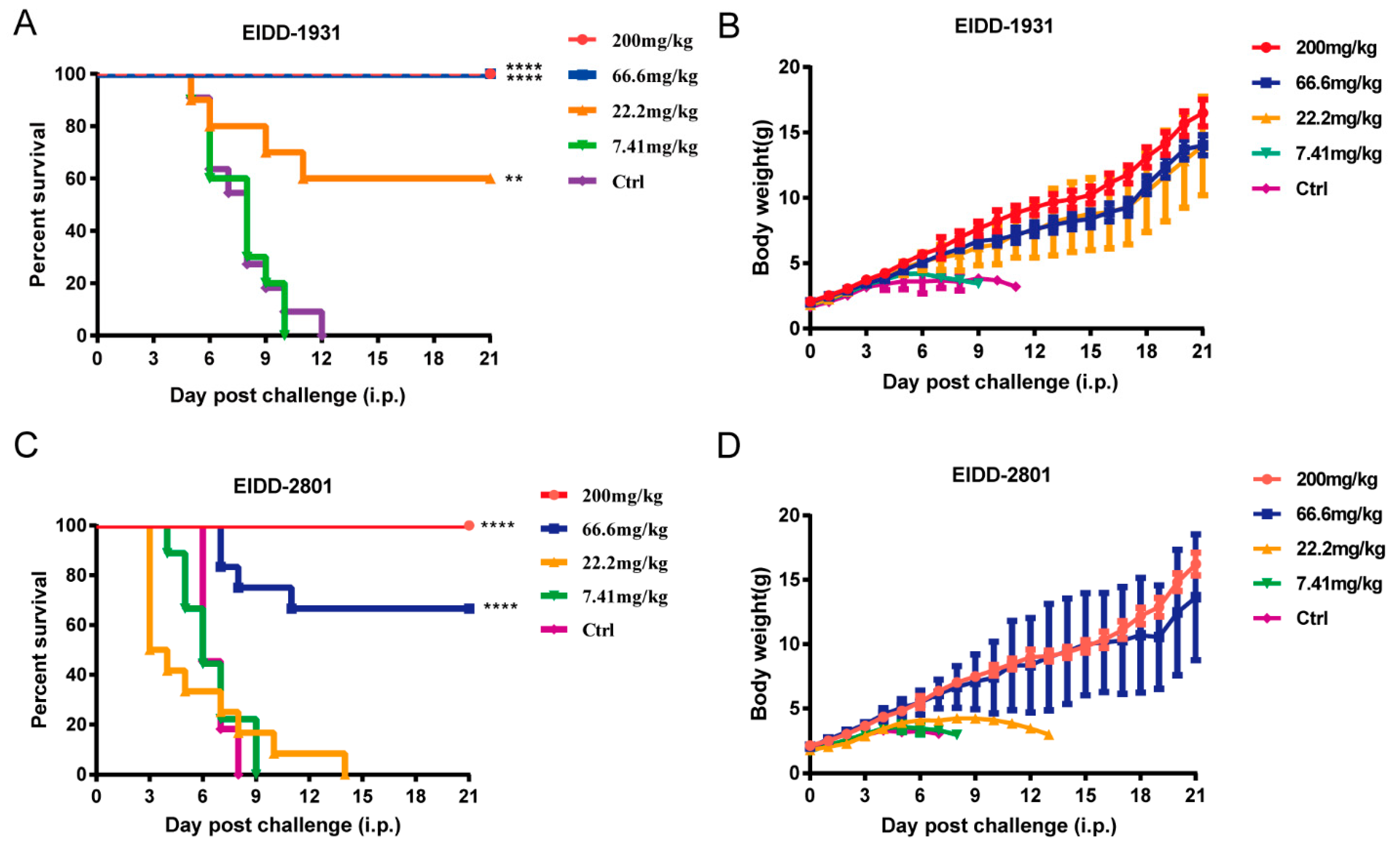
3.4. EIDD-1931 and EIDD-2801 Protected 1-Day-Old ICR Suckling Mice from Lethal EV-A71 Challenge
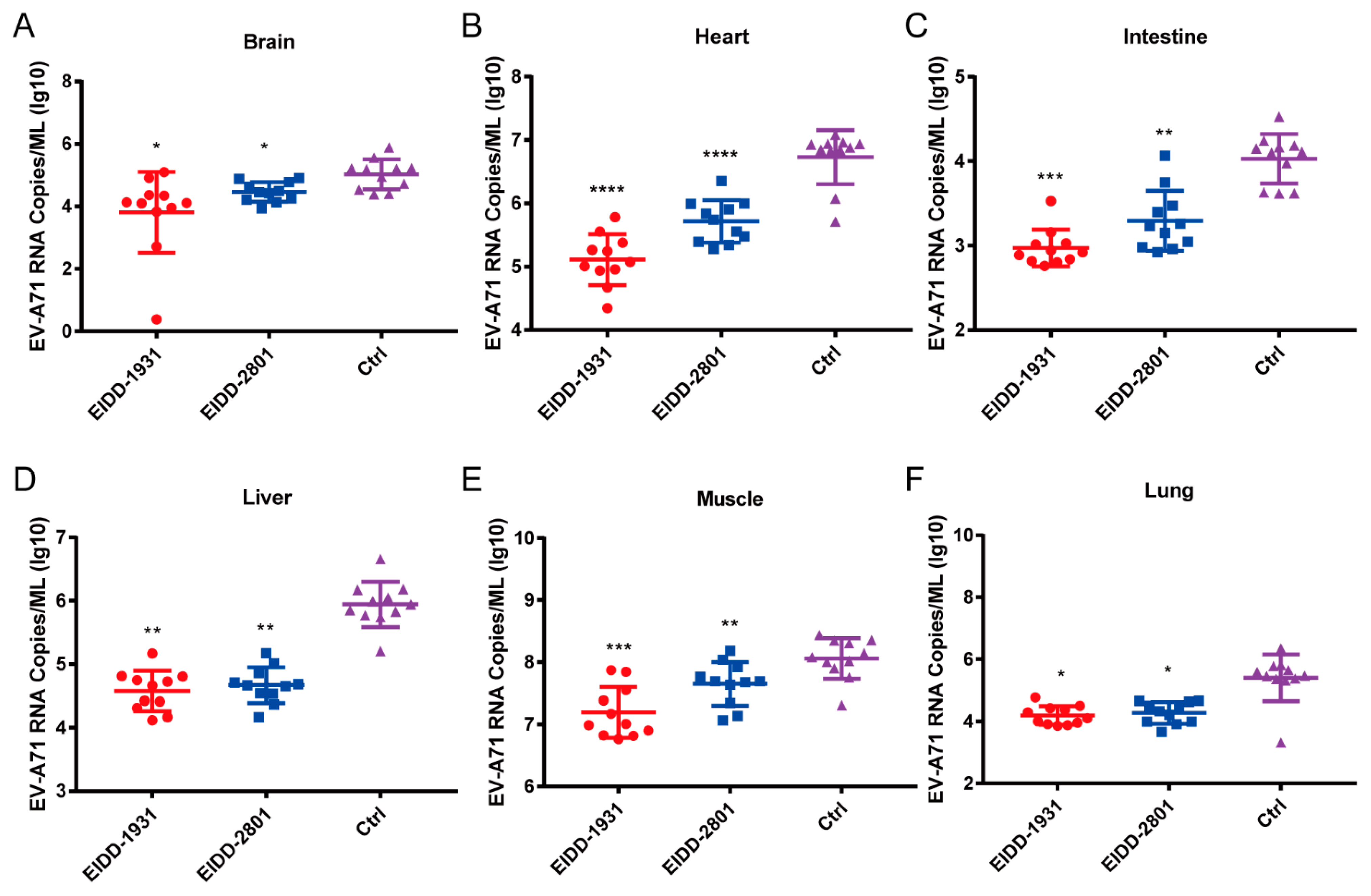
3.5. EIDD-1931 and EIDD-2801 Reduce the Viral Loads in Various Tissues of EV-A71-Infected Mice
3.6. EIDD-1931 and EIDD-2801 Have Broad-Spectrum Antiviral Activity against Multiple Enteroviruses In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khetsuriani, N.; Lamonte, A.; Oberste, M.S.; Pallansch, M. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the United States, 1983–2003. Pediatric Infect. Dis. J. 2006, 25, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008–2012: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Nichol, S.T.; Beaty, B.J.; Elliott, R.M.; Goldbach, R.; Plyusnin, A.; Schmaljohn, C.S.; Tesh, R.B. Virus Taxonomy: Classification and Nomenclature of Viruses: 8th Report of the International Committee on Taxonomy of Viruses; Academic Press: Oxford, UK, 2005. [Google Scholar]
- WPRO. A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD); WHO Western Pacific Region: Manila, PA, USA, 2011. [Google Scholar]
- Broccolo, F.; Drago, F.; Ciccarese, G.; Genoni, A.; Puggioni, A.; Rosa, G.M.; Parodi, A.; Manukyan, H.; Laassri, M.; Chumakov, K.; et al. Severe atypical hand-foot-and-mouth disease in adults due to coxsackievirus A6: Clinical presentation and phylogenesis of CV-A6 strains. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Musharrafieh, R.; Zheng, M.; Wang, J. Enterovirus D68 Antivirals: Past, Present, and Future. ACS Infect. Dis. 2020, 6, 1572–1586. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.A.; Liu, P.; Sole, M.J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ. Res. 1994, 74, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Howlett, S.E. Coxsackievirus B3-Induced Myocarditis: New Insights Into a Female Advantage. Can. J. Cardiol. 2018, 34, 354–355. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, D.; Stafford, K.A.; Sung, Y.K. Update on coxsackievirus B3 myocarditis. Curr. Opin. Rheumatol. 2012, 24, 401–407. [Google Scholar] [CrossRef]
- Huang, J.; Liao, Q.; Ooi, M.H.; Cowling, B.J.; Chang, Z.; Wu, P.; Liu, F.; Li, Y.; Luo, L.; Yu, S.; et al. Epidemiology of Recurrent Hand, Foot and Mouth Disease, China, 2008–2015. Emerg. Infect. Dis. 2018, 24, 432–442. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Kong, F.; Xin, X.; Liang, J.; Xin, H.; Dong, L.; Jiang, F. Epidemiological Characteristics of Hand, Foot, and Mouth Disease Outbreaks in Qingdao, 2009–2018. Iran. J. Public Health 2021, 50, 999–1008. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, Y.J.; Kim, T.H.; Cheon, D.S.; Nam, S.O. Clinico-radiological spectrum in enterovirus 71 infection involving the central nervous system in children. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2014, 21, 416–420. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Huang, K.; Wang, J.; Tian, H.; Song, Y.; Yang, Q.; Yan, D.; Zhu, S.; Yao, M.; et al. Two Coxsackievirus B3 outbreaks associated with hand, foot, and mouth disease in China and the evolutionary history worldwide. BMC Infect. Dis. 2019, 19, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa, I.P., Jr.; Giamberardino, H.I.; Raboni, S.M.; Debur, M.C.; de Lourdes Aguiar Oliveira, M.; Burlandy, F.M.; da Silva, E.E. Simultaneous enterovirus EV-D68 and CVA6 infections causing acute respiratory distress syndrome and hand, foot and mouth disease. Virol. J. 2021, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Z.; Rao, Q.; Wang, X.; Wang, M.; Du, T.; Tang, J.; Long, S.; Zhang, J.; Luo, J.; et al. The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming, China. Emerg. Microbes Infect. 2021, 10, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Mo, Z.; Wang, X.; Xia, J.; Liang, Z.; Zhang, Y.; Li, Y.; Mao, Q.; Wang, J.; et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014, 370, 829–837. [Google Scholar] [CrossRef]
- Zhu, F.C.; Meng, F.Y.; Li, J.X.; Li, X.L.; Mao, Q.Y.; Tao, H.; Zhang, Y.T.; Yao, X.; Chu, K.; Chen, Q.H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef]
- Reynard, O.; Nguyen, X.N.; Alazard-Dany, N.; Barateau, V.; Cimarelli, A.; Volchkov, V.E. Identification of a New Ribonucleoside Inhibitor of Ebola Virus Replication. Viruses 2015, 7, 6233–6240. [Google Scholar] [CrossRef]
- Yoon, J.J.; Toots, M.; Lee, S.; Lee, M.E.; Ludeke, B.; Luczo, J.M.; Ganti, K.; Cox, R.M.; Sticher, Z.M.; Edpuganti, V.; et al. Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses. Antimicrob. Agents Chemother. 2018, 62, e00766. [Google Scholar] [CrossRef] [Green Version]
- Painter, G.R.; Natchus, M.G.; Cohen, O.; Holman, W.; Painter, W.P. Developing a direct acting, orally available antiviral agent in a pandemic: The evolution of molnupiravir as a potential treatment for COVID-19. Curr. Opin. Virol. 2021, 50, 17–22. [Google Scholar] [CrossRef]
- Stuyver, L.J.; Whitaker, T.; McBrayer, T.R.; Hernandez-Santiago, B.I.; Lostia, S.; Tharnish, P.M.; Ramesh, M.; Chu, C.K.; Jordan, R.; Shi, J.; et al. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob. Agents Chemother. 2003, 47, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, P.; Solanki, K.; Li, Y.; Ma, Z.; Peppelenbosch, M.P.; Baig, M.S.; Pan, Q. Viral polymerase binding and broad-spectrum antiviral activity of molnupiravir against human seasonal coronaviruses. Virology 2021, 564, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Toots, M.; Yoon, J.J.; Cox, R.M.; Hart, M.; Sticher, Z.M.; Makhsous, N.; Plesker, R.; Barrena, A.H.; Reddy, P.G.; Mitchell, D.G.; et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019, 11, eaax5866. [Google Scholar] [CrossRef] [PubMed]
- Han, J.F.; Cao, R.Y.; Tian, X.; Yu, M.; Qin, E.D.; Qin, C.F. Producing infectious enterovirus type 71 in a rapid strategy. Virol. J. 2010, 7, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zheng, Q.; Li, S.; He, M.; Wu, Y.; Li, Y.; Zhu, R.; Yu, H.; Hong, Q.; Jiang, J.; et al. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody. Nat. Commun. 2017, 8, 505. [Google Scholar] [CrossRef]
- Du, R.; Mao, Q.; Hu, Y.; Lang, S.; Sun, S.; Li, K.; Gao, F.; Bian, L.; Yang, C.; Cui, B.; et al. A potential therapeutic neutralization monoclonal antibody specifically against multi-coxsackievirus A16 strains challenge. Hum. Vaccines Immunother. 2019, 15, 2343–2350. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhu, R.; Xu, L.; He, M.; Yan, X.; Liu, D.; Yin, Z.; Wu, Y.; Li, Y.; Yang, L.; et al. Atomic structures of enterovirus D68 in complex with two monoclonal antibodies define distinct mechanisms of viral neutralization. Nat. Microbiol. 2019, 4, 124–133. [Google Scholar] [CrossRef]
- Liu, J.N.; Wang, W.; Duo, J.Y.; Hao, Y.; Ma, C.M.; Li, W.B.; Lin, S.Z.; Gao, X.Z.; Liu, X.L.; Xu, Y.F.; et al. Combined peptides of human enterovirus 71 protect against virus infection in mice. Vaccine 2010, 28, 7444–7451. [Google Scholar] [CrossRef]
- Li, Z.; Xu, L.; He, D.; Yang, L.; Liu, C.; Chen, Y.; Shih, J.W.; Zhang, J.; Zhao, Q.; Cheng, T.; et al. In vivo time-related evaluation of a therapeutic neutralization monoclonal antibody against lethal enterovirus 71 infection in a mouse model. PloS ONE 2014, 9, e109391. [Google Scholar] [CrossRef]
- Hao, T.; Li, Y.; Fan, S.; Li, W.; Wang, S.; Li, S.; Cao, R.; Zhong, W. Design, synthesis and pharmacological evaluation of a novel mTOR-targeted anti-EV71 agent. Eur. J. Med. Chem. 2019, 175, 172–186. [Google Scholar] [CrossRef]
- Taguwa, S.; Maringer, K.; Li, X.; Bernal-Rubio, D.; Rauch, J.N.; Gestwicki, J.E.; Andino, R.; Fernandez-Sesma, A.; Frydman, J. Defining Hsp70 Subnetworks in Dengue Virus Replication Reveals Key Vulnerability in Flavivirus Infection. Cell 2015, 163, 1108–1123. [Google Scholar] [CrossRef] [Green Version]
- Carroll, S.S.; Tomassini, J.E.; Bosserman, M.; Getty, K.; Stahlhut, M.W.; Eldrup, A.B.; Bhat, B.; Hall, D.; Simcoe, A.L.; LaFemina, R.; et al. Inhibition of hepatitis C virus RNA replication by 2’-modified nucleoside analogs. J. Biol. Chem. 2003, 278, 11979–11984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.L.; Yeo, H.; Ye, H.Q.; Liu, S.Q.; Shang, B.D.; Gong, P.; Alonso, S.; Shi, P.Y.; Zhang, B. Inhibition of enterovirus 71 by adenosine analog NITD008. J. Virol. 2014, 88, 11915–11923. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Wang, Y.; Qing, J.; Shu, B.; Cao, L.; Lou, Z.; Gong, P.; Sun, Y.; Yin, Z. An adenosine nucleoside analogue NITD008 inhibits EV71 proliferation. Antivir. Res. 2014, 112, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, C.N.; Shih, S.R.; Ho, M.S. Characterization of a Vero cell-adapted virulent strain of enterovirus 71 suitable for use as a vaccine candidate. Vaccine 2002, 20, 2485–2493. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsieh, C.C.; Ko, W.C. Molnupiravir-A Novel Oral Anti-SARS-CoV-2 Agent. Antibiotics. 2021, 10, 1294. [Google Scholar] [CrossRef] [PubMed]


| Enterovirus | Cell Line | EIDD-1931 | EIDD-2801 | ||
|---|---|---|---|---|---|
| EC50 (μM) | SI | EC50 (μM) | SI | ||
| EV-A71 AH08/06 | Huh7 | 2.79 ± 0.89 | 12.22 | 30.29 ± 0.55 | >3.3 |
| EV-D68 STL-2014–12 | RD | 9.69 ± 0.04 | 8.3 | 86.21 ± 9.17 | >1.6 |
| CV-A6 TW-2007-00141 | RD | 15.72 ± 0.38 | 5.11 | >100 | - |
| CV-A16 190-D1 | RD | 4.14 ± 0.27 | 19.43 | 44.91 ± 4.23 | >2.23 |
| CV-B3 Nancy | RD | 3.65 ± 0.82 | 3.85 | 87.23 ± 10.84 | >1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, M.; Yan, Y.; Wang, Z.; Dai, Q.; Yang, X.; Guo, X.; Li, W.; Chen, X.; Cao, R.; et al. Molnupiravir and Its Active Form, EIDD-1931, Show Potent Antiviral Activity against Enterovirus Infections In Vitro and In Vivo. Viruses 2022, 14, 1142. https://doi.org/10.3390/v14061142
Li Y, Liu M, Yan Y, Wang Z, Dai Q, Yang X, Guo X, Li W, Chen X, Cao R, et al. Molnupiravir and Its Active Form, EIDD-1931, Show Potent Antiviral Activity against Enterovirus Infections In Vitro and In Vivo. Viruses. 2022; 14(6):1142. https://doi.org/10.3390/v14061142
Chicago/Turabian StyleLi, Yuexiang, Miaomiao Liu, Yunzheng Yan, Zhuang Wang, Qingsong Dai, Xiaotong Yang, Xiaojia Guo, Wei Li, Xingjuan Chen, Ruiyuan Cao, and et al. 2022. "Molnupiravir and Its Active Form, EIDD-1931, Show Potent Antiviral Activity against Enterovirus Infections In Vitro and In Vivo" Viruses 14, no. 6: 1142. https://doi.org/10.3390/v14061142
APA StyleLi, Y., Liu, M., Yan, Y., Wang, Z., Dai, Q., Yang, X., Guo, X., Li, W., Chen, X., Cao, R., & Zhong, W. (2022). Molnupiravir and Its Active Form, EIDD-1931, Show Potent Antiviral Activity against Enterovirus Infections In Vitro and In Vivo. Viruses, 14(6), 1142. https://doi.org/10.3390/v14061142






