Abstract
Among novel strategies proposed in pest management, synergistic agents are used to improve insecticide efficacy through an elevation of intracellular calcium concentration that activates the calcium-dependent intracellular pathway. This leads to a changed target site conformation and to increased sensitivity to insecticides while reducing their concentrations. Because virus-like particles (VLPs) increase the intracellular calcium concentration, they can be used as a synergistic agent to synergize the effect of insecticides. VLPs are self-assembled viral protein complexes, and by contrast to entomopathogen viruses, they are devoid of genetic material, which makes them non-infectious and safer than viruses. Although VLPs are well-known to be used in human health, we propose in this study the development of a promising strategy based on the use of VLPs as synergistic agents in pest management. This will lead to increased insecticides efficacy while reducing their concentrations.
1. Introduction
Today, world agriculture faces a major challenge: increasing food production to feed about 9 billion people by 2050 [1]. To improve productivity, farmers still largely rely on the extensive use of chemical insecticides to control insect pests. However, the intense use of limited number of active ingredients has generated insect-selective pressure for resistance and has led to accumulation of residual effects that impact the environment and human health [2,3,4]. Regarding insecticide resistance, hundreds of insect species have developed resistance to at least one insecticide, impacting insect control [4]. Different resistance mechanisms have been described, such as insecticide target-site mutations, detoxification enzymes overproduction, penetration resistance by cuticle modification, and behavioral resistance [5,6]. The international Insecticide-Resistance Action Committee (IRAC), including stakeholders, has been developed to improve resistance awareness and Insecticide-Resistance Management (IRM) programs in crop protection [7]. To limit the side effects due to overuse of insecticides, integrated pest management (IPM) has been implemented. It is an effective and environmental approach to pest management, including the reduction of insecticides [8].
In this context, sustainable strategies have recently been developed. This includes the use of microbial pest-control agents (viruses, bacteria, and fungi) [9]; chemical mediators such as pheromones and kairomones [10]; and/or natural substances of plant, animal, or mineral origin [11]. More recently, RNA interference (RNAi)-based strategies, which do not require plant genetic modification, have been also investigated as a new method for insect pest control [12,13]. Among additional innovative strategies, nanoscale formulations of insecticides can provide controlled release of active ingredients while efficiently enhancing permeability, stability, and solubility [14,15]. However, the use of such nanopesticides in food production has raised many questions about their safety and ecotoxicological risks [16]. Consequently, the design of innovative crop-protection strategies is required for safer and more efficient treatments.
For several years, we have proposed a new patented concept based on the use of chemical synergistic agents combined with a given insecticide, which are able to optimize, in both in vitro and in vivo, the efficacy of the treatment while reducing the concentrations [17,18,19,20,21,22,23,24,25]. Compared to classical synergists (e.g., pyperonyl butoxide, S,S,S-tributyl phosphorotrithioate (DEF), diethyl maleate), which are known to inhibit detoxification enzymes involved in the hydrolysis of insecticides [26], the synergistic agents increase the sensitivity of membrane receptors and/or ion channels to the insecticide through the activation of the calcium-dependent signaling pathways following an elevation of intracellular calcium concentration. This results in the target site conformational changes involved in the increased sensitivity to insecticides [21,25]. This alternative allows to produce a stronger effect of a given insecticide used at lower concentrations than that obtained with the same molecule but used alone in a formulation at higher concentrations.
2. Synergistic Agents as Innovative Strategies to Improve Insecticides Efficacy
As already indicated, calcium ions play a key role on the membrane target sensitivity to insecticides. They generate versatile activation of specific calcium-dependent intracellular signaling pathways that determine a large variety of functions [27] known, for some of them, to regulate the target site conformation, which thereby modulates insecticide sensitivity [25]. Many calcium-dependent cellular and molecular factors can modulate insecticide efficacy through the activation of specific, complex signaling cascades that trigger phosphorylation/dephosphorylation process. This modulatory effect has been demonstrated in vitro with different classes of conventional insecticides, including phenylpyrazoles, neonicotinoids, pyrethroids, oxadiazines, organophosphates, and carbamates, acting on insect receptors and ion channels, such as voltage-dependent sodium channel channels (NaV), nicotinic acetylcholine receptor (nAChR), gamma-aminobutyric acid receptor (GABAR), and acetylcholinesterase (AChE) [21,24,25,28,29,30,31,32,33]. These intracellular signal transduction cascades typically amplify the calcium-dependent messages via the stimulation of effector enzymes (e.g., adenylyl cyclase, phospholipase C, guanylate cyclase), which catalyze the production or, in the case of ions, release of the second messengers (e.g., calcium ions, cAMP, cGMP, diacylglycerol, and IP3). In the last case, these second messengers are present at very low concentrations in resting insect cells and can reach relatively high concentration levels when they are stimulated. In all cases, second messengers production is controlled temporally and spatially, allowing subsequent efficient activations of kinases and/or phosphatases (e.g., protein kinase A, G, C, calcium/calmodulin-dependent protein kinase II (CaM-Kinase II), protein phosphatase (PP1/2A), calcineurin), involved in the regulation of the membrane target conformation. From these results has emerged the novel synergistic agent concept [19] based on (i) the role on intracellular calcium rise and the subsequent stimulation of calcium-dependent signaling pathways, (ii) the target site-conformational changes, and (iii) the calcium-dependent increase in target sites sensitivity to insecticides. One of the most interesting features is that the synergistic agent is a chemical or microorganism (e.g., insect viruses) that has no effect or only a limited effect itself. It indirectly optimizes the efficacy of insecticides at non-toxic concentrations through the activation of specific calcium-dependent intracellular signaling pathways that are involved in the regulation of membrane receptor and ion channel functions targeted by insecticides [19]. Among chemicals, N,N-diethyl-meta-toluamide (DEET) and insect repellent (IR3535) can be considered as synergistic agents. Previous findings performed both in vitro and in vivo have reported synergism between different synergistic agents (e.g., DEET, IR3535) and insecticides, including carbamates, oxadiazine, and neonicotinoids [18,20,21,22,23,24,28,34]. All together, these results demonstrated that chemical synergistic agents combined with a given insecticide increase (i) the sensitivity of the target site to the insecticide and (ii) the mortality rate of insects compared to that obtained with insecticides used alone.
In the same context, insect viruses can also be used as biological synergistic agents [35,36]. In this case, the insect virus is not used as a bioinsecticide (i.e., replicating within host cells) [9] but used as a synergistic agent. The interaction of the virus (e.g., baculovirus, densovirus) with the cell membrane is sufficient to produce calcium influx, associated with an elevation of the intracellular calcium concentration, which produces the activation of calcium-dependent intracellular signaling pathways [17]. Previous findings have already reported that the baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) induces intracellular calcium rise through the activation of CdCl2-sensitive ion channels or membrane transports in the insect cell membrane. The results have clearly indicated that AcMNPV interaction with insect cell line plasma membrane increases acetylcholinesterase sensitivity for the organophosphate insecticide chlorpyrifos-ethyl through elevation of intracellular calcium concentration [36].
From our results, we then propose another alternative, which is based on the use of virus-like particles (VLPs) as a novel synergistic agent. By contrast to viruses, VLPs present some advantages since they are composed of viral structural proteins expressed in heterologous systems that assemble spontaneously but are lacking the viral genome [37,38]. VLPs have a structure similar to native virions of about 10–200 nm in diameter and can be enveloped or non-enveloped, spherical or filamentous, and composed of a single, double, or triple layers [39]. Interest in the design and production of VLPs has increased in recent years, and several applications have been developed, including vaccination, gene therapy, drug delivery, and nanotechnology.
3. Various Systems Used to Produce VLPs
VLPs can be produced in a variety of systems, including bacteria, yeast, mammalian cells, insect cells, and plants (Figure 1) [39,40,41,42,43]. VLPs can be also assembled in vitro from proteins produced in cell-free expression systems [44]. The characteristics, advantages, limitations, and yield ranges of the production systems are compared in Table 1 [41,45]. The correct folding and assembly of VLPs is a complex process, which is highly dependent on the structure of viral proteins, the expression system, and physical parameters, such as, for instance, pH, ionic strength, and temperature [39,46,47]. Three methods of cell culture are commonly used for VLPs production, including batch, fed-batch, and continuous cultivation. Technical challenges remain in the generation of VLPs in terms of yield, design, stability, and storage. Recently, chimeric VLPs have been developed. They are based on structural proteins originated from different viruses and generated through genetic fusion or chemical coupling [48,49]. Surface modifications of VLPs can be made to trigger an immune response of the host cells and/or to enhance specific recognition of VLPs by target cells [50].
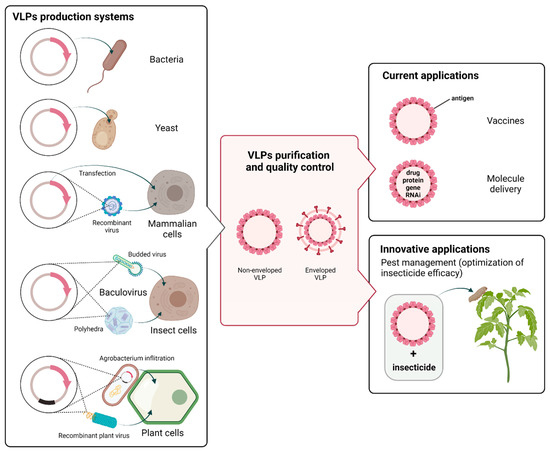
Figure 1.
Virus-like-particles (VLPs) production systems and applications. VLPs can be produced by a variety of expression platforms, including prokaryotic and eukaryotic systems. The main eukaryotic systems include yeast, mammalian cell lines, the baculovirus/insect cell system, and plants. The production stage (left part of the figure) includes cloning of the viral structural genes of interest (in red) and introduction into the host cell by plasmid transfection or viral transduction. Depending on the method used, the transgene is integrated into the host genome or replicates in an episomal form. In plants, the rapid and transient expression of recombinant proteins is commonly based on Agrobacterium-mediated infiltration and/or plant viral vectors. After expression, the self-assembly of viral structural proteins into VLPs is highly dependent of the structure of viral proteins, the expression system, and the experimental conditions. VLPs that have similar structure as native virions can be enveloped or non-enveloped (central panel), spherical or filamentous, and composed of a single, double, or triple layer. Different downstream processing steps may be required to obtain purified VLPs without residual host contaminants. VLPs have a broad range of potential applications (right part of the figure), including vaccine production, vectors for gene therapy, and targeted drug delivery. Because VLPs are considered safer than viruses and capable of triggering the calcium concentration elevation, they could also be exploited as an alternative synergistic agent co-applied with a given insecticide for optimizing insecticide efficacy (created with Biorender.com, accessed on 3 November 2021).

Table 1.
Characteristics, advantages, and disadvantages of the different VPLs platforms (modified with permission from Ref. [45], Copyright 2017 Elsevier B.V. license number 5296930551852).
3.1. Bacterial Cells
The expression system using Escherichia coli bacteria was the first established recombinant expression system. This expression system is characterized by high protein yields (Table 1), rapid cell growth, short production times, simplicity of scaling-up, and low manufacturing costs compared to eukaryotic systems. However, the inability of prokaryotic cells to undergo post-translational modifications (PTM), such as protein glycosylation and incomplete disulfide bond formation, can lead to the expression of misfolded or non-functional proteins. Bacteria can be engineered to make specific post-translational modifications, but the process then becomes expensive [52]. Furthermore, the limited solubility of recombinant viral proteins promotes the formation of inclusion bodies [51,53], and contaminant endotoxins have to be removed in the manufacturing process. Finally, protease degradation and codon bias can also contribute to lower yields. Even if E. coli remains the dominant bacterial strain in use, and various VLP vaccines generated using E. coli expression systems are under clinical trials, no VLPs are currently marketed. In addition to E. coli, VLPs have been successfully produced in some other bacterial species, such as Lactobacillus casei using a lactose-inducible promoter system for papillomavirus (HPV) L1 protein expression [77] and Pseudomonas fluorescens for the cowpea chlorotic mottle virus (CCMV) coat proteins expression [78]. Several chimeric VLPs vaccines have also been developed by antigen conjugation with bacteriophage Qβ RNA in E. coli expression platform [40].
3.2. Yeast Cells
This is the most popular expression system to produce VLPs is the yeast system due to its easy and high protein expression (Table 1), its ability to scale up, and the cost of production. Although yeasts provide a degree of PTM processes [41], the lack of complex PTM pathways is a major drawback of yeast expression systems. High mannose glycosylation, plasmid loss, and lower yields of protein compared to bacterial expression system remain additional issues to overcome [54]. The quality and quantity of yeast-produced VLPs are influenced by the choice of plasmid and promoter and by the ratio between different structural proteins produced. More than 30 types of VLPs have been produced in yeasts, particularly in Saccharomyces cerevisiae, in Pichia pastoris, and more recently in Hansenula polymorpha [41]. The yeast expression systems are generally used for generating non-enveloped VLPs. Indeed, enveloped viruses that are released through budding in mammalian cells cannot bud from yeast since the outer membrane is covered by a wall of mannoproteins and chitin [79]. However, yeast systems whose cell walls have been almost completely removed have been successfully used for HIV-1 Gag protein VLPs and Dengue virus serotype 2 VLPs [55,56]. Moreover, recent significant advances in VLPs production processes have allowed an increasing number of VLPs produced by secretion and the production of multilayered VLPs composed of more than one type of structural protein [41]. Several yeast-produced VLPs, such as papillomavirus (HPV) and hepatitis B virus (HBV) VLPs (Table 2), have already reached approval by regulatory agencies. Recently, the production of Chikungunya VLPs using the yeast Pichia pastoris has been reported [80].

Table 2.
Non-exhaustive applications of VLP in vaccines or molecule delivery.
3.3. Mammalian Cells
Although mammalian cells present lower yield for VLPs production compared to other systems (Table 1), they have the advantage to produce VLPs with appropriate PTMs essential for proper protein folding. Mammalian cells can be used to produce complex non-enveloped and enveloped VLPs composed of multiple structural proteins (up to five proteins) [48]. Several mammalian cell lines are suitable for VLPs production, including Chinese hamster ovary (CHO), baby hamster kidney-21 (BHK-21), human embryonic kidney 293 (HEK293), Vero cell lines, CAP-T cell line derived from human amniocytes, and East Lansing line-0 (ELL-0) [68]. The most frequently used CHO cell line, which is not derived from human, presents a lower risk of contamination by human viruses [68]. CHO cells have already been used for successful production of both dengue virus and hantavirus VLPs [92,93]. The HEK293 cell line is widely used to produce VLPs from rabies, HIV, and influenza viruses [66,67,94] (Table 2). More recently, Vero E6 cells has been used to produce stable SARS-CoV-2 VLPs as a candidate vaccine against the emerged disease COVID-19 [95].
VLPs are produced in cells that are previously either transiently or stably transfected or transduced with viral expression vectors (Figure 1). Although stable expression produces large amounts of protein, transient expression is preferred since high levels of proteins are obtained for shorter periods [66,67].
3.4. Baculovirus/Insect Cells
The baculovirus/insect cells system is the most commonly used expression system for large-scale production of both non-enveloped and enveloped VLPs, simple or complex, comprising up to five proteins [54]. This is a binary system consisting of a recombinant baculovirus as the vector and lepidopteran insect cells [42] (Figure 1). Baculoviruses are insect viruses not pathogenic to humans. They are easily genetically modified to express heterologous proteins in insect cells. The baculovirus AcMNPV is the most well-known virus used. Several proteins can be produced simultaneously from multiple promoters, usually under the control of the polyhedron (polh) or the p10 strong promoters [57]. In this system, VLP yields vary from 0.2 to hundreds µg/mL [48], which is similar to bacteria and yeast systems (Table 1). These high expression levels are also explained by the ability of the virus to shut off the cellular expression for the benefit of the expression of heterologous genes. The main insect cell lines used are Sf21 and Sf9 derived from Spodoptera frugiperda and the BTI-TN-5B1-4 cell line (High-Five™) derived from Trichoplusia ni [54,60]. It has been reported that High-Five™ cells have the highest heterologous protein expression yield [63,64]. Although the growth rate of insect cells is higher than mammalian cells, it is lower than yeast or bacteria. Production, therefore, requires longer times than those required for microbial systems. Culture media is more expensive compared to that used for yeast or bacteria, and production must be carried out in bioreactors. On the other hand, if the PTMs are more complex in insect cells compared to those generated in yeast and bacteria, the N-glycosylation pattern of the recombinant glycoproteins produced in this system is simpler than that of mammalian cells. Indeed, N-glycans from insect cells are not processed to terminally sialylated complex-type structures but are instead modified to a paucimannose structure [62,65]. This may result in a lower or total loss of biological function of the protein of interest, and this can be a disadvantage for some VLP applications in this production system. To solve this issue, genetic modifications of either insect cells or baculoviruses to include genes encoding N-glycosylation functions have been considered [42,58,59]. One of the main drawbacks of the baculovirus/insect cells system remains the contamination with the baculovirus particles that are also produced at the same time as VLPs [61]. To limit expensive purification steps, non-replicative baculoviruses have been developed to minimize the contamination [96]. Alternative insect cell line that avoids the use of baculovirus, such as Drosophila Schneider line 2 (S2 cells), can also be used to produce VLPs [60,97].
3.5. Plant Cells
Plant expression systems for VLPs production allow the production of properly folded complex proteins and represent a cost-effective eukaryotic system [60] that is easy to scale-up and free of mammalian pathogens. Advances in plant biotechnology have made possible the use of transgenic plants as alternatives to cell culture systems [73]. VLPs can be produced in a variety of plant species, including Solanum tuberosum (potato), Lycopersicon esculentum (tomato), Glycine max (soybean), Lupinus luteus L. (Lupin callus), Arabidopsis thaliana, and Nicotiana benthamiana or Nicotiana tabacum L. (tobacco) [70]. Plants can transiently or stably express viral proteins in the nucleus or in the chloroplast. PTMs can be performed on the viral proteins expressed from the nucleus, while chloroplast transformation enables high levels of transgene expression (up to 80% of total soluble proteins) [74] but lacks PTMs [60]. Stable expression is time-consuming and can lead to low expression yields [72]. On the other hand, transient expression obtained through either Agrobacterium infiltration or plant viral vectors is easy, quick, and highly productive [71] (Figure 1). Transient transgenic plants also have the advantage of not being classified as genetically modified organisms (GMO).
Advantages provided by transgenic plants are the low cost of production, estimated to be 10 to 50 times lower than products derived from E. coli and 140 times lower than production using baculovirus-based insect cells [69,76], and the simple scaling-up that requires few materials except for cultivation surface. Since the plants are edible, they could also serve as delivery mode for oral vaccination, thereby reducing the purification costs. Plants also present advantages in terms of storage for recombinant proteins that are protected within plant tissue. The main limitations are the long timeline for transgenic plants establishment, the low expression levels, and antigen degradation during in vivo delivery [48]. Two replicon systems in particular have been recently developed to induce strong expression of VLPs in plants: the deconstructed viral vectors composed of tobacco mosaic virus RNA replicon system (MagnICON) and the Geminiviral BeYDV DNA replicon system [98,99]. These replication systems have led to an increase of more than 80-fold in the accumulation of Norwalk virus (NV) capsid protein VLPs in transgenic tobacco and tomato [100]. Finally, both enveloped and non-enveloped as well as native and chimeric plant-derived VLPs have been produced [75]. Chimeric VLPs are composed of plant viral vectors, such as tobacco mosaic virus (TMV), cucumber mosaic virus (CMV), alfalfa mosaic virus (A1MV), cowpea mosaic virus (CPMV), papaya mosaic virus (PapMV), and the potato X virus (PVX) that carry recombinant proteins (viral or not). This strategy has enabled the production of over 100 experimental plant virus-based vaccines against a wide range of diseases in both humans and animals [101,102,103].
4. Current Applications of VLPs
Up to date, VLPs are currently used for vaccination and are in development as delivery systems for antigens, genes, nucleic acids, or drugs [37,82,104].
4.1. VLP-Based Vaccines
New generations of vaccines improve safety by using viral proteins, VLPs, or nucleic acids. Having no genomic material, VLPs have an advantage compared to classical vaccines. They can present viral epitopes via repetitive and highly organized structures, such as those expressed in natural infectious virions, without any risk of infection. VLPs interact with various components of the immune system to produce strong immune responses [37]. In addition, VLPs can have adjuvant properties [37]. There are different approaches to develop VLP-based vaccines: VLP vaccines that mimic the natural virus and chimeric VLPs (i.e., encapsulating the antigen or displaying the antigen on the exterior) [37].
Several VLP-based vaccines are already available with good results, and many others are in clinical trials or research stages [45,81]. VLPs-based vaccines that have been marketed for use in humans include recombinant vaccines for hepatitis B virus, human papillomavirus, and hepatitis E virus and in the veterinary field against porcine circovirus type 2 (Table 2). Several VLPs are under clinical trial as potential vaccines for influenza, Epstein Barr virus, and malaria and against emerging viral infections such as Ebola, avian flu, MERS, and SARS-CoV [105]. VLPs provide a promising approach for developing safe and effective flavivirus vaccines against Zika, Dengue, West Nile, and Japanese encephalitis viruses [106].
Specific vaccine antigens were generated by different expression systems to induce protective immune responses (Table 2). For example, vaccines against hepatitis B virus are produced in a yeast system to express stable HBsAg VLPs (i.e., Engenerix®) or in mammalian cells (CHO) to express three HBV antigens (S, pre-S1, and pre-S2), leading to higher immunogenicity (Sci-B-Vac®). The hepatitis E VLP-based vaccine, Hecolin®, only licensed in China, is produced using E. coli expression system, and another vaccine expressed in insect cells is under clinical trial (Table 2) [81]. Concerning the malaria vaccine Mosquirix®, enhanced efficacy was obtained with a yeast-produced direct fusion between HBsAg and CSP antigen. In this system, HBsAg is only used as a carrier matrix for the malaria antigen and does not induce antibodies against HBsAg [107].The prophylactic vaccination against HPV is based on VLPs produced by introducing the L1 gene encoding a capsid protein into eukaryotic cells (insects or yeasts). Licensed human and veterinary vaccines are based on artificial VLPs derived from human or animal viruses. However, plant virus-derived platforms can be used for the creation of novel vaccines [108]. HBV- and norovirus-derived VLPs are the most studied VLPs, produced in plant-based systems by three different species of transgenic plants: S. tuberosum [109], L. esculentum [70], and N. benthamiana [70,75,110]. Many plant virus-derived nanoparticles have also been tested as antigen carriers for different human or animal vaccines against influenza virus, hepatitis C virus, Japanese encephalitis virus, canine parvovirus, and classical swine fever virus [108]. Plant VLP vaccines are produced in the different expression systems already described, such as E. coli, and cloned in commercial plasmid, yeast, insect cells, mammalian cells, or the most commonly used plants N. tabacum, N. benthamania, and A. thaliana [43,45,108]. For influenza viruses, plant-derived VLPs are an alternative to the currently available manufacturing platforms for seasonal vaccines, and a seasonal recombinant quadrivalent VLP vaccine is currently in clinical trial phase [111] (Table 2).
4.2. Molecule Delivery
For a few decades, nanoparticle (NP)-based delivery agents, such as liposomes, polymers, dendrimers, magnetic nanoparticles, and protein-based NPs, have been used as carriers for drug delivery. These systems comprise the administration and controlled-release delivery of pharmaceutical compounds to a specific area in tissues, improving efficacy and safety [112]. Emerging research focuses on the development of protein-based NPs derived from viral capsids (VLPs) as targeted therapeutic delivery agents [39,113,114] (Table 2). Indeed, VLPs can pack and deliver therapeutic cargo such as chemotherapeutic drugs, nucleic acids, proteins, and peptides. Different strategies have been developed to carry the cargo either inside or outside the VLPs: (i) viral capsids disassembly by altering pH and buffer conditions and reassembly to encapsulate the desired cargo using buffer exchange methods, (ii) infusion of cargo due to changes in pH and salt concentrations, (iii) genetic engineering techniques utilizing genetically conjugated scaffolding proteins to encapsulate drugs, and (iv) bioconjugation using exterior surface-exposed residues [37].
Compared to classical NPs, VLPs present several advantages, including specific cell targeting. VLPs show a natural tropism to certain tissue, and a more specific targeting function can be obtained through attaching receptor-recognizing domains to the drug carriers [115] (Table 2). For example, the tumor-targeting peptide (RGD) inserted through genetic modification into the major immunodominant loop region of HBc (hepatitis B core protein) VLPs enhanced tumor-homing in mice model [116]. Furthermore, cell targeting has been achieved through functionalization of the exterior of the bacteriophage MS2 capsids with the cell penetrating HIV-1 Tat peptide [117]. In addition to specific targeting, VLPs permit efficient host cell penetration, biocompatibility, and degradability [81]. VLPs, like original viruses, have the ability to escape the endosome before lysosomal degradation occurs [115]. Indeed, after interaction with viral receptors, most viruses utilize the endocytosis pathways (clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis) to enter cells efficiently [118]. Once endocytosed, viruses can escape from late endosomes or lysosomes and release their contents to cytoplasm by different pathways, such as membrane pore formation, membrane fusion, membrane penetration, and membrane disruption. VLPs can be engineered to display cell-penetrating peptides to aid in escaping the endosome, enhancing the delivery of functional therapeutic cargo [114]. For example, MS2 VLPs have been modified with a histidine-rich fusogenic peptide (H5WYG) to promote endosomal escape of internalized VLPs. This drug delivery system is under research for delivery of doxorubicin, cisplatin, 5-fluorouracil, and siRNA for hepatocellular carcinoma treatment [91] (Table 2).
5. Future Direction in Pest Management: VLPs as Synergistic Agent of Insecticides
Up to date, VLPs are not used in the context of pest management. However, VLPs have recently received our attention because (i) they have been used worldwide in human health for over 30 years for HBV vaccination with a safety and efficacy profile more than satisfactory, taking into account the available safety data [119,120,121], and (ii) they can produce multiphasic elevation of intracellular calcium concentration in insect cells, as shown in the graph obtained by calcium imaging ratiometric method (data from our laboratory, inset Figure 2). Like classical synergistic agents [19], VLPs induce multiphasic components of the calcium elevation, suggesting the involvement of multiple molecular cellular and intracellular events (Figure 2). This includes plasma membrane calcium channel and/or receptors (e.g., voltage-dependent calcium channels, transient receptor potential (TRP), store-operated calcium entry (SOCE), and intracellular receptors (ryanodine receptor (RYR), inositol 1,4,5-trisphosphate-receptor (IP3R)) that participate in calcium rise through calcium-induced calcium release (CICR) or calcium release-activated channels (CRAC) mechanisms [122]. As stated above, the increase in intracellular calcium concentration is the essential prerequisite for producing an optimization of insecticide efficacy while reducing the concentrations used. Preliminary toxicological in vivo studies performed on the aphid Acyrthosiphon pisum revealed that the mortality rate (using an artificial diet bioassay and measured at 48 h) produced by the insecticide indoxacarb is increased by about 25% when co-applied with VLPs (5 × 109 particles/µL) (C. Deshayes, unpublished data). VLPs are considered safer than viruses due to the absence of the virus genome and capability of triggering calcium concentration elevation. For these reasons, they could be proposed as alternative synergistic agents co-applied with a given insecticide for optimizing insecticide efficacy through the elevation of the intracellular calcium concentration and be exploited in pest management. Nevertheless, ecotoxicological and environmental stability studies should be required before further applications.
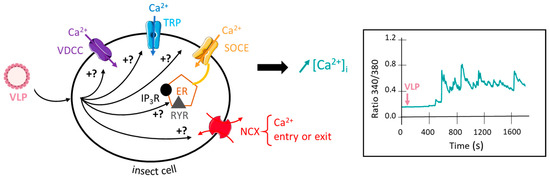
Figure 2.
VLPs induce a multicomponent intracellular calcium rise. The scheme summarizes the hypothetic mechanisms by which VLPs increase intracellular calcium concentration. Inset: Representative multicomponent effect of VLPs on intracellular calcium concentration in Fura-2 loaded isolated insect neuron cell body using the calcium imaging ratiometric method (C. Deshayes, unpublished data). VDCC, voltage-dependent calcium channel; TRP, transient receptor potential channel; SOCE, store-operated calcium entry; NCX, sodium–calcium exchanger; RYR, ryanodine receptor; IP3R, inositol triphosphate receptor; ER, endoplasmic reticulum.
Author Contributions
C.D., A.-S.G.-G., M.O., B.L. and V.A.-M. have contributed to the writing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the guest editor Miguel López-Ferber for giving us the opportunity to propose this opinion piece in the Special Issue of Viruses “Insect Viruses and Pest Management 2.0”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- OECD. OECD Environmental Outlook to 2050: The Consequences of Inaction. Available online: https://www.oecd.org/g20/topics/energy-environment-green-growth/oecdenvironmentaloutlookto2050theconsequencesofinaction.htm (accessed on 1 December 2021).
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F. Indirect Effect of Pesticides on Insects and Other Arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef] [PubMed]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Available online: https://www.epa.gov/safepestcontrol/integrated-pest-management-ipm-principles (accessed on 3 November 2021).
- Deshayes, C.; Siegwart, M.; Pauron, D.; Froger, J.A.; Lapied, B.; Apaire-Marchais, V. Microbial Pest Control Agents: Are they a Specific and Safe Tool for Insect Pest Management? Curr. Med. Chem. 2017, 24, 2959–2973. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Sharma, K.C.; Kaushal, P.; Kumar, J.; Parthiban, P.; Senthil-Nathan, S.; Mankin, R.W. Role of kairomone in biological control of crop pests—A review. Physiol. Mol. Plant Pathol. 2018, 101, 3–15. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-Based Biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef]
- Kolliopoulou, A.; Taning, C.N.T.; Smagghe, G.; Swevers, L. Viral Delivery of dsRNA for Control of Insect Agricultural Pests and Vectors of Human Disease: Prospects and Challenges. Front. Physiol. 2017, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res. Lett. 2021, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control. Insects 2021, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Fraceto, L.F.; Amorim, M.J.B.; Scott-Fordsmand, J.J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J. Hazard. Mater. 2021, 404, 124148. [Google Scholar] [CrossRef] [PubMed]
- Apaire-Marchais, V.; Ogliastro, M.; Chandre, F.; Pennetier, C.; Raymond, V.; Lapied, B. Virus and calcium: An unexpected tandem to optimize insecticide efficacy. Environ. Microbiol. Rep. 2016, 8, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.; Pennetier, C.; Duchon, S.; Lapied, B.; Corbel, V. Multi-function oxidases are responsible for the synergistic interactions occurring between repellents and insecticides in mosquitoes. Parasites Vectors 2009, 2, 17. [Google Scholar] [CrossRef]
- Deshayes, C.; Moreau, E.; Pitti-Caballero, J.; Froger, J.A.; Apaire-Marchais, V.; Lapied, B. Synergistic agent and intracellular calcium, a successful partnership in the optimization of insecticide efficacy. Curr. Opin. Insect Sci. 2018, 30, 52–58. [Google Scholar] [CrossRef]
- El-Adouzi, M.; Apaire-Marchais, V.; Raymond, V.; Lapied, B. New Insecticide Composition. Patent 16,674,692, 4 June 2020. [Google Scholar]
- Moreau, E.; Mikulska-Ruminska, K.; Goulu, M.; Perrier, S.; Deshayes, C.; Stankiewicz, M.; Apaire-Marchais, V.; Nowak, W.; Lapied, B. Orthosteric muscarinic receptor activation by the insect repellent IR3535 opens new prospects in insecticide-based vector control. Sci. Rep. 2020, 10, 6842. [Google Scholar] [CrossRef]
- Pennetier, C.; Corbel, V.; Boko, P.; Odjo, A.; N’Guessan, R.; Lapied, B.; Hougard, J.M. Synergy between repellents and non-pyrethroid insecticides strongly extends the efficacy of treated nets against Anopheles Gambiae. Malar. J. 2007, 6, 38. [Google Scholar] [CrossRef]
- Pennetier, C.; Costantini, C.; Corbel, V.; Licciardi, S.; Dabire, R.K.; Lapied, B.; Chandre, F.; Hougard, J.M. Synergy between repellents and organophosphates on bed nets: Efficacy and behavioural response of natural free-flying An. gambiae mosquitoes. PLoS ONE 2009, 4, e7896. [Google Scholar] [CrossRef]
- Pitti Caballero, J.; Murillo, L.; List, O.; Bastiat, G.; Flochlay-Sigognault, A.; Guerino, F.; Lefrançois, C.; Lautram, N.; Lapied, B.; Apaire-Marchais, V. Nanoencapsulated deltamethrin as synergistic agent potentiates insecticide effect of indoxacarb through an unusual neuronal calcium-dependent mechanism. Pestic. Biochem. Physiol. 2019, 157, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Raymond, V.; Goven, D.; Benzidane, Y.; List, O.; Lapied, B. Influence of Cellular and Molecular Factors on Membrane Target Sensitivity to Insecticides. Curr. Med. Chem. 2017, 24, 2974–2987. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, S.; Greenhalgh, R.; Tirry, L.; Clark, R.M.; Van Leeuwen, T.; Dermauw, W. The effect of insecticide synergist treatment on genome-wide gene expression in a polyphagous pest. Sci. Rep. 2017, 7, 13440. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, D.; Murray, F.; Parekh, A.B. Store-Operated Ca(2+) Channels: Mechanism, Function, Pharmacology, and Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 629–654. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ella, A.; Stankiewicz, M.; Mikulska, K.; Nowak, W.; Pennetier, C.; Goulu, M.; Fruchart-Gaillard, C.; Licznar, P.; Apaire-Marchais, V.; List, O.; et al. The Repellent DEET Potentiates Carbamate Effects via Insect Muscarinic Receptor Interactions: An Alternative Strategy to Control Insect Vector-Borne Diseases. PLoS ONE 2015, 10, e0126406. [Google Scholar] [CrossRef] [PubMed]
- Bodereau-Dubois, B.; List, O.; Calas-List, D.; Marques, O.; Communal, P.Y.; Thany, S.H.; Lapied, B. Transmembrane potential polarization, calcium influx, and receptor conformational state modulate the sensitivity of the imidacloprid-insensitive neuronal insect nicotinic acetylcholine receptor to neonicotinoid insecticides. J. Pharmacol. Exp. Ther. 2012, 341, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Houchat, J.N.; Taillebois, E.; Thany, S.H. Effects of the DAG analogue 1,2-dioctanoyl-sn-glycerol (DiC8) on nicotine- and clothianidin-evoked currents through alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors expressed on cockroach neurosecretory cells. Neurotoxicology 2020, 78, 143–151. [Google Scholar] [CrossRef]
- Lavialle-Defaix, C.; Moignot, B.; Legros, C.; Lapied, B. How does calcium-dependent intracellular regulation of voltage-dependent sodium current increase the sensitivity to the oxadiazine insecticide indoxacarb metabolite decarbomethoxylated JW062 (DCJW) in insect pacemaker neurons? J. Pharmacol. Exp. Ther. 2010, 333, 264–272. [Google Scholar] [CrossRef]
- List, O.; Calas-List, D.; Taillebois, E.; Juchaux, M.; Heuland, E.; Thany, S.H. Inhibition of PaCaMKII-E isoform in the dorsal unpaired median neurosecretory cells of cockroach reduces nicotine- and clothianidin-induced currents. J. Neurochem. 2014, 130, 507–513. [Google Scholar] [CrossRef]
- Murillo, L.; Hamon, A.; Es-Salah-Lamoureux, Z.; Itier, V.; Quinchard, S.; Lapied, B. Inhibition of protein kinase C decreases sensitivity of GABA receptor subtype to fipronil insecticide in insect neurosecretory cells. Neurotoxicology 2011, 32, 828–835. [Google Scholar] [CrossRef]
- Pennetier, C.; Costantini, C.; Corbel, V.; Licciardi, S.; Dabire, R.K.; Lapied, B.; Chandre, F.; Hougard, J.M. Mixture for controlling insecticide-resistant malaria vectors. Emerg. Infect. Dis. 2008, 14, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Lapied, B.; Pennetier, C.; Apaire-Marchais, V.; Licznar, P.; Corbel, V. Innovative applications for insect viruses: Towards insecticide sensitization. Trends Biotechnol. 2009, 27, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Licznar, P.; List, O.; Goven, D.; Nna, R.N.; Lapied, B.; Apaire-Marchais, V. A novel method using Autographa californica multiple nucleopolyhedrovirus for increasing the sensitivity of insecticide through calcium influx in insect cell line. J. Virol. Methods 2014, 195, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, N.F. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 634–641. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-derived virus-like particles in vaccine development. Npj Vaccines 2017, 2, 3. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.J. Yeast as an expression system for producing virus-like particles: What factors do we need to consider? Lett. Appl. Microbiol. 2017, 64, 111–123. [Google Scholar] [CrossRef]
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular pharming—VLPs made in plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef]
- Glass, P.J.; White, L.J.; Ball, J.M.; Leparc-Goffart, I.; Hardy, M.E.; Estes, M.K. Norwalk virus open reading frame 3 encodes a minor structural protein. J. Virol. 2000, 74, 6581–6591. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of virus-like particles for vaccines. New Biotechnol. 2017, 39, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Muller, K.M. In Vitro Assembly of Virus-Like Particles and Their Applications. Life 2021, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef]
- Roldao, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Roldão, A.; Silva, A.C.; Mellado, M.C.M.; Alves, P.M.; Carrondo, M.J.T. Viruses and Virus-Like Particles in Biotechnology: Fundamentals and Applications. Compr. Biotechnol. 2017, 1, 633–656. [Google Scholar] [CrossRef]
- Grataitong, K.; Huault, S.; Chotwiwatthanakun, C.; Jariyapong, P.; Thongsum, O.; Chawiwithaya, C.; Chakrabandhu, K.; Hueber, A.-O.; Weerachatyanukul, W. Chimeric virus-like particles (VLPs) designed from shrimp nodavirus (MrNV) capsid protein specifically target EGFR-positive human colorectal cancer cells. Sci. Rep. 2021, 11, 16579. [Google Scholar] [CrossRef]
- Lai, W.B.; Middelberg, A.P. The production of human papillomavirus type 16 L1 vaccine product from Escherichia coli inclusion bodies. Bioprocess Biosyst. Eng. 2002, 25, 121–128. [Google Scholar] [CrossRef]
- Selas Castiñeiras, T.; Williams, S.G.; Hitchcock, A.G.; Smith, D.C. E. coli strain engineering for the production of advanced biopharmaceutical products. FEMS Microbiol. Lett. 2018, 365, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Carmichael, J.; Ferguson, J.; Inglis, S.; Ashrafian, H.; Stanley, M. Expression of human papillomavirus type 16 L1 protein in Escherichia coli: Denaturation, renaturation, and self-assembly of virus-like particles in vitro. Virology 1998, 243, 423–431. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar]
- Liu, W.; Jiang, H.; Zhou, J.; Yang, X.; Tang, Y.; Fang, D.; Jiang, L. Recombinant dengue virus-like particles from Pichia pastoris: Efficient production and immunological properties. Virus Genes 2010, 40, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, S.; Goto, T.; Sano, K.; Morikawa, Y. HIV type 1 Gag virus-like particle budding from spheroplasts of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2002, 99, 7956–7961. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Fitzgerald, D.J.; Richmond, T.J. Baculovirus expression system for heterologous multiprotein complexes. Nat. Biotechnol. 2004, 22, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Jarvis, D.L. Transforming lepidopteran insect cells for improved protein processing. Methods Mol. Biol. 2007, 388, 341–356. [Google Scholar] [CrossRef]
- Jarvis, D.L.; Kawar, Z.S.; Hollister, J.R. Engineering N-glycosylation pathways in the baculovirus-insect cell system. Curr. Opin. Biotechnol. 1998, 9, 528–533. [Google Scholar] [CrossRef]
- Legastelois, I.; Buffin, S.; Peubez, I.; Mignon, C.; Sodoyer, R.; Werle, B. Non-conventional expression systems for the production of vaccine proteins and immunotherapeutic molecules. Hum. Vaccines Immunother. 2017, 13, 947–961. [Google Scholar] [CrossRef]
- Liu, F.; Wu, X.; Li, L.; Liu, Z.; Wang, Z. Use of baculovirus expression system for generation of virus-like particles: Successes and challenges. Protein Expr. Purif. 2013, 90, 104–116. [Google Scholar] [CrossRef]
- Walski, T.; De Schutter, K.; Van Damme, E.J.M.; Smagghe, G. Diversity and functions of protein glycosylation in insects. Insect Biochem. Mol. Biol. 2017, 83, 21–34. [Google Scholar] [CrossRef]
- Wang, P.; Granados, R.R.; Shuler, M.L. Studies on serum-free culture of insect cells for virus propagation and recombinant protein production. J. Invertebr. Pathol. 1992, 59, 46–53. [Google Scholar] [CrossRef]
- Yamaji, H.; Manabe, T.; Kitaura, A.; Izumoto, E.; Fukuda, H. Efficient production of recombinant protein in immobilized insect cell culture using serum-free basal media after baculovirus infection. Biochem. Eng. J. 2006, 28, 67–72. [Google Scholar] [CrossRef][Green Version]
- Zhu, F.; Li, D.; Chen, K. Structures and functions of invertebrate glycosylation. Open Biol. 2019, 9, 180232. [Google Scholar] [CrossRef] [PubMed]
- Cervera, L.; Gutierrez-Granados, S.; Martinez, M.; Blanco, J.; Godia, F.; Segura, M.M. Generation of HIV-1 Gag VLPs by transient transfection of HEK 293 suspension cell cultures using an optimized animal-derived component free medium. J. Biotechnol. 2013, 166, 152–165. [Google Scholar] [CrossRef]
- Fontana, D.; Kratje, R.; Etcheverrigaray, M.; Prieto, C. Immunogenic virus-like particles continuously expressed in mammalian cells as a veterinary rabies vaccine candidate. Vaccine 2015, 33, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Bell, G.; Coy, D.H.; Brunicardi, F.C. Activation of human somatostatin receptor type 2 causes inhibition of cell growth in transfected HEK293 but not in transfected CHO cells. J. Surg. Res. 1997, 71, 13–18. [Google Scholar] [CrossRef]
- Cid, R.; Bolívar, J. Platforms for Production of Protein-Based Vaccines: From Classical to Next-Generation Strategies. Biomolecules 2021, 11, 1072. [Google Scholar] [CrossRef]
- Huang, Z.; Elkin, G.; Maloney, B.J.; Beuhner, N.; Arntzen, C.J.; Thanavala, Y.; Mason, H.S. Virus-like particle expression and assembly in plants: Hepatitis B and Norwalk viruses. Vaccine 2005, 23, 1851–1858. [Google Scholar] [CrossRef]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar] [CrossRef]
- Lossl, A.G.; Waheed, M.T. Chloroplast-derived vaccines against human diseases: Achievements, challenges and scopes. Plant Biotechnol. J. 2011, 9, 527–539. [Google Scholar] [CrossRef]
- Mason, H.S.; Warzecha, H.; Mor, T.; Arntzen, C.J. Edible plant vaccines: Applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 2002, 8, 324–329. [Google Scholar] [CrossRef]
- Oey, M.; Lohse, M.; Kreikemeyer, B.; Bock, R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. Cell Mol. Biol. 2009, 57, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Scotti, N.; Rybicki, E.P. Virus-like particles produced in plants as potential vaccines. Expert Rev. Vaccines 2013, 12, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Towler, M.; Weathers, P.J. Platforms for Plant-Based Protein Production. Bioprocess. Plant Vitr. Syst. 2018, 509–548. [Google Scholar] [CrossRef]
- Aires, K.A.; Cianciarullo, A.M.; Carneiro, S.M.; Villa, L.L.; Boccardo, E.; Perez-Martinez, G.; Perez-Arellano, I.; Oliveira, M.L.; Ho, P.L. Production of human papillomavirus type 16 L1 virus-like particles by recombinant Lactobacillus casei cells. Appl. Environ. Microbiol. 2006, 72, 745–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phelps, J.P.; Dao, P.; Jin, H.; Rasochova, L. Expression and self-assembly of cowpea chlorotic mottle virus-like particles in Pseudomonas fluorescens. J. Biotechnol. 2007, 128, 290–296. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Saraswat, S.; Athmaram, T.N.; Parida, M.; Agarwal, A.; Saha, A.; Dash, P.K. Expression and Characterization of Yeast Derived Chikungunya Virus Like Particles (CHIK-VLPs) and Its Evaluation as a Potential Vaccine Candidate. PLOS Negl. Trop. Dis. 2016, 10, e0004782. [Google Scholar] [CrossRef]
- Qian, C.; Liu, X.; Xu, Q.; Wang, Z.; Chen, J.; Li, T.; Zheng, Q.; Yu, H.; Gu, Y.; Li, S.; et al. Recent Progress on the Versatility of Virus-Like Particles. Vaccines 2020, 8, 139. [Google Scholar] [CrossRef]
- Shirbaghaee, Z.; Bolhassani, A. Different applications of virus-like particles in biology and medicine: Vaccination and delivery systems. Biopolymers 2016, 105, 113–132. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus. Viruses 2020, 12, 826. [Google Scholar] [CrossRef]
- Tagliamonte, M.; Visciano, M.L.; Tornesello, M.L.; De Stradis, A.; Buonaguro, F.M.; Buonaguro, L. HIV-Gag VLPs presenting trimeric HIV-1 gp140 spikes constitutively expressed in stable double transfected insect cell line. Vaccine 2011, 29, 4913–4922. [Google Scholar] [CrossRef] [PubMed]
- BioSpace. Medicago Develops Plant-Based Coronavirus Vaccine Candidate. Available online: https://www.biospace.com/article/medicago-successfully-produces-a-viable-vaccine-candidate-for-covid-19/ (accessed on 4 October 2021).
- Mi, Y.; Xie, T.; Zhu, B.; Tan, J.; Li, X.; Luo, Y.; Li, F.; Niu, H.; Han, J.; Lv, W.; et al. Production of SARS-CoV-2 Virus-Like Particles in Insect Cells. Vaccines 2021, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Mattei, A.A.; Karuppannan, A.K.; Halbur, P.G. Future perspectives on swine viral vaccines: Where are we headed? Porc. Health Manag. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zochowska, M.; Paca, A.; Schoehn, G.; Andrieu, J.P.; Chroboczek, J.; Dublet, B.; Szolajska, E. Adenovirus dodecahedron, as a drug delivery vector. PLoS ONE 2009, 4, e5569. [Google Scholar] [CrossRef] [PubMed]
- Zochowska, M.; Piguet, A.C.; Jemielity, J.; Kowalska, J.; Szolajska, E.; Dufour, J.F.; Chroboczek, J. Virus-like particle-mediated intracellular delivery of mRNA cap analog with in vivo activity against hepatocellular carcinoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 67–76. [Google Scholar] [CrossRef]
- Pan, Y.; Jia, T.; Zhang, Y.; Zhang, K.; Zhang, R.; Li, J.; Wang, L. MS2 VLP-based delivery of microRNA-146a inhibits autoantibody production in lupus-prone mice. Int. J. Nanomed. 2012, 7, 5957–5967. [Google Scholar] [CrossRef]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef]
- Li, C.; Liu, F.; Liang, M.; Zhang, Q.; Wang, X.; Wang, T.; Li, J.; Li, D. Hantavirus-like particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine 2010, 28, 4294–4300. [Google Scholar] [CrossRef]
- Purdy, D.E.; Chang, G.J. Secretion of noninfectious dengue virus-like particles and identification of amino acids in the stem region involved in intracellular retention of envelope protein. Virology 2005, 333, 239–250. [Google Scholar] [CrossRef]
- Thompson, C.M.; Petiot, E.; Lennaertz, A.; Henry, O.; Kamen, A.A. Analytical technologies for influenza virus-like particle candidate vaccines: Challenges and emerging approaches. Virol. J. 2013, 10, 141. [Google Scholar] [CrossRef]
- Xu, R.; Shi, M.; Li, J.; Song, P.; Li, N. Construction of SARS-CoV-2 Virus-Like Particles by Mammalian Expression System. Front. Bioeng. Biotechnol. 2020, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Marek, M.; van Oers, M.M.; Devaraj, F.F.; Vlak, J.M.; Merten, O.W. Engineering of baculovirus vectors for the manufacture of virion-free biopharmaceuticals. Biotechnol. Bioeng. 2011, 108, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, Y.; Li, X.; Huang, X.; Liu, J.; Ding, H.; Zhu, P.; Zhou, P. HIV-1 virus-like particles produced by stably transfected Drosophila S2 cells: A desirable vaccine component. J. Virol. 2012, 86, 7662–7676. [Google Scholar] [CrossRef]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A new platform for expressing recombinant vaccines in plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, Q.; Hjelm, B.; Arntzen, C.; Mason, H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol. Bioeng. 2009, 103, 706–714. [Google Scholar] [CrossRef]
- Santi, L.; Batchelor, L.; Huang, Z.; Hjelm, B.; Kilbourne, J.; Arntzen, C.J.; Chen, Q.; Mason, H.S. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine 2008, 26, 1846–1854. [Google Scholar] [CrossRef]
- Balke, I.; Zeltins, A. Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. WIREs Nanomed. Nanobiotechnology 2020, 12, e1587. [Google Scholar] [CrossRef]
- Santoni, M.; Zampieri, R.; Avesani, L. Plant Virus Nanoparticles for Vaccine Applications. Curr. Protein Pept. Sci. 2020, 21, 344–356. [Google Scholar] [CrossRef]
- Hill, B.D.; Zak, A.; Khera, E.; Wen, F. Engineering Virus-like Particles for Antigen and Drug Delivery. Curr. Protein Pept. Sci. 2018, 19, 112–127. [Google Scholar] [CrossRef]
- Jeong, H.; Seong, B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017, 55, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, C.; Jiang, S.; Du, L. Recent Advances in the Development of Virus-Like Particle-Based Flavivirus Vaccines. Vaccines 2020, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef]
- Balke, I.; Zeltins, A. Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv. Drug Deliv. Rev. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Kong, Q.; Richter, L.; Yang, Y.F.; Arntzen, C.J.; Mason, H.S.; Thanavala, Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 2001, 98, 11539–11544. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Ball, J.M.; Shi, J.J.; Jiang, X.; Estes, M.K.; Arntzen, C.J. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5335–5340. [Google Scholar] [CrossRef]
- Medicago. Medicago Announces Phase 3 Study of VLP Quadrivalent Influenza Vaccine. Available online: https://medicago.com/en/press-release/medicago-announces-phase-3-study-of-vlp-quadrivalent-influenza-vaccine/ (accessed on 6 October 2021).
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Comas-Garcia, M.; Colunga-Saucedo, M.; Rosales-Mendoza, S. The Role of Virus-Like Particles in Medical Biotechnology. Mol. Pharm. 2020, 17, 4407–4420. [Google Scholar] [CrossRef]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Chroboczek, J. Virus-like particles as drug delivery vectors. Acta Biochim. Pol. 2016, 63, 469–473. [Google Scholar] [CrossRef]
- Shan, W.; Zhang, D.; Wu, Y.; Lv, X.; Hu, B.; Zhou, X.; Ye, S.; Bi, S.; Ren, L.; Zhang, X. Modularized peptides modified HBc virus-like particles for encapsulation and tumor-targeted delivery of doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wei, Y.; Zhang, K.; Wang, J.; Xu, R.; Zhan, S.; Lin, G.; Wang, W.; Liu, M.; Wang, L.; et al. Development of an antisense RNA delivery system using conjugates of the MS2 bacteriophage capsids and HIV-1 TAT cell-penetrating peptide. Biomed. Pharmacother. Biomed. Pharmacother. 2009, 63, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Qiushi, L.; Kuroda, S.I. Current Progress of Virus-mimicking Nanocarriers for Drug Delivery. Nanotheranostics 2017, 1, 415–429. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hepatitis B vaccines: WHO position paper—July 2017—Vaccins anti-hépatite B: Note de synthèse de l’OMS—juillet 2017. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2017, 92, 369–392. [Google Scholar]
- CDC. Hepatitis B Vaccines, Safety Information. Available online: https://www.cdc.gov/vaccinesafety/vaccines/hepatitis-b-vaccine.html (accessed on 12 April 2022).
- World Health Organization. Hepatitis B vaccines: WHO position paper--recommendations. Vaccine 2010, 28, 589–590. [Google Scholar] [CrossRef]
- Heine, M.; Heck, J.; Ciuraszkiewicz, A.; Bikbaev, A. Dynamic compartmentalization of calcium channel signalling in neurons. Neuropharmacology 2020, 169, 107556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).