Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus Strains and Replication-Defective Virus Construction
2.3. Mice
2.4. Immunization and Challenge
2.5. Post-Challenge Assessments
2.6. Quantitation of Serum Antibodies
2.7. Statistical Analyses
3. Results
3.1. Construction of KOS-NA/8-
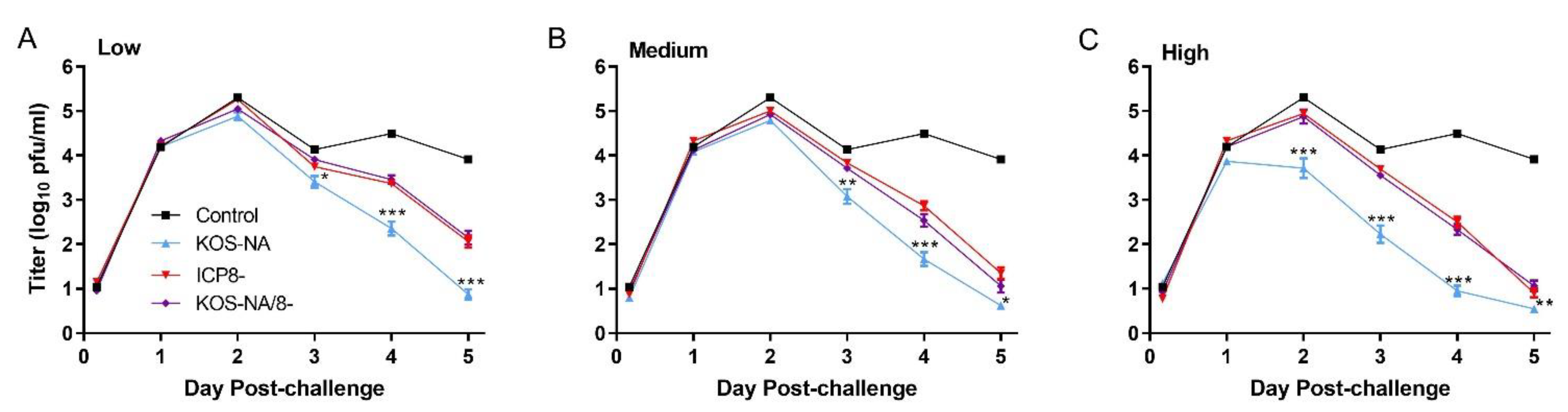
3.2. Effect of Vaccine Strains on Challenge Virus Replication in the Cornea
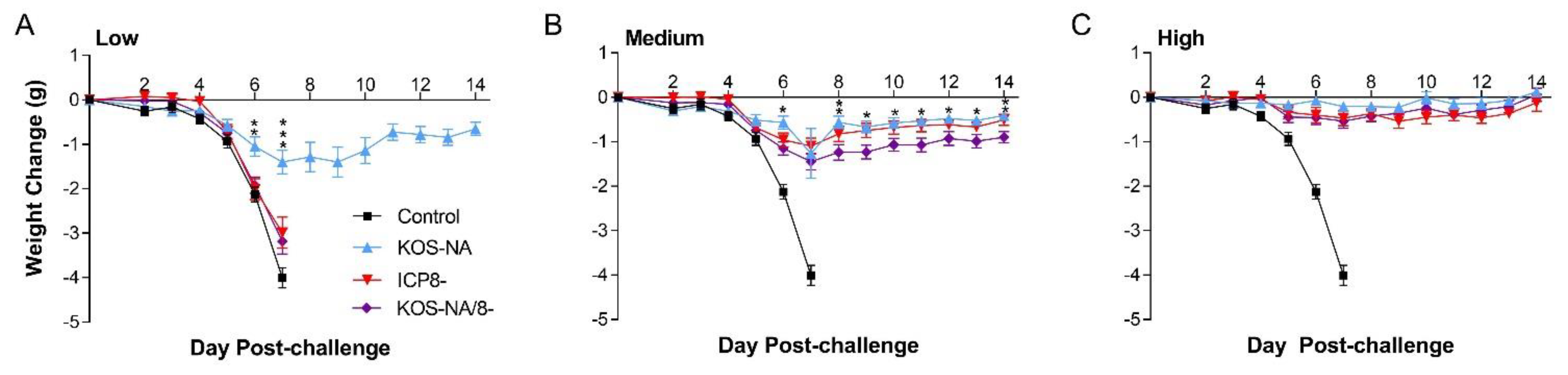
3.3. Effect of Vaccine Strains on Eye Diseases
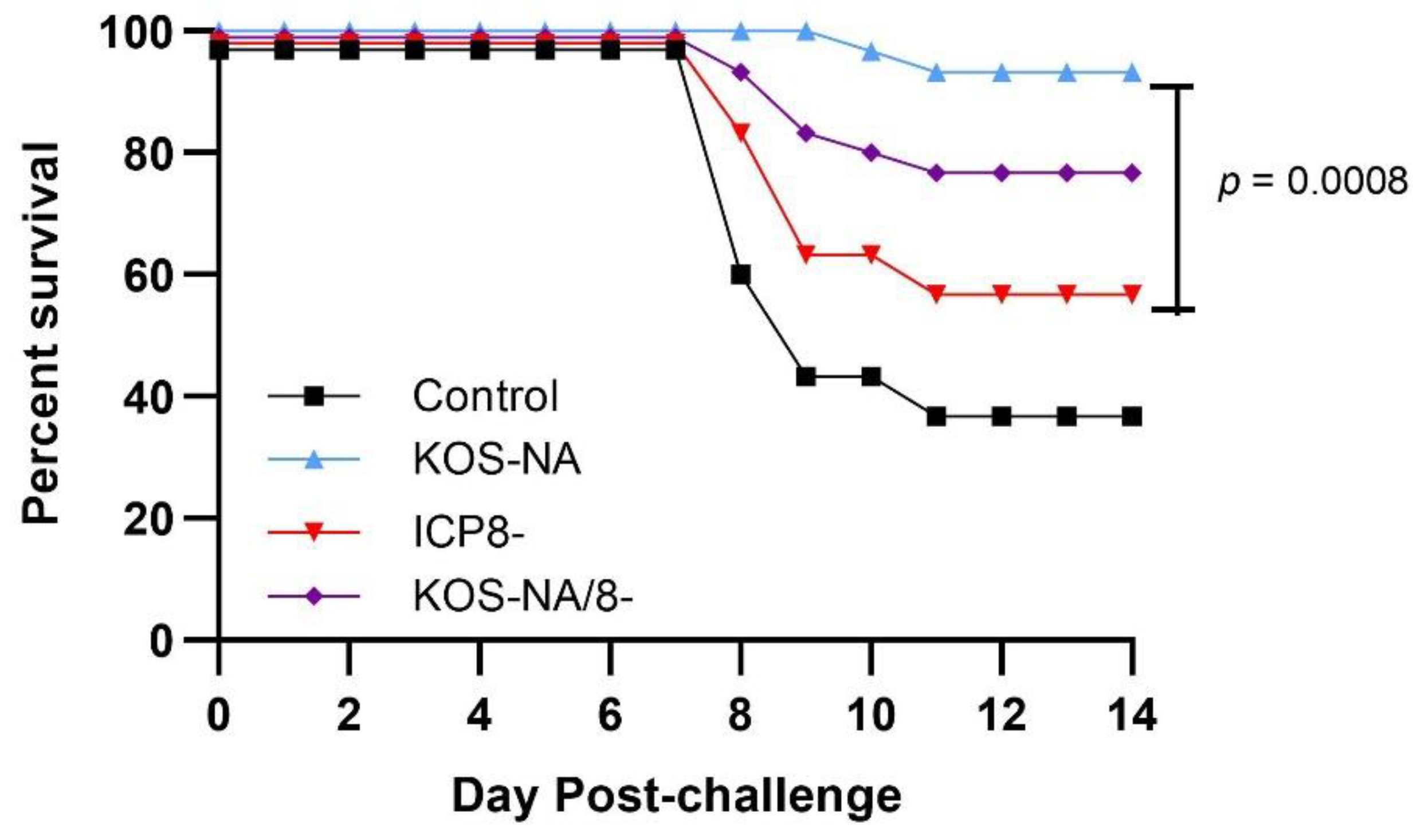
3.4. Effect of Vaccine Strains on Health and Survival
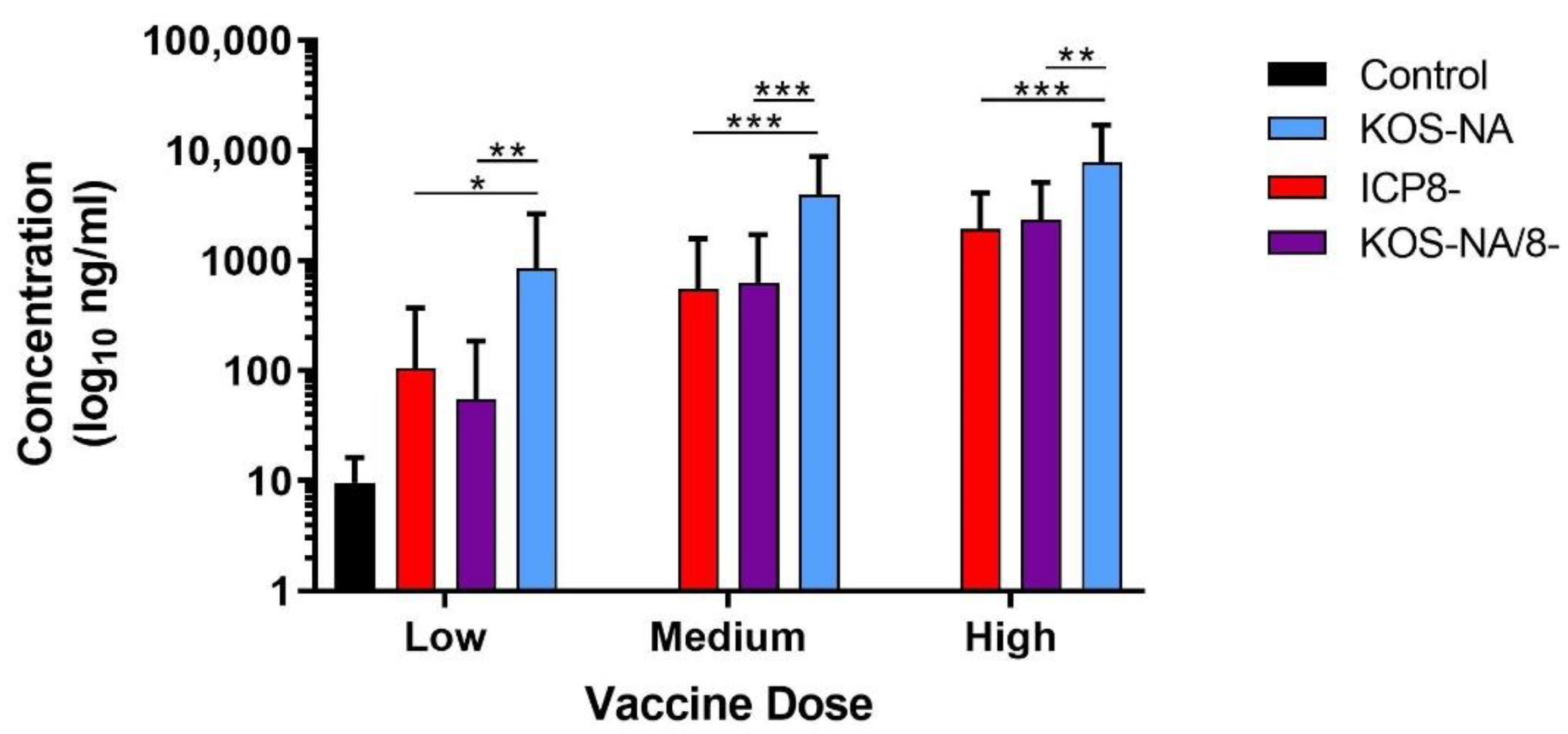
3.5. Antibody Response to Immunization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSV-1 | herpes simplex virus 1 |
| ICP8 | infected cell protein 8 |
| Pfu | plaque-forming units |
References
- Roizman, R.; Knipe, D.M.; Whitley, R.J. Herpes simplex viruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: New York, NY, USA, 2007; pp. 2501–2601. [Google Scholar]
- Dana, M.R.; Qian, Y.; Hamrah, P. Twenty-five-year panorama of corneal immunology: Emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000, 19, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Liesegang, T.J. Herpes simplex virus epidemiology and ocular importance. Cornea 2001, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barron, B.A.; Gee, L.; Hauck, W.W.; Kurinij, N.; Dawson, C.R.; Jones, D.B.; Wilhelmus, K.R.; Kaufman, H.E.; Sugar, J.; Hyndiuk, R.A.; et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 1994, 101, 1871–1882. [Google Scholar] [CrossRef]
- Chou, T.Y.; Hong, B.Y. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Ther. Clin. Risk Manag. 2014, 10, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmus, K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015, 1, CD002898. [Google Scholar] [CrossRef]
- Ike, A.C.; Onu, C.J.; Ononugbo, C.M.; Reward, E.E.; Muo, S.O. Immune response to herpes simplex virus infection and vaccine development. Vaccines 2020, 8, 302. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Herpevac Trial for Women. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef]
- Awasthi, S.; Hook, L.M.; Shaw, C.E.; Pahar, B.; Stagray, J.A.; Liu, D.; Veazey, R.S.; Friedman, H.M. An HSV-2 trivalent vaccine is immunogenic in rhesus macaques and highly efficacious in guinea pigs. PLoS Pathog. 2017, 13, e1006141. [Google Scholar] [CrossRef]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 2019, 4, eaaw7083. [Google Scholar] [CrossRef]
- Ghiasi, H.; Bahri, S.; Nesburn, A.B.; Wechsler, S.L. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Ophthalmol. Vis. Sci. 1995, 36, 1352–1360. [Google Scholar]
- Peng, T.; Ponce-de-Leon, M.; Jiang, H.; Dubin, G.; Lubinski, J.M.; Eisenberg, R.J.; Cohen, G.H. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 1998, 72, 65–72. [Google Scholar] [CrossRef]
- Hook, L.M.; Awasthi, S.; Dubin, J.; Flechtner, J.; Long, D.; Friedman, H.M. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine 2019, 37, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J. Virol. 2013, 87, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, N.A.; Daheshia, M.; Marconi, P.C.; Krisky, D.M.; Rouse, R.J.; Glorioso, J.C.; Manican, E.; Rouse, B.T. Modulation of mucosal and systemic immunity by enteric administration of nonreplicating herpes simplex virus expressing cytokines. Virology 1998, 240, 245–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz, F.M.; Knipe, D.M. Protection from genital herpes disease, seroconversion and latent infection in a non-lethal murine genital infection model by immunization with an HSV-2 replication-defective mutant virus. Virology 2016, 488, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Dropulic, L.K.; Oestreich, M.C.; Pietz, H.L.; Laing, K.J.; Hunsberger, S.; Lumbard, K.; Garabedian, D.; Turk, S.P.; Chen, A.; Hornung, R.L.; et al. A randomized, double-blinded, placebo-controlled, phase 1 study of a replication-defective herpes simplex virus (HSV) type 2 vaccine, HSV529, in adults with or without HSV infection. J. Infect. Dis. 2019, 220, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Zumbrun, E.E.; Si, H.; Wang, F.; Shaw, C.E.; Cai, M.; Lubinski, J.M.; Barrett, S.M.; Balliet, J.W.; Flynn, J.A.; et al. Live attenuated herpes simplex virus 2 glycoprotein E deletion mutant as a vaccine candidate defective in neuronal spread. J. Virol. 2012, 86, 4586–4598. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.E.; McLean, C.S.; Harley, C.; Efstathiou, S.; Inglis, S.; Minson, A.C. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J. Virol. 1994, 68, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, N.L.M.; Visciano, M.; Hunte, R.; Loh, L.N.; Burn Aschner, C.; Jacobs, W.R., Jr.; Herold, B.C. A single-cycle glycoprotein D deletion viral vaccine candidate, DeltagD-2, elicits polyfunctional antibodies that protect against ocular herpes simplex virus. J. Virol. 2020, 94, e00335-20. [Google Scholar] [CrossRef]
- Petro, C.; González, P.A.; Cheshenko, N.; Jandl, T.; Khajoueinejad, N.; Bénard, A.; Sengupta, M.; Herold, B.C.; Jacobs, W.R. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 2015, 10, e06054. [Google Scholar] [CrossRef]
- Naidu, S.K.; Nabi, R.; Cheemarla, N.R.; Stanfield, B.A.; Rider, P.J.; Jambunathan, N.; Chouljenko, V.N.; Carter, R.; Del Piero, F.; Langohr, I.; et al. Intramuscular vaccination of mice with the human herpes simplex virus type-1(HSV-1) VC2 vaccine, but not its parental strain HSV-1(F) confers full protection against lethal ocular HSV-1 (McKrae) pathogenesis. PLoS ONE 2020, 15, e0228252. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.J.; Gurung, H.R.; Jinkins, J.K.; Geltz, J.J.; Wu, J.L.; Halford, W.P.; Carr, D.J.J. A highly efficacious herpes simplex virus 1 vaccine blocks viral pathogenesis and prevents corneal immunopathology via humoral immunity. J. Virol. 2016, 90, 5514–5529. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.H.; Thompson, T.W.; Konen, A.J.; Haenchen, S.D.; Hilliard, J.G.; Macdonald, S.J.; Morrison, L.A.; Davido, D.J. Herpes simplex virus 1 mutant with point mutations in UL39 is impaired for acute viral replication in mice, establishment of latency, and explant-induced reactivation. J. Virol. 2018, 92, e01654-17. [Google Scholar] [CrossRef] [PubMed]
- Davido, D.J.; Tu, E.M.; Wang, H.; Korom, M.; Gazquez Casals, A.; Reddy, P.J.; Mostafa, H.H.; Combs, B.; Haenchen, S.D.; Morrison, L.A. Attenuated herpes simplex virus 1 (HSV-1) expressing a mutant form of ICP6 stimulates a strong immune response that protects mice against HSV-1-induced corneal disease. J. Virol. 2018, 92, e01036-18. [Google Scholar] [CrossRef]
- Gao, M.; Knipe, D.M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 1989, 63, 5258–5267. [Google Scholar] [CrossRef]
- Knipe, D.M.; Spang, A.E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 1982, 43, 314–324. [Google Scholar] [CrossRef]
- Morrison, L.A.; Da Costa, X.J.; Knipe, D.M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 1998, 243, 178–187. [Google Scholar] [CrossRef]
- Walker, J.; Leib, D.A. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine 1998, 16, 1–5. [Google Scholar] [CrossRef]
- Geiss, B.J.; Smith, T.J.; Leib, D.A.; Morrison, L.A. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J. Virol. 2000, 74, 11137–11144. [Google Scholar] [CrossRef]
- Hoshino, Y.; Dalai, S.K.; Wang, K.; Pesnicak, L.; Lau, T.Y.; Knipe, D.M.; Cohen, J.I.; Straus, S.E. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 2005, 79, 410–418. [Google Scholar] [CrossRef]
- Hoshino, Y.; Pesnicak, L.; Dowdell, K.C.; Burbelo, P.D.; Knipe, D.M.; Straus, S.E.; Cohen, J.I. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J. Infect. Dis. 2009, 200, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, J.E.; Tu, E.M.; Wang, H.; Wong, Y.M.; Morrison, L.A. B7 costimulation molecules encoded by replication-defective, vhs-deficient HSV-1 improve vaccine-induced protection against corneal disease. PLoS ONE 2011, 6, e22772. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W.; Dana, M.R.; Ksander, B.R. Immunity causing blindness: Five different paths to herpes stromal keratitis. Immunol. Today 1997, 18, 443–449. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Davido, D.J.; Mostafa, H.H.; Morrison, L.A. Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication. Viruses 2022, 14, 869. https://doi.org/10.3390/v14050869
Wang H, Davido DJ, Mostafa HH, Morrison LA. Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication. Viruses. 2022; 14(5):869. https://doi.org/10.3390/v14050869
Chicago/Turabian StyleWang, Hong, David J. Davido, Heba H. Mostafa, and Lynda A. Morrison. 2022. "Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication" Viruses 14, no. 5: 869. https://doi.org/10.3390/v14050869
APA StyleWang, H., Davido, D. J., Mostafa, H. H., & Morrison, L. A. (2022). Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication. Viruses, 14(5), 869. https://doi.org/10.3390/v14050869





