HIV UTR, LTR, and Epigenetic Immunity
Abstract
1. Introduction
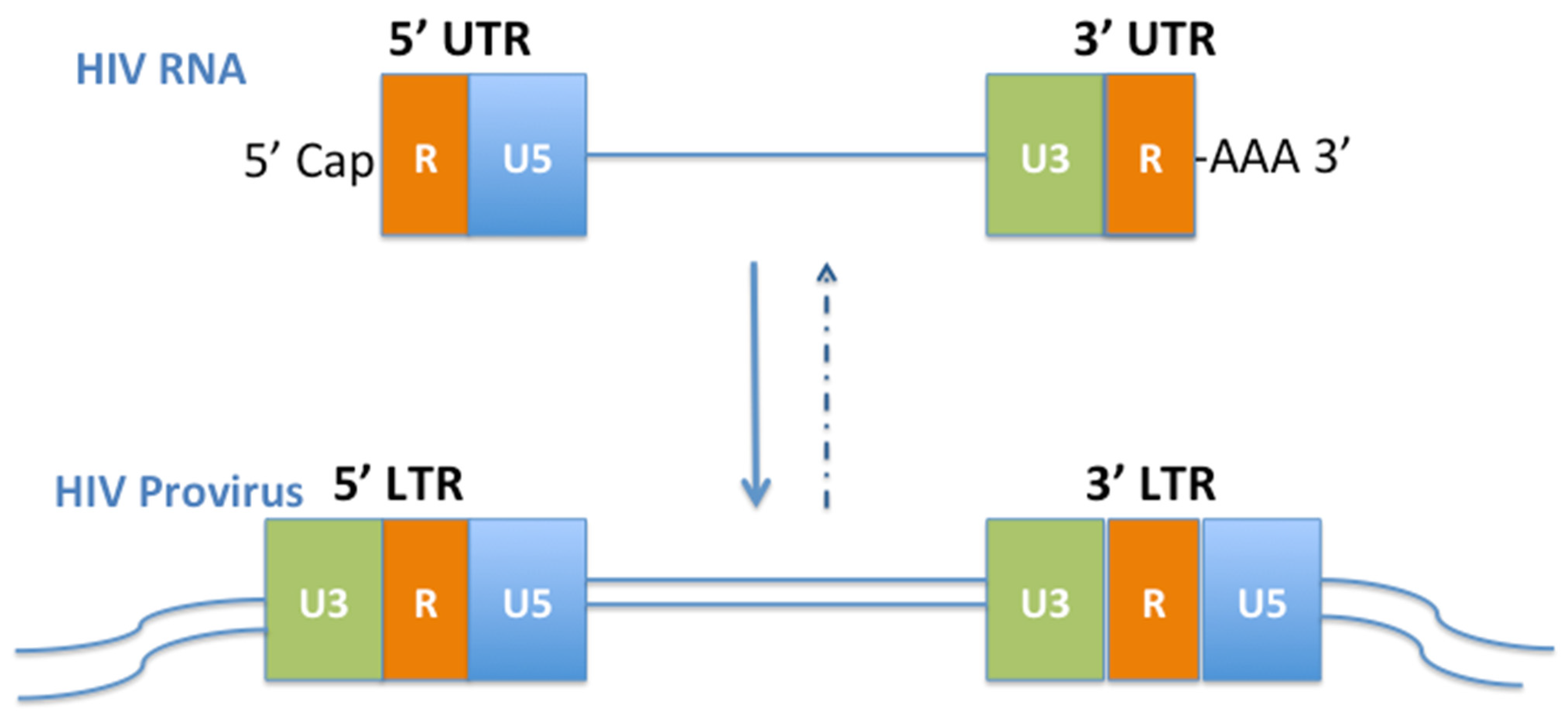
2. HIV UTRs
2.1. 5′UTR
2.2. 3′UTR
3. HIV LTRs
3.1. 5′LTR
3.2. 3′LTR
3.3. Impact of LTR and UTR in Viral Tropism
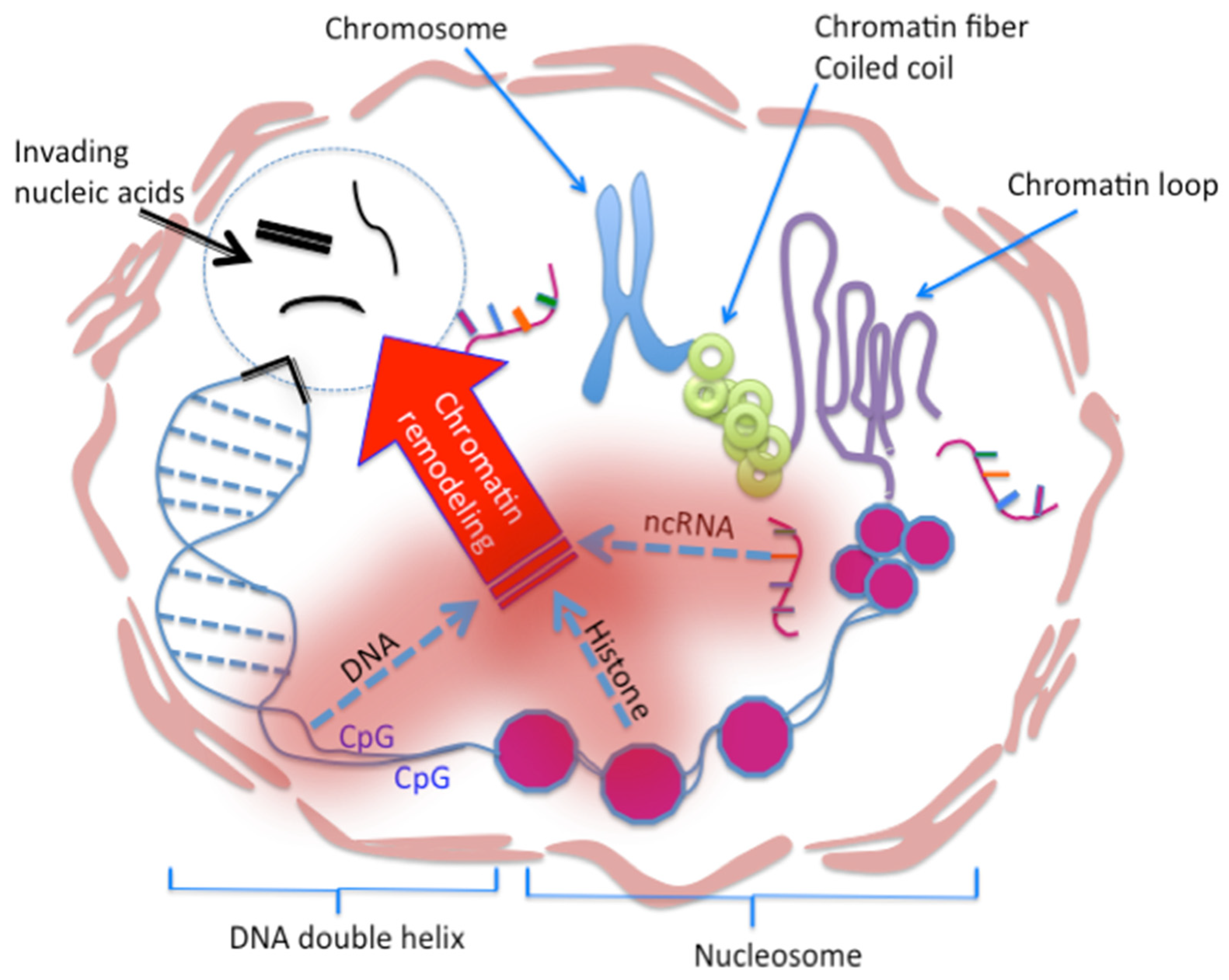
4. Armamentaria of the Human Host
4.1. Epigenetic Immunity
4.2. Chromatin Vaccine (cVaccine)
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sadaie, M.R.; Kalyanaraman, V.S.; Mukopadhayaya, R.; Tschachler, E.; Gallo, R.C.; Wong-Staal, F. Biological characterization of noninfectious HIV-1 particles lacking the envelope protein. Virology 1992, 187, 604–611. [Google Scholar] [CrossRef]
- Ribeiro, R.M.; Mohri, H.; Ho, D.D.; Perelson, A.S. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: Why are CD4+ but not CD8+ T cells depleted? Proc. Natl. Acad. Sci. USA 2002, 99, 15572–15577. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Welsh, T.M.; Peterlin, B.M. The human immunodeficiency virus type 1 long terminal repeat specifies two different transcription complexes, only one of which is regulated by Tat. J. Virol. 1993, 67, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, L.; Roebuck, K.A. Human immunodeficiency virus type-1 transcription: Role of the 5’-untranslated leader region (review). Int. J. Mol. Med. 1998, 1, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sodroski, J.; Rosen, C.; Wong-Staal, F.; Salahuddin, S.Z.; Popovic, M.; Arya, S.; Gallo, R.C.; Haseltine, W.A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science 1985, 227, 171–173. [Google Scholar] [CrossRef]
- Nabel, G.; Baltimore, D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 1987, 326, 711–713, Erratum in Nature 1990, 344, 178. [Google Scholar] [CrossRef]
- Kaufman, P.A.; Weinberg, J.B.; Greene, W.C. Nuclear expression of the 50- and 65-kD Rel-related subunits of nuclear factor-kappa B is differentially regulated in human monocytic cells. J. Clin. Investig. 1992, 90, 121–129. [Google Scholar] [CrossRef]
- Henderson, A.J.; Connor, R.I.; Calame, K.L. C/EBP activators are required for HIV-1 replication and proviral induction in monocytic cell lines. Immunity 1996, 5, 91–101. [Google Scholar] [CrossRef]
- Zhang, J.; Scadden, D.T.; Crumpacker, C.S. Primitive hematopoietic cells resist HIV-1 infection via p21. J. Clin. Investig. 2007, 117, 473–481. [Google Scholar] [CrossRef]
- Zhang, J.L.; Crumpacker, C.S. Towards a Cure, does host immunity play a role? mSphere 2017, 2, e00138-17. [Google Scholar] [CrossRef]
- Song, R.; Kafaie, J.; Laughrea, M. Role of the 5’ TAR stem--loop and the U5-AUG duplex in dimerization of HIV-1 genomic RNA. Biochemistry 2008, 47, 3283–3293. [Google Scholar] [CrossRef]
- Soto-Rifo, R.; Limousin, T.; Rubilar, P.S.; Ricci, E.P.; Décimo, D.; Moncorgé, O.; Trabaud, M.A.; André, P.; Cimarelli, A.; Ohlmann, T. Different effects of the TAR structure on HIV-1 and HIV-2 genomic RNA translation. Nucleic Acids Res. 2012, 40, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, J. Structure of the HIV-1 5’ untranslated region dimer alone and in complex with gold nanocolloids: Support of a TAR-TAR-containing 5’ dimer linkage site (DLS) and a 3’ DIS-DIS-containing DLS. Biochemistry 2011, 50, 6170–6177. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, J.; Gendron, K.; Ferbeyre, G.; Brakier-Gingras, L. The 5’ UTR of HIV-1 full-length mRNA and the Tat viral protein modulate the programmed -1 ribosomal frameshift that generates HIV-1 enzymes. RNA 2012, 18, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Fay, P.J.; Bambara, R.A. The kissing hairpin sequence promotes recombination within the HIV-I 5’ leader region. J. Biol. Chem. 2001, 276, 36482–36492. [Google Scholar] [CrossRef]
- Cockrell, A.S.; van Praag, H.; Santistevan, N.; Ma, H.; Kafri, T. The HIV-1 Rev/RRE system is required for HIV-1 5’ UTR cis elements to augment encapsidation of heterologous RNA into HIV-1 viral particles. Retrovirology 2011, 8, 51. [Google Scholar] [CrossRef]
- Didierlaurent, L.; Racine, P.J.; Houzet, L.; Chamontin, C.; Berkhout, B.; Mougel, M. Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles. Nucleic Acids Res. 2011, 39, 8915–8927. [Google Scholar] [CrossRef]
- Ka, W.H.; Jeong, Y.Y.; You, J.C. Identification of the HIV-1 packaging RNA sequence (Ψ) as a major determinant for the translation inhibition conferred by the HIV-1 5’ UTR. Biochem. Biophys. Res. Commun. 2012, 417, 501–507. [Google Scholar] [CrossRef]
- Barraud, P.; Gaudin, C.; Dardel, F.; Tisné, C. New insights into the formation of HIV-1 reverse transcription initiation complex. Biochimie 2007, 89, 1204–1210. [Google Scholar] [CrossRef]
- Buck, C.B.; Shen, X.; Egan, M.A.; Pierson, T.C.; Walker, C.M.; Siliciano, R.F. The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site. J. Virol. 2001, 75, 181–191. [Google Scholar] [CrossRef]
- Waysbort, A.; Bonnal, S.; Audigier, S.; Estève, J.P.; Prats, A.C. Pyrimidine tract binding protein and La autoantigen interact differently with the 5’ untranslated regions of lentiviruses and oncoretrovirus mRNAs. FEBS Lett. 2001, 490, 54–58. [Google Scholar] [CrossRef]
- Abbink, T.E.; Berkhout, B. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J. Biol. Chem. 2003, 278, 11601–11611. [Google Scholar] [CrossRef] [PubMed]
- Balvay, L.; Soto Rifo, R.; Ricci, E.P.; Decimo, D.; Ohlmann, T. Structural and functional diversity of viral IRESes. Biochim. Biophys. Acta 2009, 1789, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Johnson, A.T.; Howard, J.L.; Purcell, D.F. Both linear and discontinuous ribosome scanning are used for translation initiation from bicistronic human immunodeficiency virus type 1 env mRNAs. J. Virol. 2007, 81, 4664–4676. [Google Scholar] [CrossRef]
- Obayashi, C.M.; Shinohara, Y.; Masuda, T.; Kawai, G. Influence of the 5′-terminal sequences on the 5′-UTR structure of HIV-1 genomic RNA. Sci. Rep. 2021, 11, 10920. [Google Scholar] [CrossRef]
- Yamamoto, M.; Harada, K.; Shoji, S.; Misumi, S.; Takamune, N. Identification of essential cis element in 5’UTR of Nef mRNA for Nef translation. Curr. HIV Res. 2014, 12, 213–219. [Google Scholar] [CrossRef]
- Barrera, A.; Olguín, V.; Vera-Otarola, J.; López-Lastra, M. Cap-independent translation initiation of the unspliced RNA of retro. Viruses Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194583. [Google Scholar] [CrossRef]
- Comandur, R.; Olson, E.D.; Musier-Forsyth, K. Conservation of tRNA mimicry in the 5’-untranslated region of distinct HIV-1 subtypes. RNA 2017, 23, 1850–1859. [Google Scholar] [CrossRef]
- Sampathkumar, R.; Scott-Herridge, J.; Liang, B.; Kimani, J.; Plummer, F.A.; Luo, M. HIV-1 Subtypes and 5’LTR-Leader Sequence Variants Correlate with Seroconversion Status in Pumwani Sex Worker Cohort. Viruses 2017, 10, 4. [Google Scholar] [CrossRef]
- Mbondji-Wonje, C.; Dong, M.; Zhao, J.; Wang, X.; Nanfack, A.; Ragupathy, V.; Sanchez, A.M.; Denny, T.N.; Hewlett, I. Genetic variability of the U5 and downstream sequence of major HIV-1 subtypes and circulating recombinant forms. Sci. Rep. 2020, 10, 13214. [Google Scholar] [CrossRef]
- Gee, A.H.; Kasprzak, W.; Shapiro, B.A. Structural differentiation of the HIV-1 polyA signals. J. Biomol. Struct. Dyn. 2006, 23, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, S.; Garyu, L.; Connor, R.; Summers, M.F. Potential intra- and intermolecular interactions involving the unique-5’ region of the HIV-1 5’-UTR. Biochemistry 2008, 47, 13064–13073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comas-Garcia, M.; Davis, S.R.; Rein, A. On the Selective Packaging of Genomic RNA by HIV-1. Viruses 2016, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Barksdale, S.K.; Baker, C.C. The human immunodeficiency virus type 1 Rev protein and the Rev-responsive element counteract the effect of an inhibitory 5’ splice site in a 3’ untranslated region. Mol. Cell Biol. 1995, 15, 2962–2971. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.T.; Gilmartin, G.M.; Venkatarama, K.; Arriagada, G.; Goff, S.P. HIV-1 mRNA 3’ end processing is distinctively regulated by eIF3f, CDK11, and splice factor 9G8. Mol. Cell 2009, 36, 279–289. [Google Scholar] [CrossRef] [PubMed]
- De Breyne, S.; Chamond, N.; Décimo, D.; Trabaud, M.A.; André, P.; Sargueil, B.; Ohlmann, T. In vitro studies reveal that different modes of initiation on HIV-1 mRNA have different levels of requirement for eukaryotic initiation factor 4F. FEBS J. 2012, 279, 3098–3111. [Google Scholar] [CrossRef] [PubMed]
- Schopman, N.C.; Willemsen, M.; Liu, Y.P.; Bradley, T.; van Kampen, A.; Baas, F.; Berkhout, B.; Haasnoot, J. Deep sequencing of virus-infected cells reveals HIV-encoded small RNAs. Nucleic Acids Res. 2012, 40, 414–427. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. Emerging roles for natural microRNA sponges. Curr. Biol. 2010, 20, R858–R861. [Google Scholar] [CrossRef]
- Kennedy, E.M.; Bogerd, H.P.; Kornepati, A.V.; Kang, D.; Ghoshal, D.; Marshall, J.B.; Poling, B.C.; Tsai, K.; Gokhale, N.S.; Horner, S.M.; et al. Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Cell Host Microbe 2016, 19, 675–685, Erratum in Cell Host Microbe 2017, 22, 830. [Google Scholar] [CrossRef]
- Garcia-Moreno, M.; Sanz, M.A.; Carrasco, L. A Viral mRNA Motif at the 3’-Untranslated Region that Confers Translatability in a Cell-Specific Manner. Implications for Virus Evolution. Sci. Rep. 2016, 6, 19217. [Google Scholar] [CrossRef]
- Tsai, K.; Bogerd, H.P.; Kennedy, E.M.; Emery, A.; Swanstrom, R.; Cullen, B.R. Epitranscriptomic addition of m6A regulates HIV-1 RNA stability and alternative splicing. Genes Dev. 2021, 35, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; Sanz, M.A.; González-Almela, E. The Regulation of Translation in Alphavirus-Infected Cells. Viruses 2018, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Nejati, A.; Shahmahmoodi, S.; Arefian, E.; Shoja, Z.; Marashi, S.M.; Tabatabaie, H.; Mollaei-Kandelous, Y.; Soleimani, M.; Nategh, R. Efficient inhibition of human immunodeficiency virus replication using novel modified microRNA-30a targeting 3′-untranslated region transcripts. Exp. Ther. Med. 2016, 11, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Nathans, R.; Chu, C.Y.; Serquina, A.K.; Lu, C.C.; Cao, H.; Rana, T.M. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol. Cell. 2009, 34, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Hunter, E. HIV transmission. Cold Spring Harb. Perspect. Med. 2012, 2, a006965. [Google Scholar] [CrossRef]
- Musich, T.; Jones, J.C.; Keele, B.F.; Jenkins, L.M.M.; Demberg, T.; Uldrick, T.S.; Yarchoan, R.; Robert-Guroff, M. Flow virometric sorting and analysis of HIV quasispecies from plasma. JCI Insight 2017, 2, e90626. [Google Scholar] [CrossRef]
- Franza, B.R., Jr.; Josephs, S.F.; Gilman, M.Z.; Ryan, W.; Clarkson, B. Characterization of cellular proteins recognizing the HIV enhancer using a microscale DNA-affinity precipitation assay. Nature 1987, 330, 391–395. [Google Scholar] [CrossRef]
- Tong-Starksen, S.E.; Luciw, P.A.; Peterlin, B.M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc. Natl. Acad. Sci. USA 1987, 84, 6845–6849. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Valandra, G.; Roderiquez, G.; Bushar, G.; Giri, C.; Norcross, M.A. Phorbol ester enhances human immunodeficiency virus-promoted gene expression and acts on a repeated 10-base-pair functional enhancer element. Mol. Cell Biol. 1987, 7, 3759–3766. [Google Scholar] [CrossRef]
- Dinter, H.; Chiu, R.; Imagawa, M.; Karin, M.; Jones, K.A. In vitro activation of the HIV-1 enhancer in extracts from cells treated with a phorbol ester tumor promoter. EMBO J. 1987, 6, 4067–4071. [Google Scholar] [CrossRef]
- Böhnlein, E.; Lowenthal, J.W.; Siekevitz, M.; Ballard, D.W.; Franza, B.R.; Greene, W.C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell 1988, 53, 827–836. [Google Scholar] [CrossRef]
- Jakobovits, A.; Smith, D.H.; Jakobovits, E.B.; Capon, D.J. A discrete element 3’ of human immunodeficiency virus 1 (HIV-1) and HIV-2 mRNA initiation sites mediates transcriptional activation by an HIV trans activator. Mol. Cell Biol. 1988, 8, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Hazan, U.; Thomas, D.; Alcami, J.; Bachelerie, F.; Israel, N.; Yssel, H.; Virelizier, J.L.; Arenzana-Seisdedos, F. Stimulation of a human T-cell clone with anti-CD3 or tumor necrosis factor induces NF-kappa B translocation but not human immunodeficiency virus 1 enhancer-dependent transcription. Proc. Natl. Acad. Sci. USA 1990, 87, 7861–7865. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Zhao, M.J.; Nagase, T.; Maekawa, T.; Ishizaki, R.; Tabata, S.; Ishii, S. HIV-EP2, a new member of the gene family encoding the human immunodeficiency virus type 1 enhancer-binding protein. Comparison with HIV-EP1/PRDII-BF1/MBP-1. J. Biol. Chem. 1991, 266, 8590–8594. [Google Scholar] [CrossRef]
- Verdin, E. DNase I-hypersensitive sites are associated with both long terminal repeats and with the intragenic enhancer of integrated human immunodeficiency virus type 1. J. Virol. 1991, 65, 6790–6799. [Google Scholar] [CrossRef]
- Berkhout, B.; Jeang, K.T. Functional roles for the TATA promoter and enhancers in basal and Tat-induced expression of the human immunodeficiency virus type 1 long terminal repeat. J. Virol. 1992, 66, 139–149. [Google Scholar] [CrossRef]
- Proudfoot, N.J.; Lee, B.A.; Monks, J. Multiple SP1 binding sites confer enhancer-independent, replication-activated transcription of HIV-1 and globin gene promoters. New Biol. 1992, 4, 369–381. [Google Scholar]
- Fiume, G.; Vecchio, E.; De Laurentiis, A.; Trimboli, F.; Palmieri, C.; Pisano, A.; Falcone, C.; Pontoriero, M.; Rossi, A.; Scialdone, A.; et al. Human immunodeficiency virus-1 Tat activates NF-κB via physical interaction with IκB-α and p65. Nucleic Acids Res. 2012, 40, 3548–3562. [Google Scholar] [CrossRef]
- Stein, B.; Baldwin, A.S., Jr.; Ballard, D.W.; Greene, W.C.; Angel, P.; Herrlich, P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993, 12, 3879–3891. [Google Scholar] [CrossRef]
- Mondal, D.; Alam, J.; Prakash, O. NF-kappa B site-mediated negative regulation of the HIV-1 promoter by CCAAT/enhancer binding proteins in brain-derived cells. J. Mol. Neurosci. 1994, 5, 241–258. [Google Scholar] [CrossRef]
- Vacca, A.; Farina, M.; Maroder, M.; Alesse, E.; Screpanti, I.; Frati, L.; Gulino, A. Human immunodeficiency virus type-1 tat enhances interleukin-2 promoter activity through synergism with phorbol ester and calcium-mediated activation of the NF-AT cis-regulatory motif. Biochem. Biophys. Res. Commun. 1994, 205, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Calame, K.L. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4(+) T cells. Proc. Natl. Acad. Sci. USA 1997, 94, 8714–8719. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, B.E.; Khalili, K.; Amini, S. Transcription of the human immunodeficiency virus type 1 (HIV-1) promoter in central nervous system cells: Effect of YB-1 on expression of the HIV-1 long terminal repeat. J. Gen. Virol. 1998, 79, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Popik, W.; Hesselgesser, J.E.; Pitha, P.M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 1998, 72, 6406–6413. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Rogers, L.; Nakata, K.; Zhao, B.Y.; Pine, R.; Nakai, Y.; Kurosu, K.; Rom, W.N.; Weiden, M. Type I interferon induces inhibitory 16-kD CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: Pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J. Exp. Med. 1998, 188, 1255–1265. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Yull, F.E.; Chen, C.L.; Venkatakrishnan, A.; Blackwell, T.R.; Hicks, D.J.; Lancaster, L.H.; Christman, J.W.; Kerr, L.D. Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am. J. Respir. Crit. Care Med. 2000, 162, 1095–1101. [Google Scholar] [CrossRef]
- Rosati, M.; Valentin, A.; Patenaude, D.J.; Pavlakis, G.N. CCAAT-enhancer-binding protein beta (C/EBP beta) activates CCR5 promoter: Increased C/EBP beta and CCR5 in T lymphocytes from HIV-1-infected individuals. J. Immunol. 2001, 167, 1654–1662. [Google Scholar] [CrossRef]
- Lee, E.S.; Zhou, H.; Henderson, A.J. Endothelial cells enhance human immunodeficiency virus type 1 replication in macrophages through a C/EBP-dependent mechanism. J. Virol. 2001, 75, 9703–9712. [Google Scholar] [CrossRef]
- Fang, G.; Burger, H.; Chappey, C.; Rowland-Jones, S.; Visosky, A.; Chen, C.H.; Moran, T.; Townsend, L.; Murray, M.; Weiser, B. Analysis of transition from long-term nonprogressive to progressive infection identifies sequences that may attenuate HIV type 1. AIDS Res. Hum. Retroviruses 2001, 17, 1395–1404. [Google Scholar] [CrossRef]
- Hoshino, Y.; Nakata, K.; Hoshino, S.; Honda, Y.; Tse, D.B.; Shioda, T.; Rom, W.N.; Weiden, M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J. Exp. Med. 2002, 195, 495–505. [Google Scholar] [CrossRef]
- Reed-Inderbitzin, E.; Maury, W. Cellular specificity of HIV-1 replication can be controlled by LTR sequences. Virology 2003, 314, 680–695. [Google Scholar] [CrossRef]
- Dandekar, D.H.; Ganesh, K.N.; Mitra, D. HIV-1 Tat directly binds to NFkappaB enhancer sequence: Role in viral and cellular gene expression. Nucleic Acids Res. 2004, 32, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.L.; Barthel, K.K.; Wu, Y.; Kalhor, R.; Stroud, J.C.; Giffin, M.J.; Chen, L. Crystal structure of NFAT bound to the HIV-1 LTR tandem kappaB enhancer element. Structure 2008, 16, 684–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kogan, M.; Haine, V.; Ke, Y.; Wigdahl, B.; Fischer-Smith, T.; Rappaport, J. Macrophage colony stimulating factor regulation by nuclear factor kappa B: A relevant pathway in human immunodeficiency virus type 1 infected macrophages. DNA Cell Biol. 2012, 31, 280–289. [Google Scholar] [CrossRef]
- Bhargavan, B.; Woollard, S.M.; Kanmogne, G.D. Toll-like receptor-3 mediates HIV-1 transactivation via NFκB and JNK pathways and histone acetylation, but prolonged activation suppresses Tat and HIV-1 replication. Cell Signal. 2016, 28, 7–22. [Google Scholar] [CrossRef]
- Li, W.; Notani, D.; Rosenfeld, M.G. Enhancers as non-coding RNA transcription units: Recent insights and future perspectives. Nat. Rev. Genet. 2016, 17, 207–223. [Google Scholar] [CrossRef]
- Yi, H.; Liu, S.; Kashiwagi, Y.; Ikegami, D.; Huang, W.; Kanda, H.; Iida, T.; Liu, C.H.; Takahashi, K.; Lubarsky, D.A.; et al. Phosphorylated CCAAT/Enhancer Binding Protein β Contributes to Rat HIV-Related Neuropathic Pain: In Vitro and In Vivo Studies. J. Neurosci. 2018, 38, 555–574. [Google Scholar] [CrossRef]
- Acchioni, C.; Remoli, A.L.; Marsili, G.; Acchioni, M.; Nardolillo, I.; Orsatti, R.; Farcomeni, S.; Palermo, E.; Perrotti, E.; Barreca, M.L.; et al. Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus. J. Virol. 2019, 93, e00495-19. [Google Scholar] [CrossRef]
- Böhnlein, S.; Hauber, J.; Cullen, B.R. Identification of a U5-specific sequence required for efficient polyadenylation within the human immunodeficiency virus long terminal repeat. J. Virol. 1989, 63, 421–424. [Google Scholar] [CrossRef]
- Eggermont, J.; Proudfoot, N.J. Tat-dependent occlusion of the HIV poly(A) site. EMBO J. 1993, 12, 2119–2128. [Google Scholar]
- Ashe, M.P.; Griffin, P.; James, W.; Proudfoot, N.J. Poly(A) site selection in the HIV-1 provirus: Inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev. 1995, 9, 3008–3025. [Google Scholar] [CrossRef] [PubMed]
- Katano, H.; Sato, Y.; Hoshino, S.; Tachikawa, N.; Oka, S.; Morishita, Y.; Ishida, T.; Watanabe, T.; Rom, W.N.; Mori, S.; et al. Integration of HIV-1 caused STAT3-associated B cell lymphoma in an AIDS patient. Microbes. Infect. 2007, 9, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.J.; Lusic, M.; Mitar, I.; Giacca, M.; Proudfoot, N.J. Transcription-dependent gene looping of the HIV-1 provirus is dictated by recognition of pre-mRNA processing signals. Mol. Cell. 2008, 29, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.C.; Campilongo, F.; Barclay, R.A.; DeMarino, C.; Iglesias-Ussel, M.D.; Kashanchi, F.; Romerio, F. The Human Immunodeficiency Virus 1 ASP RNA promotes viral latency by recruiting the Polycomb Repressor Complex 2 and promoting nucleosome assembly. Virology 2017, 506, 34–44. [Google Scholar] [CrossRef]
- Ma, G.; Yasunaga, J.I.; Shimura, K.; Takemoto, K.; Watanabe, M.; Amano, M.; Nakata, H.; Liu, B.; Zuo, X.; Matsuoka, M. Human retroviral antisense mRNAs are retained in the nuclei of infected cells for viral persistence. Proc. Natl. Acad. Sci. USA 2021, 118, e2014783118. [Google Scholar] [CrossRef]
- Canchi, S.; Swinton, M.K.; Rissman, R.A.; Fields, J.A. Transcriptomic analysis of brain tissues identifies a role for CCAAT enhancer binding protein β in HIV-associated neurocognitive disorder. J. Neuroinflamm. 2020, 17, 112. [Google Scholar] [CrossRef]
- Roy, S.; Ganguly, N.; Banerjee, S. Exploring clinical implications and role of non-coding RNAs in lung carcinogenesis. Mol. Biol. Rep. 2022, 1–13. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef]
- Wadley, G.D.; Lamon, S.; Alexander, S.E.; McMullen, J.R.; Bernardo, B.C. Noncoding RNAs regulating cardiac muscle mass. J. Appl. Physiol. 2019, 127, 633–644. [Google Scholar] [CrossRef]
- Widlak, P.; Gaynor, R.B.; Garrard, W.T. In vitro chromatin assembly of the HIV-1 promoter. ATP-dependent polar repositioning of nucleosomes by Sp1 and NFkappaB. J. Biol. Chem. 1997, 272, 17654–17661. [Google Scholar] [CrossRef]
- Van Lint, C.; Amella, C.A.; Emiliani, S.; John, M.; Jie, T.; Verdin, E. Transcription factor binding sites downstream of the human immunodeficiency virus type 1 transcription start site are important for virus infectivity. J. Virol. 1997, 71, 6113–6127. [Google Scholar] [CrossRef] [PubMed]
- Angelov, D.; Charra, M.; Seve, M.; Côté, J.; Khochbin, S.; Dimitrov, S. Differential remodeling of the HIV-1 nucleosome upon transcription activators and SWI/SNF complex binding. J. Mol. Biol. 2000, 302, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Weiser, B.; Kemal, K.S.; Burger, H.; Ramirez, C.M.; Korn, K.; Anastos, K.; Kaul, R.; Kovacs, C.; Doerfler, W. Epigenetic analysis of HIV-1 proviral genomes from infected individuals: Predominance of unmethylated CpG’s. Virology 2014, 449, 181–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sen, D.R.; Kaminski, J.; Barnitz, R.A.; Kurachi, M.; Gerdemann, U.; Yates, K.B.; Tsao, H.W.; Godec, J.; LaFleur, M.W.; Brown, F.D.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Miklík, D.; Šenigl, F.; Hejnar, J. Proviruses with Long-Term Stable Expression Accumulate in Transcriptionally Active Chromatin Close to the Gene Regulatory Elements: Comparison of ASLV-, HIV- and MLV-Derived Vectors. Viruses 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.C.; Zhang, Q.; Li, Z.; Tiwari, S.K.; Qin, Y.; Yau, E.; Sanchez, A.; Singh, G.; Chang, K.; Kaul, M.; et al. The Long Noncoding RNA HEAL Regulates HIV-1 Replication through Epigenetic Regulation of the HIV-1 Promoter. mBio 2019, 10, e02016-19. [Google Scholar] [CrossRef]
- Doerfler, W. Epigenetic consequences of foreign DNA insertions: De novo methylation and global alterations of methylation patterns in recipient genomes. Rev. Med. Virol. 2011, 21, 336–346. [Google Scholar] [CrossRef]
- Weber, S.; Jung, S.; Doerfler, W. DNA methylation and transcription in HERV (K, W, E) and LINE sequences remain unchanged upon foreign DNA insertions. Epigenomics 2016, 8, 157–165. [Google Scholar] [CrossRef]
- Zhang, J.; Attar, E.; Cohen, K.; Crumpacker, C.; Scadden, D. Silencing p21(Waf1/Cip1/Sdi1) expression increases gene transduction efficiency in primitive human hematopoietic cells. Gene Ther. 2005, 12, 1444–1452. [Google Scholar] [CrossRef]
- Bergamaschi, A.; David, A.; Le Rouzic, E.; Nisole, S.; Barré-Sinoussi, F.; Pancino, G. The CDK inhibitor p21Cip1/WAF1 is induced by FcgammaR activation and restricts the replication of human immunodeficiency virus type 1 and related primate lentiviruses in human macrophages. J. Virol. 2009, 83, 12253–12265. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Huang, J.; Cung, T.; Seiss, K.; Beamon, J.; Carrington, M.F.; Porter, L.C.; Burke, P.S.; Yang, Y.; et al. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J. Clin. Investig. 2011, 121, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Crumpacker, C. Eradication of HIV and Cure of AIDS, Now and How? Front. Immunol. 2013, 4, 337. [Google Scholar] [CrossRef] [PubMed]
- Reichel, A.; Stilp, A.C.; Scherer, M.; Reuter, N.; Lukassen, S.; Kasmapour, B.; Schreiner, S.; Cicin-Sain, L.; Winterpacht, A.; Stamminger, T. Chromatin-Remodeling Factor SPOC1 Acts as a Cellular Restriction Factor against Human Cytomegalovirus by Repressing the Major Immediate Early Promoter. J. Virol. 2018, 92, e00342-18. [Google Scholar] [CrossRef] [PubMed]
- Niller, H.H.; Banati, F.; Salamon, D.; Minarovits, J. Epigenetic Alterations in Epstein-Barr Virus-Associated Diseases. Adv. Exp. Med. Biol. 2016, 879, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Doerfler, W.; Hohlweg, U.; Müller, K.; Remus, R.; Heller, H.; Hertz, J. Foreign DNA integration--perturbations of the genome--oncogenesis. Ann. N. Y. Acad. Sci. 2001, 945, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Doerfler, W.; Casadesús, J. (Eds.) Epigenetics of Infectious Diseases; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-55021-3. [Google Scholar] [CrossRef]
- Doerfler, W.; Böhm, P. (Eds.) Epigenetics—A Different Way of Looking at Genetics; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-27186-6. Available online: https://link.springer.com/book/10.1007/978-3-319-27186-6 (accessed on 20 March 2022).
- Minarovits, J.; Banati, F.; Szenthe, K.; Niller, H.H. Epigenetic Regulation. Adv. Exp. Med. Biol. 2016, 879, 1–25. [Google Scholar] [CrossRef]
- Chang, H.D.; Radbruch, A. Targeting pathogenic T helper cell memory. Ann. Rheum. Dis. 2011, 70 (Suppl. S1), i85–i87. [Google Scholar] [CrossRef]
- Chang, H.D.; Tokoyoda, K.; Hoyer, B.; Alexander, T.; Khodadadi, L.; Mei, H.; Dörner, T.; Hiepe, F.; Burmester, G.R.; Radbruch, A. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol. 2019, 61, 86–91. [Google Scholar] [CrossRef]
- Hirahara, K.; Vahedi, G.; Ghoreschi, K.; Yang, X.P.; Nakayamada, S.; Kanno, Y.; O’Shea, J.J.; Laurence, A. Helper T-cell differentiation and plasticity: Insights from epigenetics. Immunology 2011, 134, 235–245. [Google Scholar] [CrossRef]
- Nakayamada, S.; Kanno, Y.; Takahashi, H.; Jankovic, D.; Lu, K.T.; Johnson, T.A.; Sun, H.W.; Vahedi, G.; Hakim, O.; Handon, R.; et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 2011, 35, 919–931. [Google Scholar] [CrossRef]
- Nakayamada, S.; Takahashi, H.; Kanno, Y.; O’Shea, J.J. Helper T cell diversity and plasticity. Curr. Opin. Immunol. 2012, 24, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, K.; Poholek, A.; Vahedi, G.; Laurence, A.; Kanno, Y.; Milner, J.D.; O’Shea, J.J. Mechanisms underlying helper T-cell plasticity: Implications for immune-mediated disease. J. Allergy Clin. Immunol. 2013, 131, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wei, L.; Zhu, J.; Zang, C.; Hu-Li, J.; Yao, Z.; Cui, K.; Kanno, Y.; Roh, T.Y.; Watford, W.T.; et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 2009, 30, 155–167. [Google Scholar] [CrossRef]
- Vahedi, G.; Kanno, Y.; Sartorelli, V.; O’Shea, J.J. Transcription factors and CD4 T cells seeking identity: Masters, minions, setters and spikers. Immunology 2013, 139, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Georgakilas, G.; Petrovic, J.; Kurachi, M.; Cai, S.; Harly, C.; Pear, W.S.; Bhandoola, A.; Wherry, E.J.; Vahedi, G. Lineage-Determining Transcription Factor TCF-1 Initiates the Epigenetic Identity of T Cells. Immunity 2018, 48, 243–257.e10. [Google Scholar] [CrossRef]
- Durek, P.; Nordström, K.; Gasparoni, G.; Salhab, A.; Kressler, C.; De Almeida, M.; Bassler, K.; Ulas, T.; Schmidt, F.; Xiong, J.; et al. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity 2016, 45, 1148–1161. [Google Scholar] [CrossRef]
- Issuree, P.D.; Day, K.; Au, C.; Raviram, R.; Zappile, P.; Skok, J.A.; Xue, H.H.; Myers, R.M.; Littman, D.R. Stage-specific epigenetic regulation of CD4 expression by coordinated enhancer elements during T cell development. Nat. Commun. 2018, 9, 3594. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Pioger, L.; El Aabidine, A.Z.; Karasu, N.; Molitor, A.M.; Dao, L.T.M.; Charbonnier, G.; van Laethem, F.; Fenouil, R.; Koch, F.; et al. Alternative Enhancer Usage and Targeted Polycomb Marking Hallmark Promoter Choice during T Cell Differentiation. Cell Rep. 2020, 32, 108048. [Google Scholar] [CrossRef]
- Witte, S.; O’Shea, J.J.; Vahedi, G. Super-enhancers: Asset management in immune cell genomes. Trends Immunol. 2015, 36, 519–526. [Google Scholar] [CrossRef]
- Paiano, J.J.; Johnson, J.L.; Vahedi, G. Enhancing our understanding of enhancers in T-helper cells. Eur. J. Immunol. 2015, 45, 2998–3001. [Google Scholar] [CrossRef]
- Haftmann, C.; Riedel, R.; Porstner, M.; Wittmann, J.; Chang, H.D.; Radbruch, A.; Mashreghi, M.F. Direct uptake of Antagomirs and efficient knockdown of miRNA in primary B and T lymphocytes. J. Immunol. Methods 2015, 426, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, S.; Feil, R. Long noncoding RNAs in human disease: Emerging mechanisms and therapeutic strategies. Epigenomics 2015, 7, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Sanli, I.; Feil, R. Chromatin mechanisms in the developmental control of imprinted gene expression. Int. J. Biochem. Cell Biol. 2015, 67, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Feil, R. Environmental effects on chromatin repression at imprinted genes and endogenous retroviruses. Curr. Opin. Chem. Biol. 2018, 45, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, M.; Tarakhovsky, A. Epigenetic control of Immunity. Cold Spring Harb. Perspect. Biol. 2014, 6, a019307. [Google Scholar] [CrossRef]
- Whyte, W.A.; Bilodeau, S.; Orlando, D.A.; Hoke, H.A.; Frampton, G.M.; Foster, C.T.; Cowley, S.M.; Young, R.A. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature 2012, 482, 221–225. [Google Scholar] [CrossRef]
- Smith, E.; Shilatifard, A. Enhancer biology and enhanceropathies. Nat. Struct. Mol. Biol. 2014, 21, 210–219. [Google Scholar] [CrossRef]
- Carter, A.C.; Xu, J.; Nakamoto, M.Y.; Wei, Y.; Zarnegar, B.J.; Shi, Q.; Broughton, J.P.; Ransom, R.C.; Salhotra, A.; Nagaraja, S.D.; et al. Spen links RNA-mediated endogenous retrovirus silencing and X chromosome inactivation. eLife 2020, 9, e54508. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.H.; et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef]
- Zhang, J.L.; Crumpacker, C.S. An integrative immunobiology and inflammation study on cytomegalovirus. Integr. Immunobiol. Inflamm. 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Crumpacker, C. HIV UTR, LTR, and Epigenetic Immunity. Viruses 2022, 14, 1084. https://doi.org/10.3390/v14051084
Zhang J, Crumpacker C. HIV UTR, LTR, and Epigenetic Immunity. Viruses. 2022; 14(5):1084. https://doi.org/10.3390/v14051084
Chicago/Turabian StyleZhang, Jielin, and Clyde Crumpacker. 2022. "HIV UTR, LTR, and Epigenetic Immunity" Viruses 14, no. 5: 1084. https://doi.org/10.3390/v14051084
APA StyleZhang, J., & Crumpacker, C. (2022). HIV UTR, LTR, and Epigenetic Immunity. Viruses, 14(5), 1084. https://doi.org/10.3390/v14051084




