Evaluation of Molecular Test for the Discrimination of “Naked” DNA from Infectious Parvovirus B19 Particles in Serum and Bone Marrow Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Samples and Ethical Aspects
2.2. Diagnostic Criteria of B19V Infection
2.3. Endonuclease Pretreatment
2.4. B19V DNA Extraction and qPCR
2.5. Statistical Analysis
3. Results
3.1. Determination of the Optimal Benzonase® Concentration
3.2. Effect of Benzonase® Pretreatment on the B19V DNA Levels in Serum Samples from Infected Patients
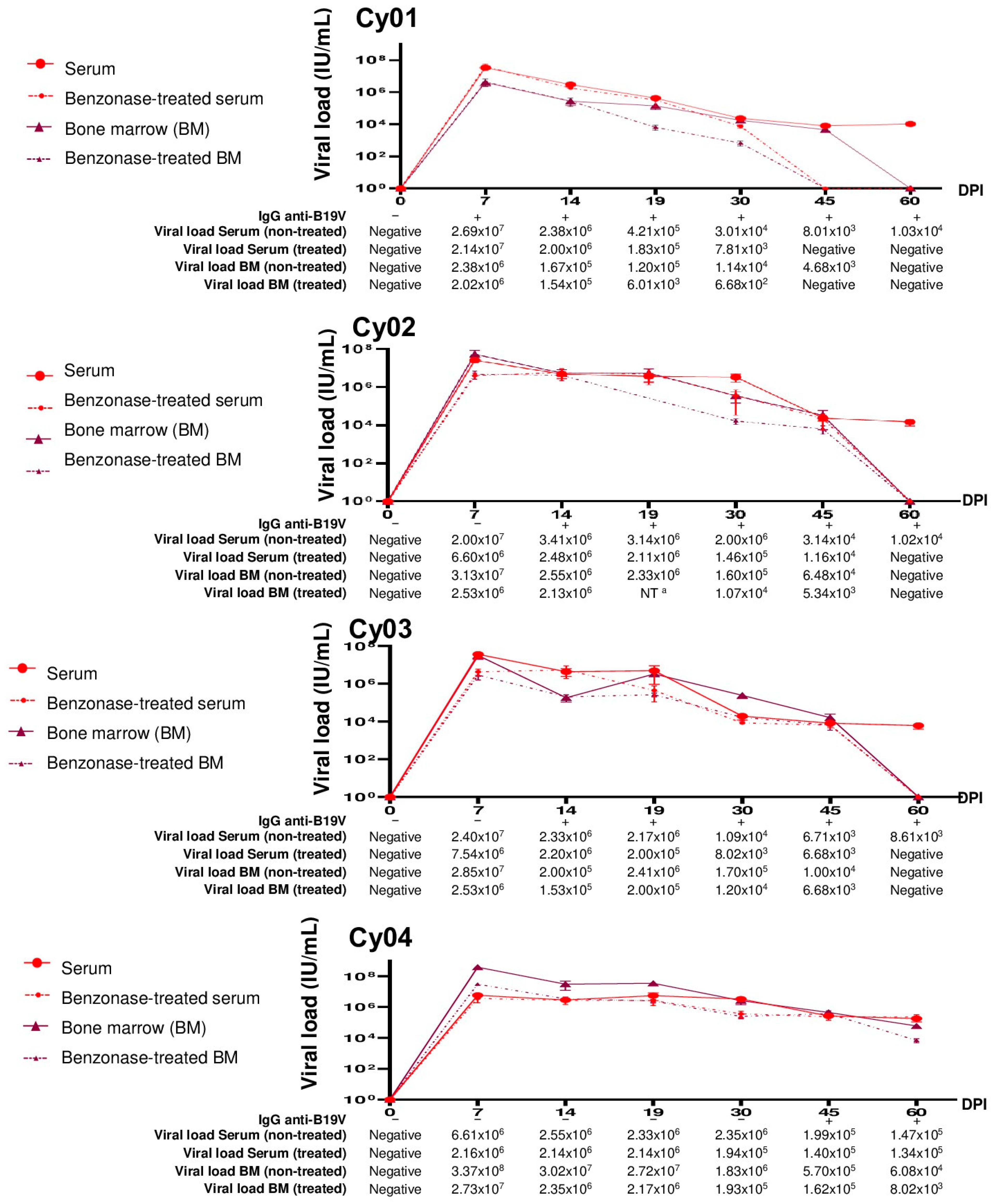
3.3. Evaluation of B19V DNA in Serum and Bone Marrow Samples from Experimentally Infected Cynomolgus Monkeys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Bua, G.; Manaresi, E.; Bonvicini, F.; Gallinella, G. Parvovirus B19 Replication and Expression in Differentiating Erythroid Progenitor Cells. PLoS ONE 2016, 11, e0148547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heegaard, E.D.; Brown, K.E. Human parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J.; Higgins, P.G.; Davis, L.R.; Willman, J.S.; Jones, S.E.; Kidd, I.M.; Pattison, J.R.; Tyrrell, D.A.J. Experimental parvoviral infection in humans. J. Infect. Dis. 1985, 152, 257–265. [Google Scholar] [CrossRef]
- Woolf, A.D.; Campion, G.V.; Chishick, A.; Wise, S.; Cohen, B.J.; Klouda, P.T.; Caul, O.; Dieppe, P.A. Clinical manifestations of human parvovirus B19 in adults. Arch. Intern. Med. 1989, 149, 1153–1156. [Google Scholar] [CrossRef]
- Pattison, J.R. The pathogenesis of diseases associated with B19 virus. Behring Inst. Mitt. 1990, 85, 55–59. [Google Scholar]
- Brown, K.E.; Green, S.W.; Antunez de Mayolo, J.; Young, N.S.; Bellanti, J.A.; Smith, S.D.; Smith, T.J. Congenital anaemia after transplacental B19 parvovirus infection. Lancet 1994, 343, 895–896. [Google Scholar] [CrossRef]
- White, F.V.; Jordan, J.; Dickman, P.S.; Knisely, A.S. Fetal parvovirus B19 infection and liver disease of antenatal onset in an infant with Ebstein’s anomaly. Pediatr. Pathol. Lab. Med. 1995, 15, 121–129. [Google Scholar] [CrossRef]
- Young, N.S.; Brown, K.E. Parvovirus B19. N. Engl. J. Med. 2004, 350, 586–597. [Google Scholar] [CrossRef]
- Cassinotti, P.; Siegl, G. Quantitative evidence for persistence of human parvovirus B19 DNA in an immunocompetent individual. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 886–887. [Google Scholar] [CrossRef] [PubMed]
- Norja, P.; Hokynar, K.; Aaltonen, L.M.; Chen, R.; Ranki, A.; Partio, E.K.; Kiviluoto, O.; Davidkin, I.; Leivo, T.; Eis-Hübinger, A.M.; et al. Bioportfolio: Lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc. Natl. Acad. Sci. USA 2006, 103, 7450–7453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson-Small, L.A.; Ignatovich, I.V.; Laemmerhirt, M.G.; Hobbs, J.A. Persistent parvovirus B19 infection in non-erythroid tissues: Possible role in the inflammatory and disease process. Virus Res. 2014, 190, 8–16. [Google Scholar] [CrossRef]
- Alves, A.D.; Melgaço, J.G.; Cássia Nc Garcia, R.; Raposo, J.V.; de Paula, V.S.; Araújo, C.C.; Pinto, M.A.; Amado, L.A. Persistence of Parvovirus B19 in liver from transplanted patients with acute liver failure. Future Microbiol. 2020, 15, 307–317. [Google Scholar] [CrossRef]
- Söderlund, M.; von Essen, R.; Haapasaari, J.; Kiistala, U.; Kiviluoto, O.; Hedman, K. Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet 1997, 349, 1063–1065. [Google Scholar] [CrossRef]
- Molenaar-de Backer, M.W.; Russcher, A.; Kroes, A.C.; Koppelman, M.H.; Lanfermeijer, M.; Zaaijer, H.L. Detection of parvovirus B19 DNA in blood: Viruses or DNA remnants? J. Clin. Virol. 2016, 84, 19–23. [Google Scholar] [CrossRef]
- Reber, U.; Moser, O.; Dilloo, D.; Eis-Hubinger, A.M. On the utility of the benzonase treatment for correct laboratory diagnosis of parvovirus B19 infection. J. Clin. Virol. 2017, 95, 10–11. [Google Scholar] [CrossRef]
- Leon, L.A.; Marchevsky, R.S.; Gaspar, A.M.; Garcia Rde, C.; Almeida, A.J.; Pelajo-Machado, M.; De Castro, T.X.; Nascimento, J.P.D.; Brown, K.E.; Pinto, M.A. Cynomolgus monkeys (Macaca fascicularis) experimentally infected with B19V and hepatitis A virus: No evidence of the co-infection as a cause of acute liver failure. Mem. Inst. Oswaldo Cruz 2016, 111, 258–266. [Google Scholar] [CrossRef]
- Virion\Serion. SERION ELISA Classic Parvovirus B19 IgG/IgM 2022. Available online: https://www.serion-diagnostics.de/index.php?eID=dumpFile&t=f&f=5684&token=7c18512b5138b8f2e5dec0a78e0f8d4edc94ccd4 (accessed on 30 June 2021).
- Guidance for Industry, Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications, U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research [February 2010]. Available online: https://www.fda.gov/media/78428/download (accessed on 7 April 2022).
- Lyashchenko, K.P.; Singh, M.; Colangeli, R.; Gennaro, M.L. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 2000, 242, 91–100. [Google Scholar] [CrossRef]
- Alves, A.D.R.; Cubel Garcia, R.D.C.N.; Cruz, O.G.; Pinto, M.A.; Amado Leon, L.A. Quantitative real-time PCR for differential diagnostics of parvovirus B19 infection in acute liver failure patients. Expert Rev. Mol. Diagn. 2019, 19, 259–266. [Google Scholar] [CrossRef]
- Juhl, D.; Görg, S.; Hennig, H. Persistence of Parvovirus B19 (B19V) DNA and humoral immune response in B19V-infected blood donors. Vox Sang. 2014, 107, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Curran, M.D.; Moore, J.E.; Coyle, P.V.; Ferguson, W.P. Persistent parvovirus B19 infection. Lancet 1995, 345, 1118. [Google Scholar] [CrossRef]
- Musiani, M.; Zerbini, M.; Gentilomi, G.; Plazzi, M.; Gallinella, G.; Venturoli, S. Parvovirus B19 clearance from peripheral blood after acute infection. J. Infect. Dis. 1995, 172, 1360–1363. [Google Scholar] [CrossRef]
- Gravelsina, S.; Nora-Krukle, Z.; Svirskis, S.; Cunskis, E.; Murovska, M. Presence of B19V in Patients with Thyroid Gland Disorders. Medicina 2019, 55, 774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santonja, C.; Santos-Briz, A.; Palmedo, G.; Kutzner, H.; Requena, L. Detection of human parvovirus B19 DNA in 22% of 1815 cutaneous biopsies of a wide variety of dermatological conditions suggests viral persistence after primary infection and casts doubts on its pathogenic significance. Br. J. Dermatol. 2017, 177, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Elsanhoury, A.; Klein, O.; Sosnowski, M.; Miteva, K.; Lassner, D.; Abou-El-Enein, M.; Pieske, B.; Kühl, U.; Tschöpe, C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Heart Fail. 2018, 5, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Maple, P.A.; Hedman, L.; Dhanilall, P.; Kantola, K.; Nurmi, V.; Söderlund-Venermo, M.; Brown, K.E.; Hedman, K. Identification of past and recent parvovirus B19 infection in immunocompetent individuals by quantitative PCR and enzyme immunoassays: A dual-laboratory study. J. Clin. Microbiol. 2014, 52, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Bredl, S.; Plentz, A.; Wenzel, J.J.; Pfister, H.; Möst, J.; Modrow, S. False-negative serology in patients with acute parvovirus B19 infection. J. Clin. Virol. 2011, 51, 115–120. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph.Eur.). B19 VIRUS DNA for NAT Testing BRP (Y0000285); European Pharmacopoeia: Strasbourg, France, 2019; p. 3. [Google Scholar]
- Food and Drug Administration (FDA). Nucleic Acid Testing (NAT) to Reduce the Possible Risk of Human Parvovirus B19 Transmission by Plasma-Derived Products (HFM-40); Outreach and Development (OCOD) (HFM-40): Rockville, MD, USA, 2009; p. 7.
- Lefrère, J.J.; Maniez-Montreuil, M.; Morel, P.; Defer, C.; Laperche, S. Safety of blood products and B19 parvovirus. Transfus. Clin. Biol. 2006, 13, 235–241. [Google Scholar] [CrossRef]
- Lefrère, J.J.; Servant-Delmas, A.; Candotti, D.; Mariotti, M.; Thomas, I.; Brossard, Y.; Lefreère, F.; Girot, R.; Allain, J.-P.; Laperche, S. Persistent B19 infection in immunocompetent individuals: Implications for transfusion safety. Blood 2005, 106, 2890–2895. [Google Scholar] [CrossRef]
- Bonvicini, F.; Manaresi, E.; Di Furio, F.; De Falco, L.; Gallinella, G. Parvovirus b19 DNA CpG dinucleotide methylation and epigenetic regulation of viral expression. PLoS ONE 2012, 7, e33316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcioli, F.; Zakrzewska, K.; Rinieri, A.; Fanci, R.; Innocenti, M.; Civinini, R.; De Giorgi, V.; Di Lollo, S.; Azzi, A. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J. Med. Virol. 2008, 80, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Schenk, T.; Enders, M.; Pollak, S.; Hahn, R.; Huzly, D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J. Clin. Microbiol. 2009, 47, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganaie, S.S.; Qiu, J. Recent Advances in Replication and Infection of Human Parvovirus B19. Front. Cell. Infect. Microbiol. 2018, 8, 166. [Google Scholar] [CrossRef] [Green Version]

| ID | Serum (IU/mL) | DNA a (IU/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nontreated (Mean ± SD) | 0.1 (Mean ± SD) | 0.5 (Mean ± SD) | 1.0 (Mean ± SD) | 1.5 (Mean ± SD) | 2.0 (Mean ± SD) | 2.5 (Mean ± SD) | 0.1 (Mean ± SD) | 0.5 (Mean ± SD) | 1.0 (Mean ± SD) | |
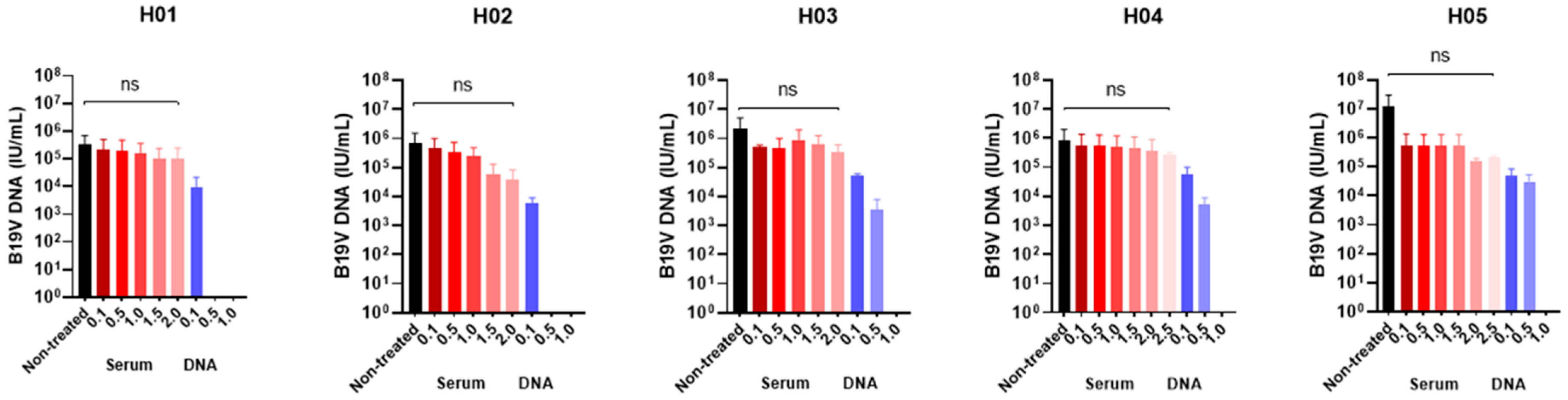
| H01 | 3.5 × 105 ± 3.3 × 105 | 2.1 × 105 ± 2.0 × 105 | 1.9 × 105 ± 1.9 × 105 | 1.5 × 105 ± 1.5 × 105 | 1.0 × 105 ± 1.4 × 105 | 1.1 × 105 ± 1.4 × 105 | NT | 9.4 × 103 ± 8.8 × 103 | Negative | Negative |
| H02 | 6.6 × 106 ± 5.7 × 106 | 4.6 × 105 ± 3.8 × 105 | 3.4 × 105 ± 2.7 × 105 | 2.4 × 105 ± 1.9 × 105 | 5.6 × 104 ± 6.2 × 104 | 3.9 × 104 ± 3.4 × 104 | NT | 6.1 × 103 ± 2.1 × 103 | Negative | Negative |
| H03 | 2.2 × 106 ± 2.0 × 106 | 4.9 × 105 ± 8.9 × 104 | 4.8 × 105 ± 3.6 × 105 | 8.9 × 105 ± 7.7 × 105 | 6.4 × 105 ± 4.8 × 105 | 3.3 × 105 ± 2.1 × 105 | NT | 5.1 × 104 ± 6.4 × 103 | 3.4 × 103 ± 3.2 × 103 | Negative |
| H04 | 8.6 × 105 ± 8.4 × 105 | 5.8 × 105 ± 6.5 × 105 | 5.4 × 105 ± 6.1 × 105 | 4.9 × 105 ± 5.7 × 105 | 4.7 × 105 ± 5.2 × 105 | 3.8 × 105 ± 4.3 × 105 | 2.1 × 105 ± 4.8 × 104 | 5.6 × 104 ± 3.4 × 104 | 5.2 × 103 ± 2.6 × 103 | Negative |
| H05 | 1.3 × 107 ± 1.7 × 107 | 5.4 × 106 ± 6.7 × 106 | 5.6 × 105 ± 5.9 × 105 | 5.4 × 105 ± 6.0 × 105 | 5.2 × 105 ± 6.2 × 105 | 1.5 × 105 ± 3.1 × 104 | 1.1 × 105 ± 1.0 × 104 | 4.9 × 104 ± 2.9 × 104 | 3.1 × 104 ± 1.8 × 104 | Negative |
| ID | Sera Collected at 0 dpa | Sera Collected at 30 dpa | |||||
|---|---|---|---|---|---|---|---|
| B19V Load (IU/mL) (Mean ± SD) | B19V Load Treated (IU/mL) (Mean ± SD) | p-Value | B19V Load (IU/mL) (Mean ± SD) | B19V Load Treated (IU/mL) (Mean ± SD) | p-Value | ||
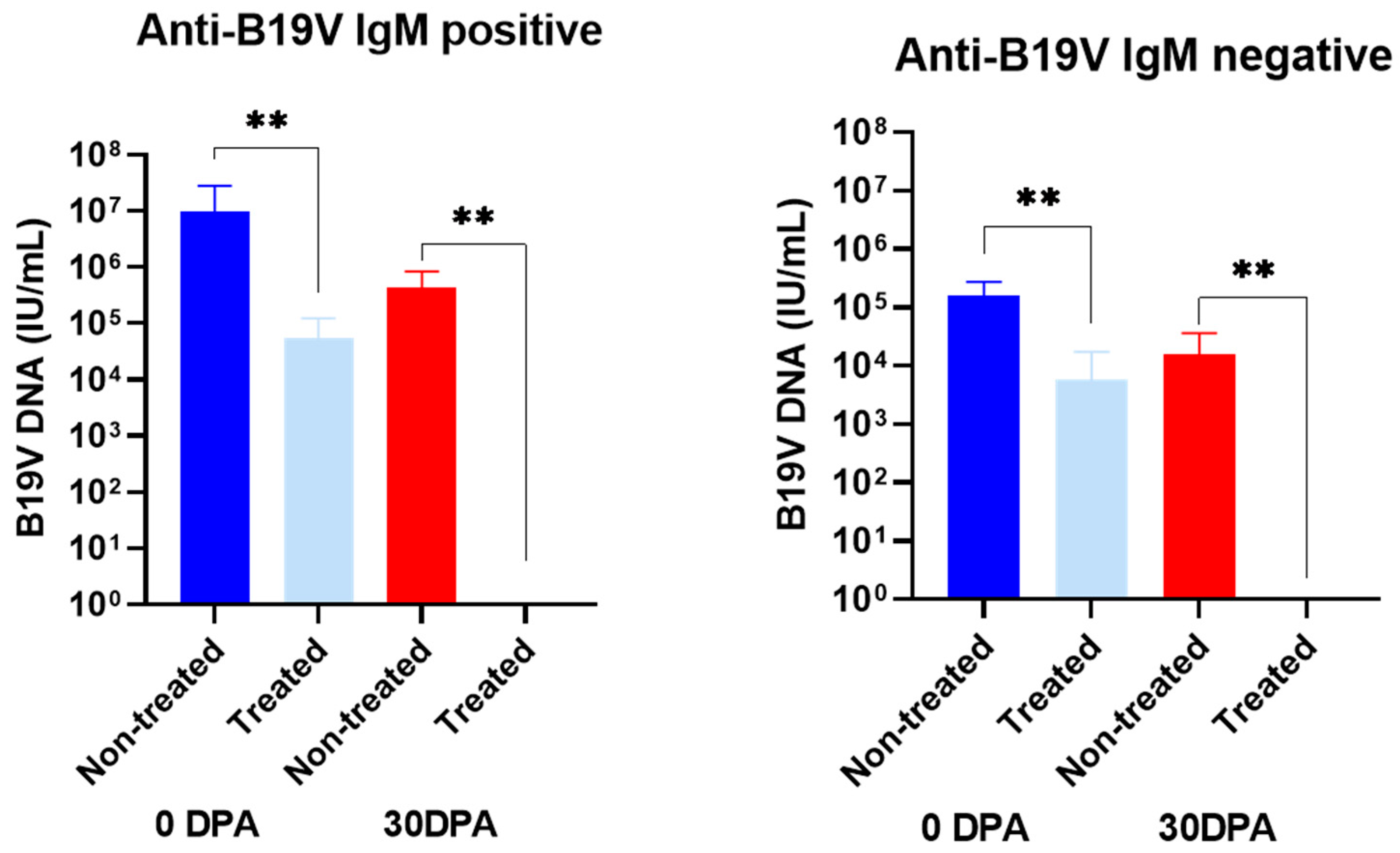
| Anti-B19V IgM positive | H06 | 9.3 × 105 ± 8.7 × 105 | 1.6 × 104 ± 1.4 × 104 | <0.01 | 1.7 × 105 ± 1.4 × 105 | Negative | <0.01 |
| H07 | 4.3 × 107 ± 3.1 × 107 | 6.4 × 104 ± 4.1 × 104 | 2.1 × 105 ± 1.9 × 105 | Negative | |||
| H08 | 1.9 × 106 ± 2.3 × 106 | 2.4 × 104 ± 1.9 × 104 | 9.0 × 105 ± 7.8 × 105 | Negative | |||
| H09 | 1.7 × 106 ± 1.5 × 106 | Negative | 8.4 × 105 ± 8.2 × 105 | Negative | |||
| H10 | 2.3 × 105 ± 1.7 × 105 | 1.7 × 105 ± 1.6 × 105 | 2.2 × 103 ± 1.6 × 103 | Negative | |||
| Anti-B19V IgM negative | H11 | 1.9 × 105 ± 2.7 × 105 | Negative | <0.01 | Negative | Negative | <0.01 |
| H12 | 1.8 × 105 ± 1.3 × 105 | 1.8 × 105 ± 1.7 × 105 | 3.7 × 104 ± 2.9 × 104 | Negative | |||
| H13 | 3.3 × 105 ± 8.4 × 105 | 1.8 × 105 ± 1.7 × 105 | Negative | Negative | |||
| H14 | 4.6 × 104 ± 2.5 × 104 | Negative | Negative | Negative | |||
| H15 | 5.7 × 104 ± 4.9 × 104 | Negative | 4.0 × 104 ± 3.9 × 104 | Negative | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.D.R.; Langella, B.B.; Lima, M.M.d.S.; Coelho, W.L.d.C.N.P.; Cubel Garcia, R.d.C.N.; Cardoso, C.A.A.; Marchevsky, R.S.; Pinto, M.A.; Amado, L.A. Evaluation of Molecular Test for the Discrimination of “Naked” DNA from Infectious Parvovirus B19 Particles in Serum and Bone Marrow Samples. Viruses 2022, 14, 843. https://doi.org/10.3390/v14040843
Alves ADR, Langella BB, Lima MMdS, Coelho WLdCNP, Cubel Garcia RdCN, Cardoso CAA, Marchevsky RS, Pinto MA, Amado LA. Evaluation of Molecular Test for the Discrimination of “Naked” DNA from Infectious Parvovirus B19 Particles in Serum and Bone Marrow Samples. Viruses. 2022; 14(4):843. https://doi.org/10.3390/v14040843
Chicago/Turabian StyleAlves, Arthur Daniel Rocha, Barbara Barbosa Langella, Mariana Magaldi de Souza Lima, Wagner Luís da Costa Nunes Pimentel Coelho, Rita de Cássia Nasser Cubel Garcia, Claudete Aparecida Araújo Cardoso, Renato Sergio Marchevsky, Marcelo Alves Pinto, and Luciane Almeida Amado. 2022. "Evaluation of Molecular Test for the Discrimination of “Naked” DNA from Infectious Parvovirus B19 Particles in Serum and Bone Marrow Samples" Viruses 14, no. 4: 843. https://doi.org/10.3390/v14040843
APA StyleAlves, A. D. R., Langella, B. B., Lima, M. M. d. S., Coelho, W. L. d. C. N. P., Cubel Garcia, R. d. C. N., Cardoso, C. A. A., Marchevsky, R. S., Pinto, M. A., & Amado, L. A. (2022). Evaluation of Molecular Test for the Discrimination of “Naked” DNA from Infectious Parvovirus B19 Particles in Serum and Bone Marrow Samples. Viruses, 14(4), 843. https://doi.org/10.3390/v14040843






