Protection of Ducklings from Duck Hepatitis A Virus Infection with ELPylated Duck Interferon-α
Abstract
:1. Introduction
2. Materials and Methods
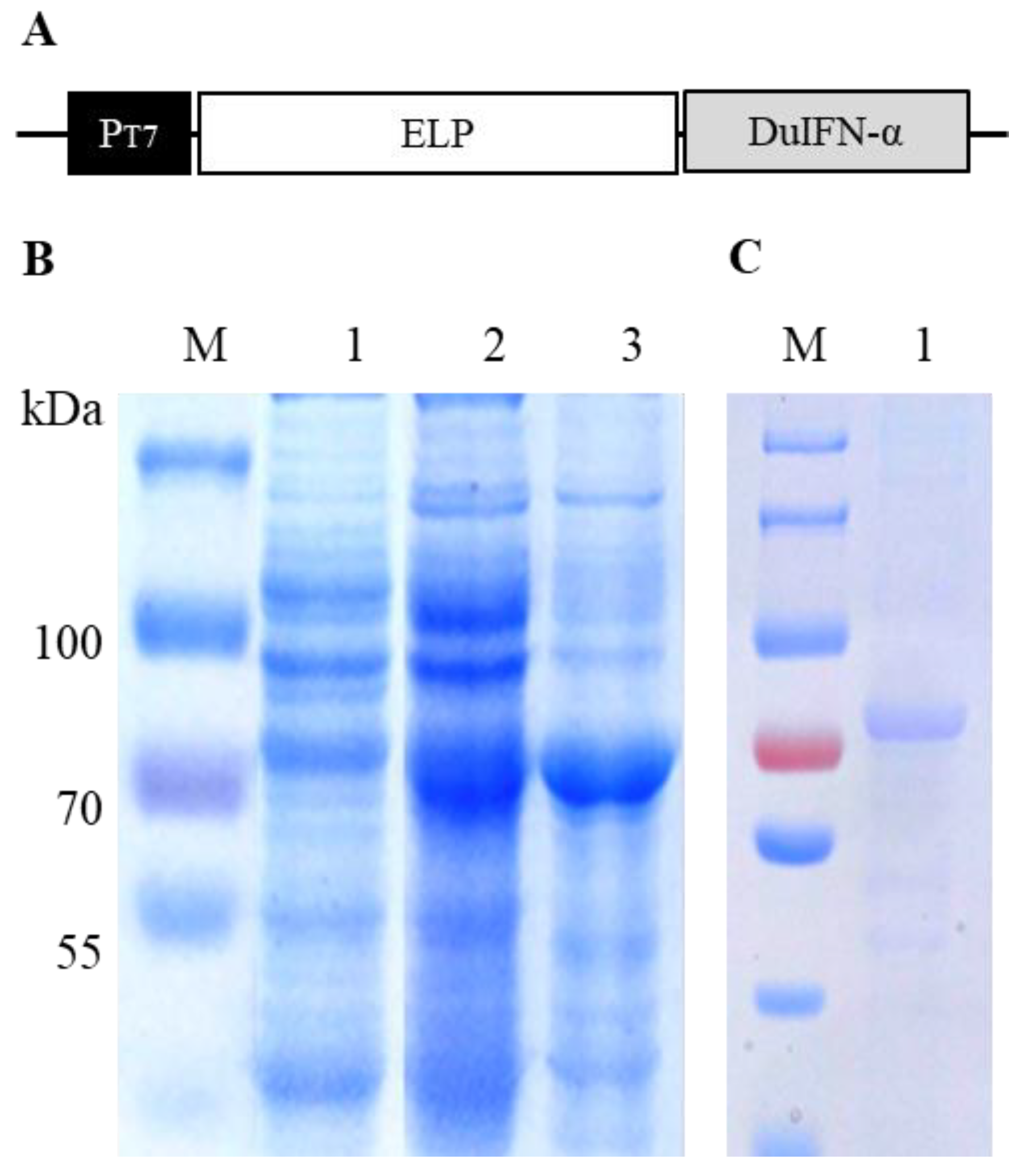
2.1. Protein Expression
2.2. Protein Purification
2.3. Cytotoxicity Assay
2.4. Virus Proliferation
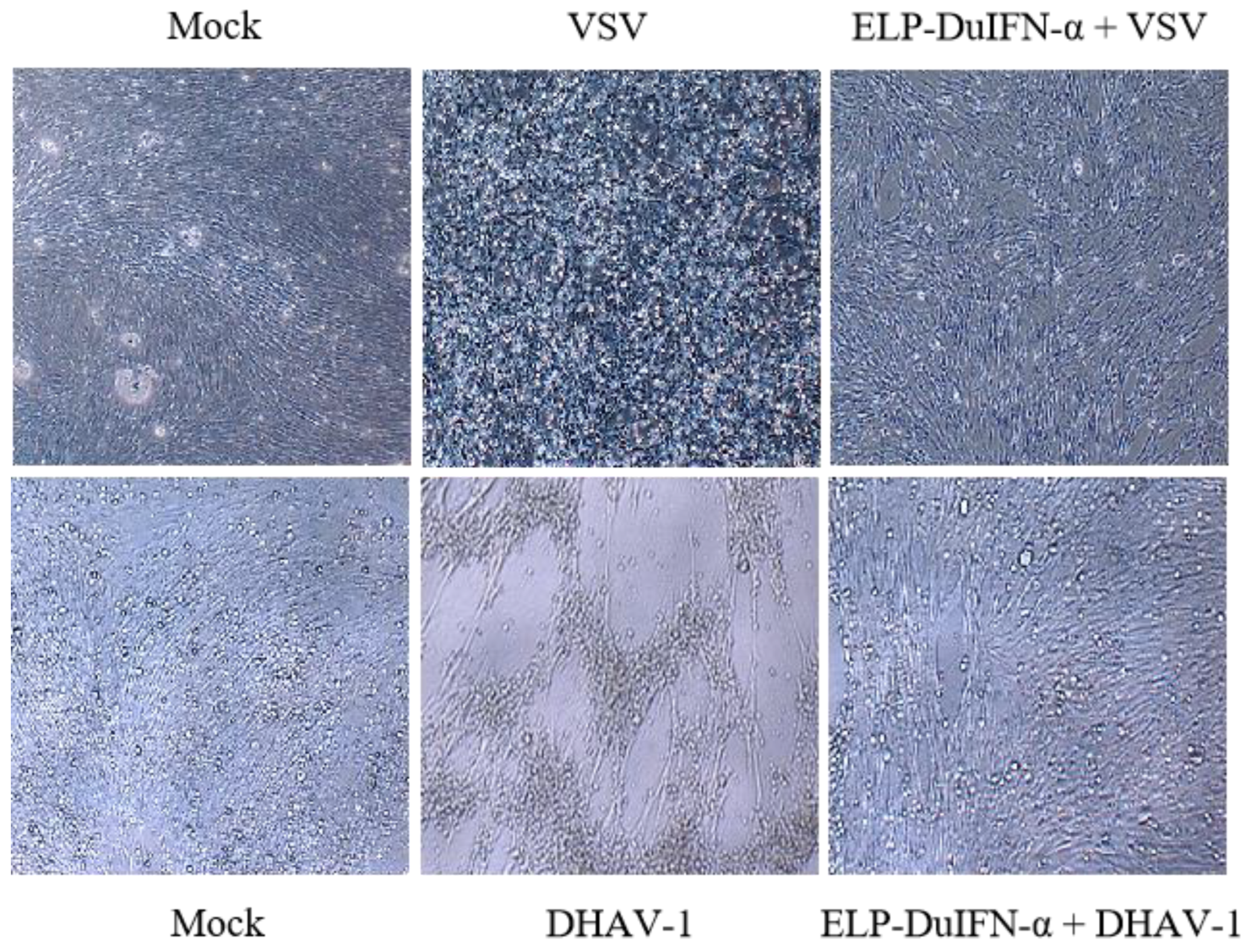
2.5. In Vitro Antiviral Assay
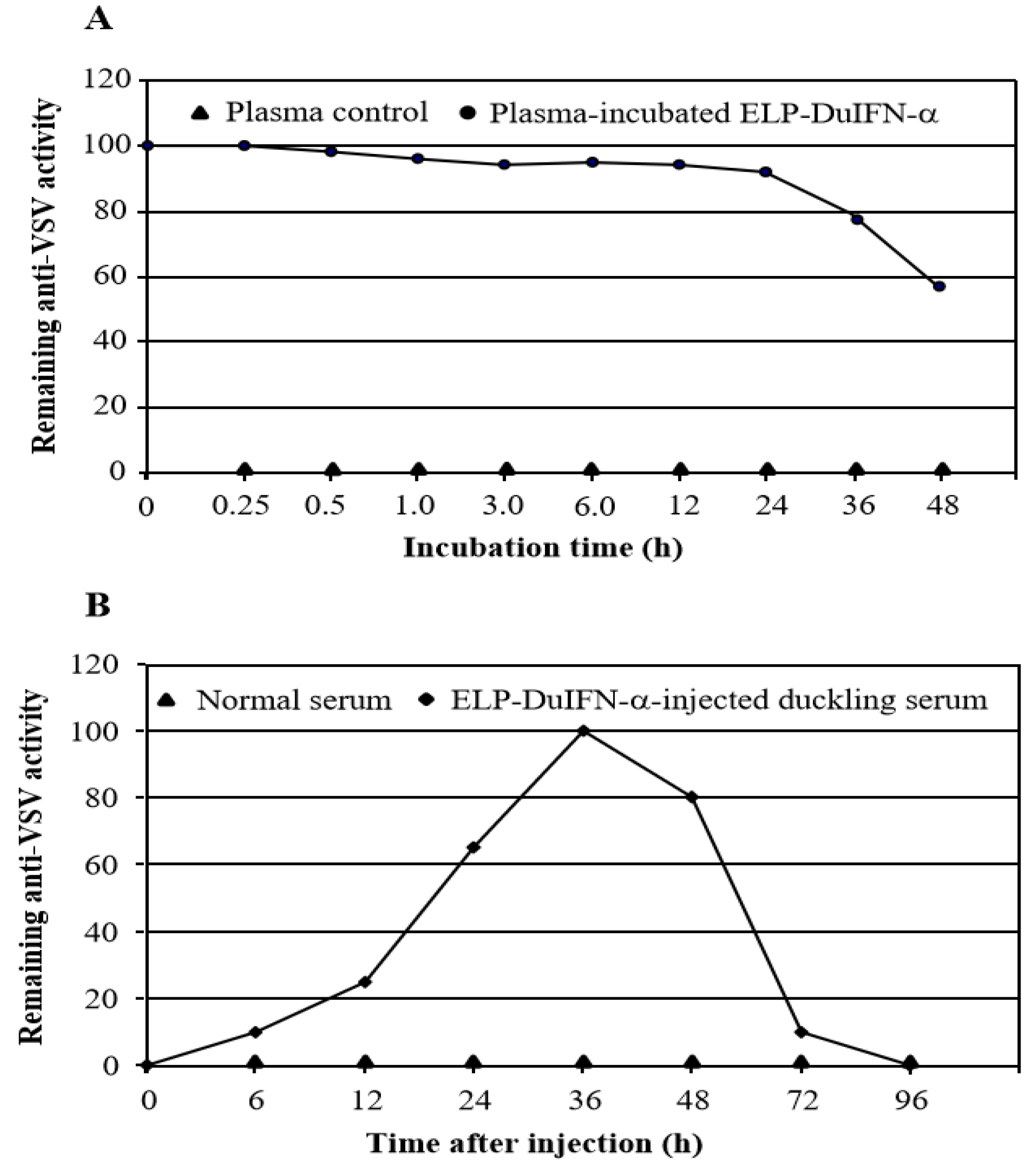
2.6. Plasma Stability Assay
2.7. Serum Half-Life Assay
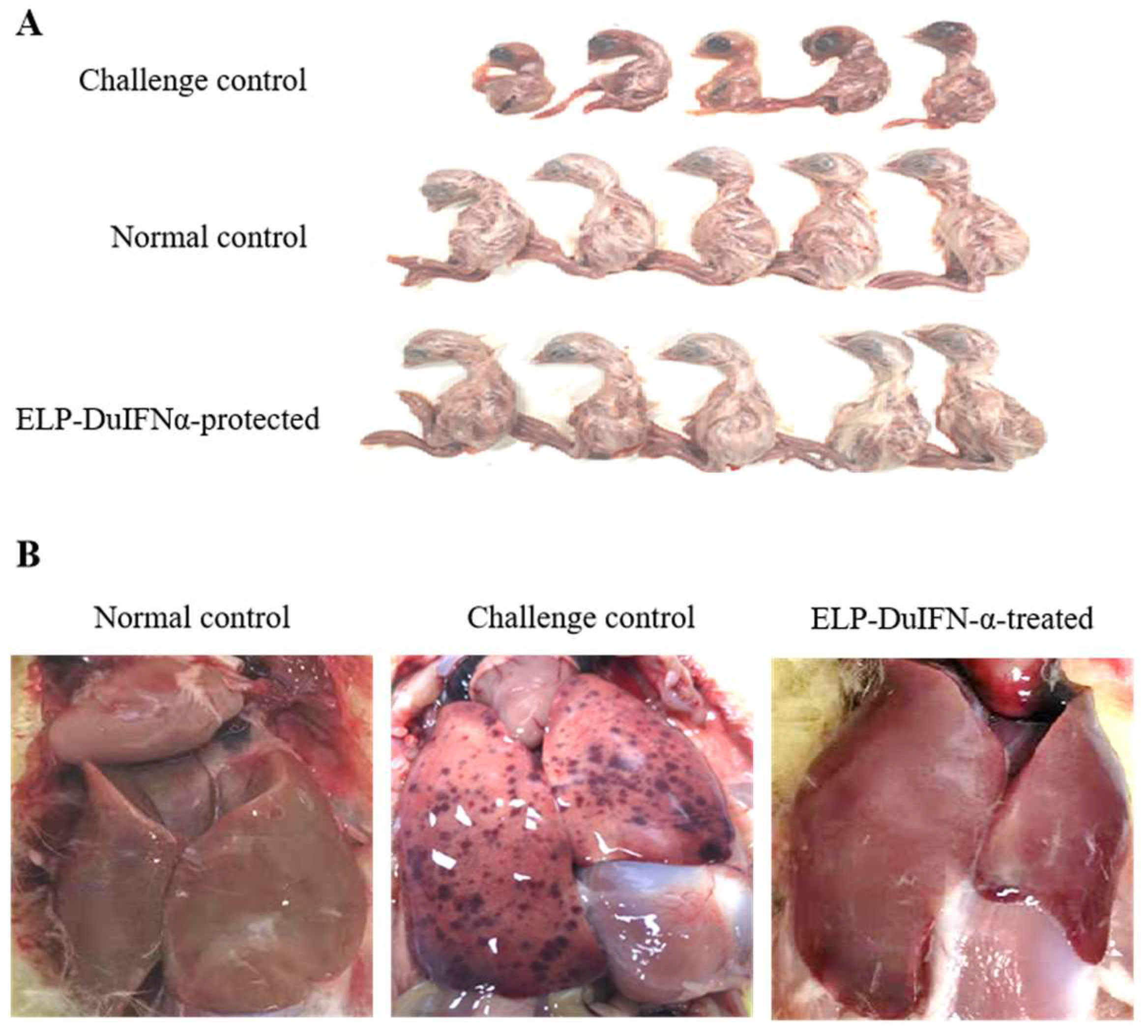
2.8. Virus Infection Protection Assay in Embryos
2.9. Virus Infection Protection Assay in Ducklings
2.10. Interferon Stimulated Genes Assay in Experimental Livers
3. Results
3.1. ELP-DuIFN-α Expression and Purification
3.2. Cytotoxicity of ELP-DuIFN-α
3.3. In Vitro Antiviral Activities of ELP-DuIFN-α
3.4. Plasma and Serum Half-Lives of ELP-DuIFN-α
3.5. Protection of Embryos from DHAV-1 Infection with ELP-DuIFN-α
3.6. Protection of Ducklings from DHAV-1 Infection with ELP-DuIFN-α
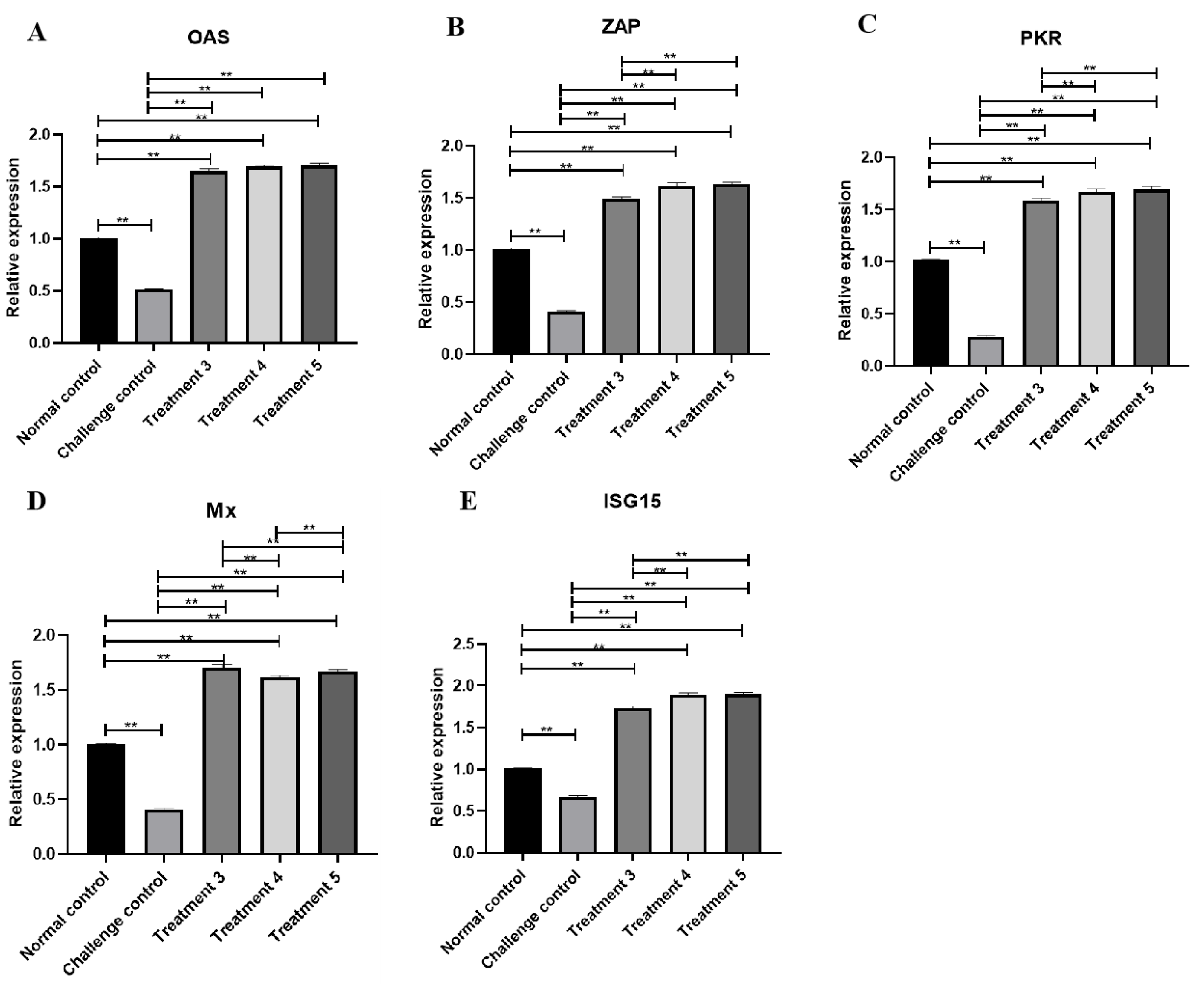
3.7. ISGs Transcription Level Changes in Livers with ELP-DuIFN-α
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Cheng, A.; Wang, M.; Chen, S.; Jia, R.; Zhu, D.; Liu, M.; Sun, K.; Yang, Q.; Chen, X. Development of an indirect ELISA method based on the VP3 protein of duck hepatitis A virus type 1 (DHAV-1) for dual detection of DHAV-1 and DHAV-3 antibodies. J. Virol. Methods 2015, 225, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Yugo, D.M.; Hauck, R.; Shivaprasad, H.L.; Meng, X.-J. Hepatitis Virus Infections in Poultry. Avian Dis. 2016, 60, 576–588. [Google Scholar] [CrossRef]
- Chen, L.-L.; Xu, Q.; Zhang, R.-H.; Yang, L.; Li, J.-X.; Xie, Z.-J.; Zhu, Y.-L.; Jiang, S.-J.; Si, X.-K. Improved duplex RT-PCR assay for differential diagnosis of mixed infection of duck hepatitis A virus type 1 and type 3 in ducklings. J. Virol. Methods 2013, 192, 12–17. [Google Scholar] [CrossRef]
- Lin, S.-L.; Cong, R.-C.; Zhang, R.-H.; Chen, J.-H.; Xia, L.-L.; Xie, Z.-J.; Wang, Y.; Zhu, Y.-L.; Jiang, S.-J. Circulation and in vivo distribution of duck hepatitis A virus types 1 and 3 in infected ducklings. Arch. Virol. 2015, 161, 405–416. [Google Scholar] [CrossRef]
- Soliman, M.; Alfajaro, M.M.; Lee, M.-H.; Jeong, Y.-J.; Kim, D.-S.; Deok-Song, K.; Kwon, J.; Choi, J.-S.; Lim, J.-S.; Lee, T.-U.; et al. The prevalence of duck hepatitis A virus types 1 and 3 on Korean duck farms. Arch. Virol. 2014, 160, 493–498. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, M.J.; Kwon, Y.K.; Lindberg, A.M.; Joh, S.J.; Kwon, H.M.; Li, Y.J.; Kwon, J.H. Development of duck hepatitis A virus type 3 vaccine and its use to protect ducklings against infections. Vaccine 2009, 27, 6688–6694. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bai, J.; Wang, J.; He, M.; Xiong, W.; Yuan, W.; Qiao, M.; Ming, K.; Wu, Y.; Wang, D.; et al. Assessment of the hepatocyte protective effects of gypenoside and its phosphorylated derivative against DHAV-1 infection on duck embryonic hepatocytes. BMC Veter. Res. 2019, 15, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, R.; Buronfosse, T.; Schultz, U.; Chevallier-Gueyron, P.; Guerret, S.; Chevallier, M.; Saade, F.; Ndeboko, B.; Trepo, C.; Zoulim, F.; et al. Rise in gamma interferon expression during resolution of duck hepatitis B virus infection. J. Gen. Virol. 2006, 87, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. New characteristics and prevention strategies of duck disease. J. Anim. Husb. Vet. Med. 2018, 39, 30–32. [Google Scholar]
- Chun, X.; Jianchun, W.; Zhiguang, W.; Ming, W.; Yupu, G. Molecular cloning and sequence analysis of the type-I IFN from Beijing duck. Acta. Vet. Zoot. Sina. 2000, 6, 567–570. [Google Scholar]
- Gao, P.; Xiang, B.; Li, Y.; Li, Y.; Sun, M.; Kang, Y.; Xie, P.; Chen, L.; Lin, Q.; Liao, M.; et al. Therapeutic Effect of Duck Interferon-Alpha Against H5N1 Highly Pathogenic Avian Influenza Virus Infection in Peking Ducks. J. Interf. Cytokine Res. 2018, 38, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.F.; Lin, C.Y.; Yang, T.Y.; Shi, X.J.; Xia, C. Molecular cloning and expression of Peking duck IFN-α gene. Chin. J. Vet. Med. 2004, 12, 11–13. [Google Scholar]
- Schultz, U.; Summers, J.; Staeheli, P.; Chisari, F.V. Elimination of Duck Hepatitis B Virus RNA-Containing Capsids in Duck Interferon-Alpha-Treated Hepatocytes. J. Virol. 1999, 73, 5459–5465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Chen, J.; Zhang, J.; Yang, Y.; Li, P.; Lan, J.; Xie, Z.; Jiang, S. Novel duck hepatitis A virus type 1 isolates from adult ducks showing egg drop syndrome. Veter. Microbiol. 2018, 221, 33–37. [Google Scholar] [CrossRef]
- Tian, S.; Xu, C.; Yao, W.B. Research progress of long acting interferon. China Biotech. 2010, 30, 122–127. [Google Scholar]
- Cheng, T.Y.; Jin, W.W.; Gao, X.D. Progress of Modifying Interferon by Protein Engineering. World Notes Antibiot. 2011, 32, 156–160. [Google Scholar]
- Meyer, D.E.; Chilkoti, A. Genetically Encoded Synthesis of Protein-Based Polymers with Precisely Specified Molecular Weight and Sequence by Recursive Directional Ligation: Examples from the Elastin-like Polypeptide System. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 1992, 57, 23–57. [Google Scholar] [CrossRef]
- Mackay, J.A.; Chilkoti, A. Temperature sensitive peptides: Engineering hyperthermia-directed therapeutics. Int. J. Hyperth. 2008, 24, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Wang, G.; Liu, X.; Gao, W. Enhancing Pharmacokinetics, Tumor Accumulation, and Antitumor Efficacy by Elastin-Like Polypeptide Fusion of Interferon Alpha. Adv. Mater. 2015, 27, 7320–7324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, X.; Zong, Y.; Lu, H.; Zhang, X.; Xia, X.; Sun, H. Enhancing purification and plasma stability of porcine interferon-α/γ by fusion to elastin-like polypeptide. Veter. Immunol. Immunopathol. 2018, 203, 60–64. [Google Scholar] [CrossRef]
- Liu, W.J.; Wu, Q.; Xu, B.; Zhang, X.Y.; Xia, X.L.; Sun, H.C. Single-step purification of recombinant proteins using elastin-like peptide-mediated inverse transition cycling and self-processing module from Neisseria meningitides FrpC. Protein Expr. Purif. 2014, 98, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Grote, A.; Hiller, K.; Scheer, M.; Münch, R.; Nörtemann, B.; Hempel, D.C.; Jahn, D. JCat: A novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005, 33, 526–531. [Google Scholar] [CrossRef]
- Osborn, B.L.; Olsen, H.S.; Nardelli, B.; Murray, J.H.; Zhou, J.X.H.; Garcia, A.; Moody, G.; Zaritskaya, L.S.; Sung, C. Pharmacokinetic and Pharmacodynamic Studies of a Human Serum Albumin-Interferon-α Fusion Protein in Cynomolgus Monkeys. J. Pharmacol. Exp. Ther. 2002, 303, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.P.; Liu, Y.; Luo, F.; Mei, X.G. Investigation on tolerability and safety of interferonα-2b dry powder inhalers in rats. Pharm. J. Chin. PLA 2010, 26, 393–395. [Google Scholar]
- Wang, Y.; Zhu, S.; Hong, W.; Wang, A.; Zuo, W. A multiplex PCR for detection of six viruses in ducks. J. Virol. Methods 2017, 248, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, Q.; Yao, W.; Xu, C. Construction and characterization of a potent, long-lasting recombinant human serum albumin-interferon α1 fusion protein expressed in Pichia Pastor. Protein Expr. Purif. 2013, 90, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Gaberc-Porekar, V.; Zore, I.; Podobnik, B.; Menart, V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr. Opin. Drug Discov. Dev. 2008, 11, 242–250. [Google Scholar]
- Li, Y. Self-cleaving fusion tags for recombinant protein production. Biotechnol. Lett. 2011, 33, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.M.; Beilharz, M.W.; Krakowka, S. Oral use of interferon. J. Interferon Cytokine Res. 1999, 19, 853–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Yang, Y.; Lan, J.; Xie, Z.; Zhang, X.; Jiang, S. Evidence of possible vertical transmission of duck hepatitis A virus type 1 in ducks. Transbound. Emerg. Dis. 2020, 68, 267–275. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence (5′-3′) |
|---|---|
| GAPDH | F:GCACTGTCAAGGCTGAGAACG R:GATGATAACACGCTTAGCACCAC |
| OAS | F:GCGGTGAAGCAGACGGTGAA R:CGATGATGGCGAGGATGTG |
| Mx | F:AACGCTGCTCAGGTCAGAAT R:GTGAAGCACATCCAAAAGCA |
| PKR | F:CCTCTGCTGGCCTTACTGTCA R:AAGAGAGGCAGAAGGAATAATTTGCC |
| ZAP | F:ATCGCTTTACCTTTCCTTG R:GTGCCATCGTATCATCTTCA |
| ISG15 | F:TCGCAGCAGCTCCTATGAGGTC R:GCCAGAACTGGTCCGCTTGC |
| ELP-DuIFNα (µg/mL) | Growth Inhibition (%) | |
|---|---|---|
| MDCK Cells | DEF Cells | |
| 200 | 0 | 0 |
| 20 | 0 | 0 |
| 2 | 0 | 0 |
| 2 × 10−1 | 0 | 0 |
| 2 × 10−2 | 0 | 0 |
| 2 × 10−3 | 0 | 0 |
| 2 × 10−4 | 0 | 0 |
| 2 × 10−5 | 0 | 0 |
| 2 × 10−6 | 0 | 0 |
| 2 × 10−7 | 0 | 0 |
| Testing System | Antiviral Activity (IU/mg Protein) |
|---|---|
| MDCK-VSV | 1.25 × 106 |
| DEF-VSV | 1.25 × 107 |
| DEF-DHAV-1 | 6.0 × 104 |
| Group | No. of Duck Embryos | Death of Duck Embryos | Protection (%) |
|---|---|---|---|
| Normal control | 5 | 0 | |
| Challenge control | 5 | 5 | 0 |
| Treatment 1 | 5 | 0 | 100 |
| Treatment 2 | 5 | 0 | 100 |
| Treatment 3 | 5 | 1 | 80 |
| Group | No. of Ducklings | Route of Treatment | Time of Treatment | Death of Ducklings | Protection Rate (%) |
|---|---|---|---|---|---|
| Normal control | 5 | 0 | |||
| Challenge control | 5 | 5 | |||
| Treatment 1 | 5 | Intramuscular | Co-infection | 2 | 60 |
| Treatment 2 | 5 | Oral | Co-infection | 1 | 80 |
| Treatment 3 | 5 | Oral | Pre-infection | 2 | 60 |
| Treatment 4 | 5 | Oral | Co-infection | 1 | 80 |
| Treatment 5 | 5 | Oral | Post-infection | 1 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Guo, Y.; Wang, H.; Wu, Z.; Hong, W.; Sun, H.; Zhu, S. Protection of Ducklings from Duck Hepatitis A Virus Infection with ELPylated Duck Interferon-α. Viruses 2022, 14, 633. https://doi.org/10.3390/v14030633
Wang Y, Guo Y, Wang H, Wu Z, Hong W, Sun H, Zhu S. Protection of Ducklings from Duck Hepatitis A Virus Infection with ELPylated Duck Interferon-α. Viruses. 2022; 14(3):633. https://doi.org/10.3390/v14030633
Chicago/Turabian StyleWang, Yongjuan, Yanli Guo, Haowei Wang, Zhi Wu, Weiming Hong, Huaichang Sun, and Shanyuan Zhu. 2022. "Protection of Ducklings from Duck Hepatitis A Virus Infection with ELPylated Duck Interferon-α" Viruses 14, no. 3: 633. https://doi.org/10.3390/v14030633
APA StyleWang, Y., Guo, Y., Wang, H., Wu, Z., Hong, W., Sun, H., & Zhu, S. (2022). Protection of Ducklings from Duck Hepatitis A Virus Infection with ELPylated Duck Interferon-α. Viruses, 14(3), 633. https://doi.org/10.3390/v14030633





