Tripartite-Motif 21 (TRIM21) Deficiency Results in a Modest Loss of Herpes Simplex Virus (HSV)-1 Surveillance in the Trigeminal Ganglia Following Cornea Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Infection
2.2. Virus and Plaque Assay
2.3. Real Time Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
2.4. Spectral Domain-Optical Coherence Tomography (SD-OCT)
2.5. Corneal Sensitivity
2.6. Cornea Pathology
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
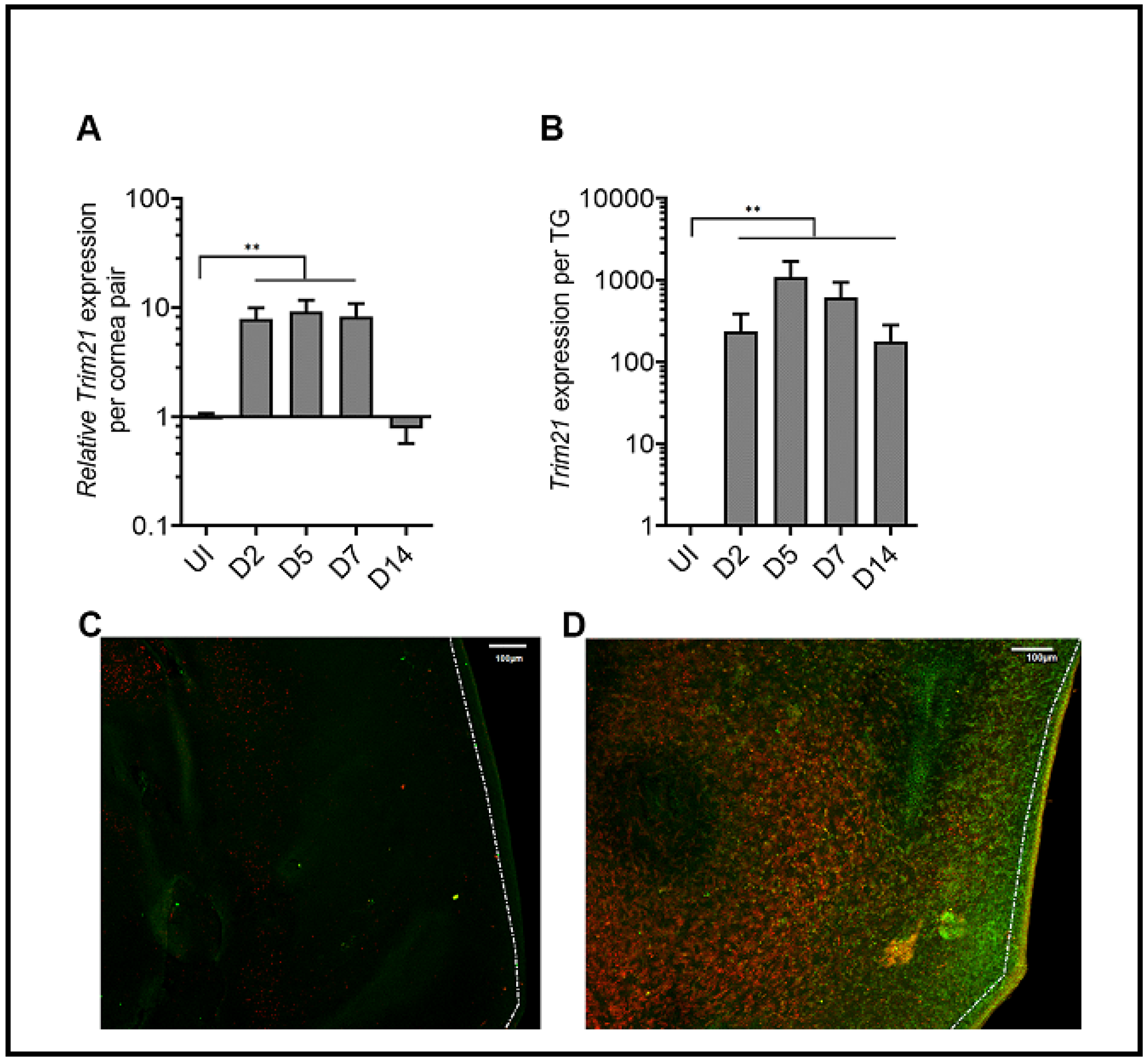
3.1. HSV-1 Infection Induces TRIM21 Expression in the Cornea
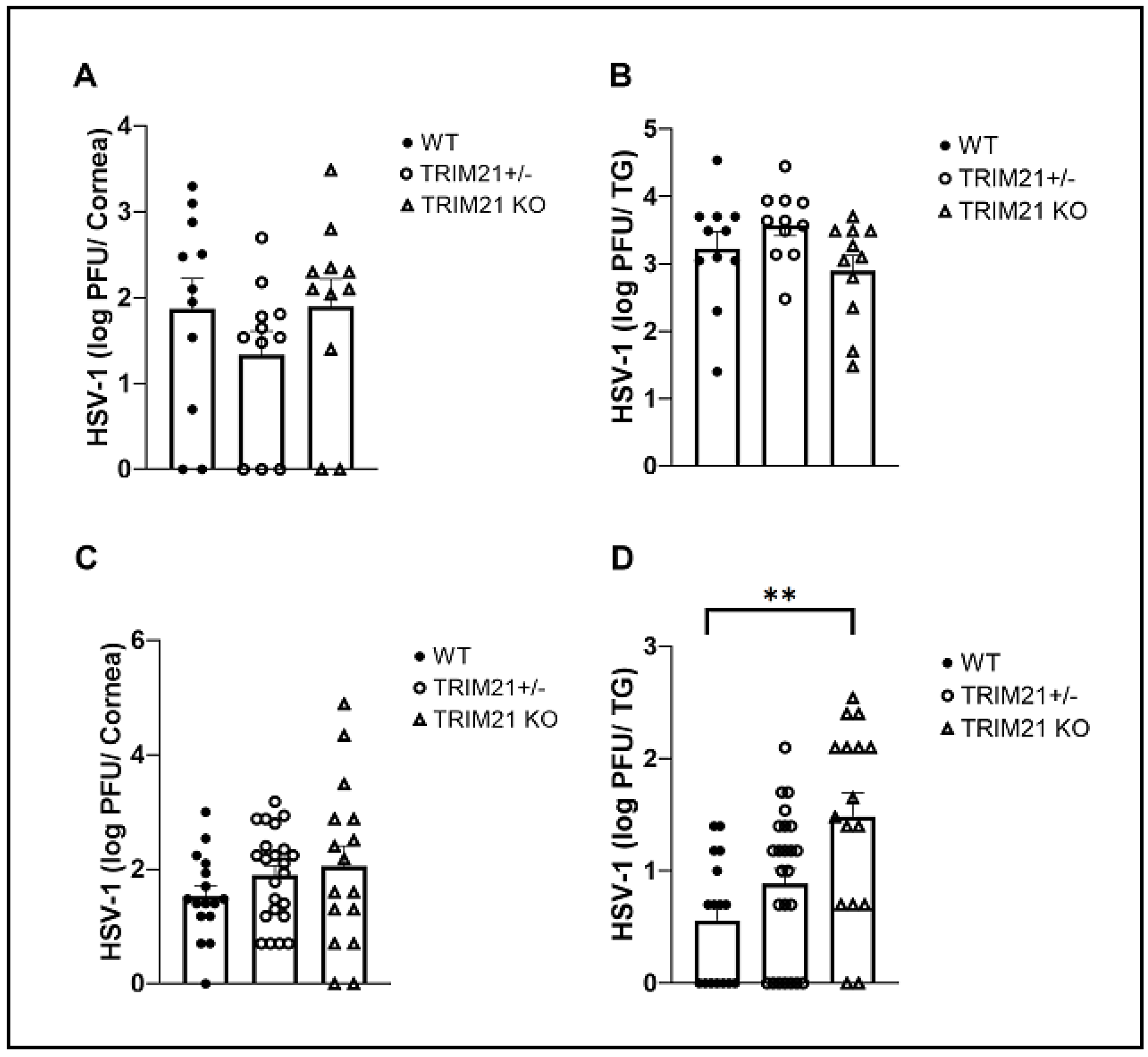
3.2. The Absence of TRIM21 Is Reflected in a Loss of Virus Surveillance in the TG, but Not in Cornea of HSV-1-Infected Mice
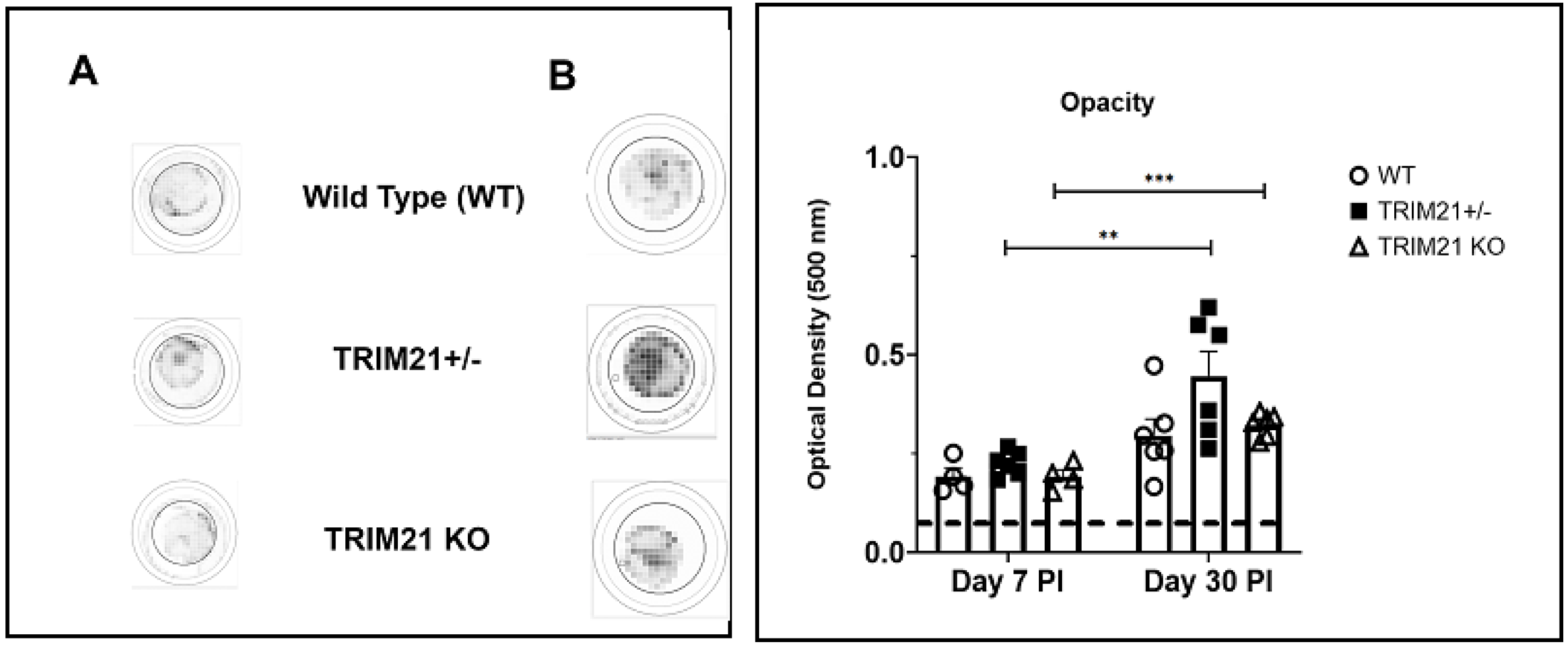
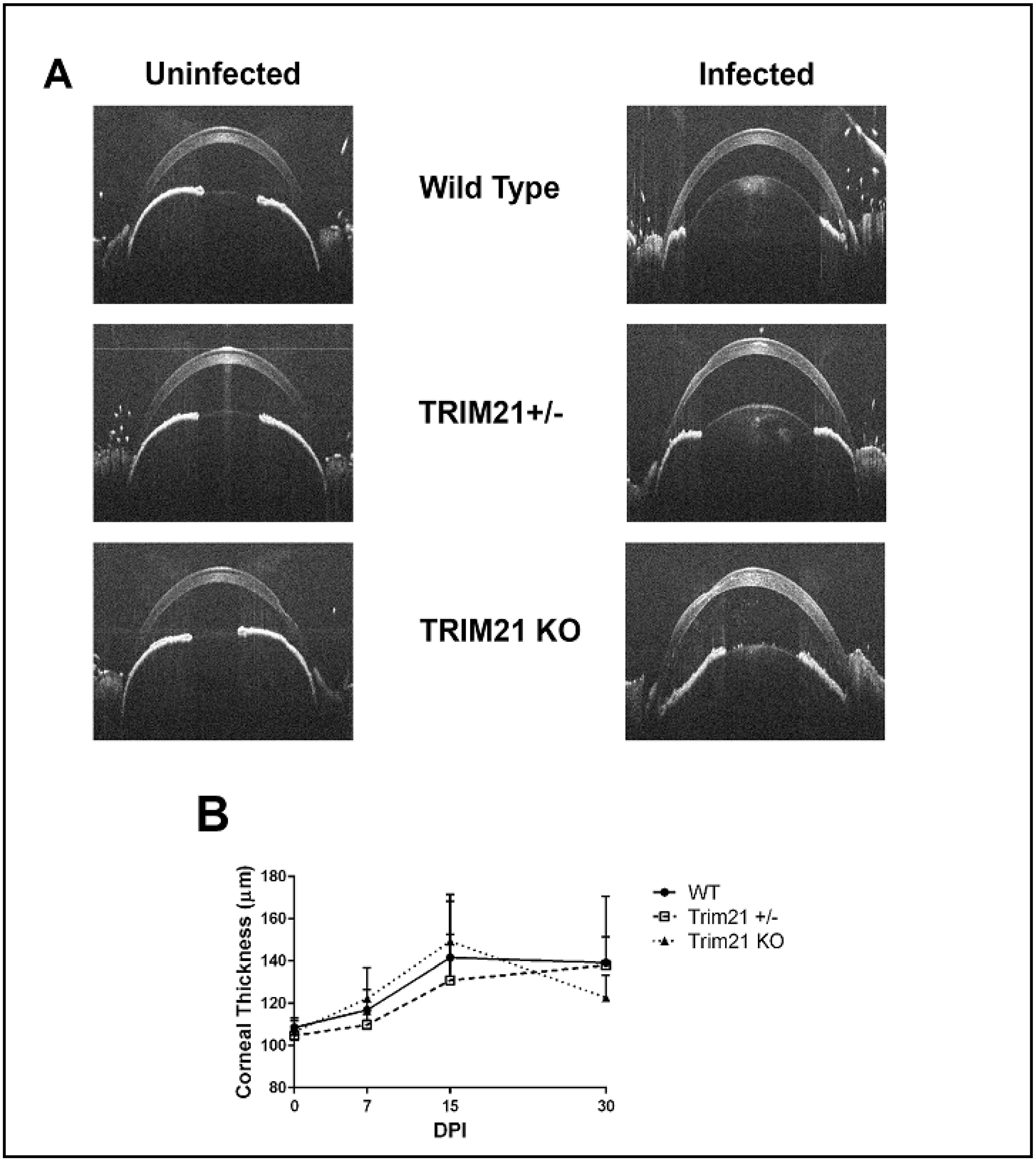
3.3. The Loss of TRIM21 Does Not Modify Corneal Pathology Compared to WT Mice
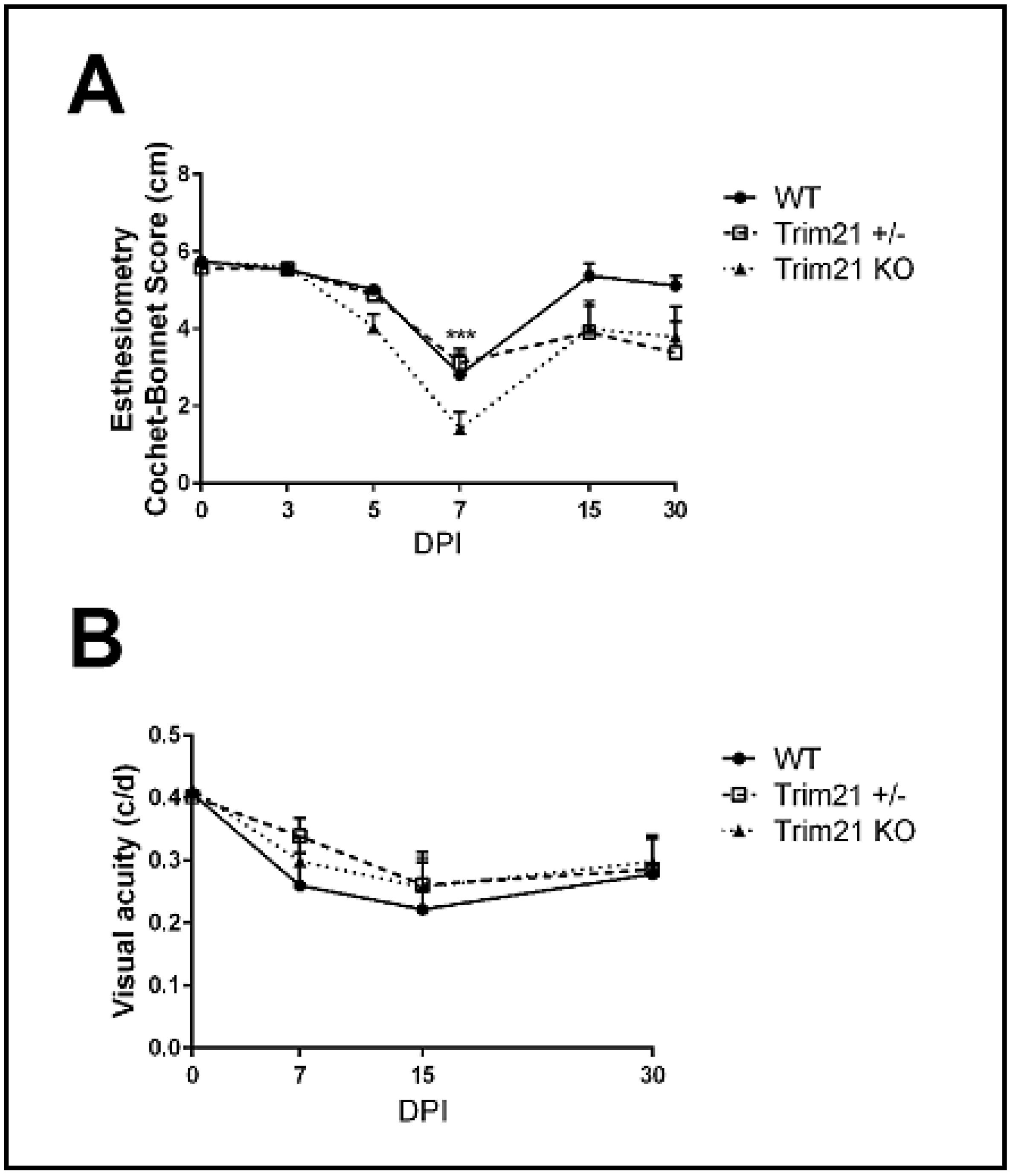
3.4. Visual Axis Function Is Reduced in WT and TRIM21 KO Mice Following HSV-1 Infectionn
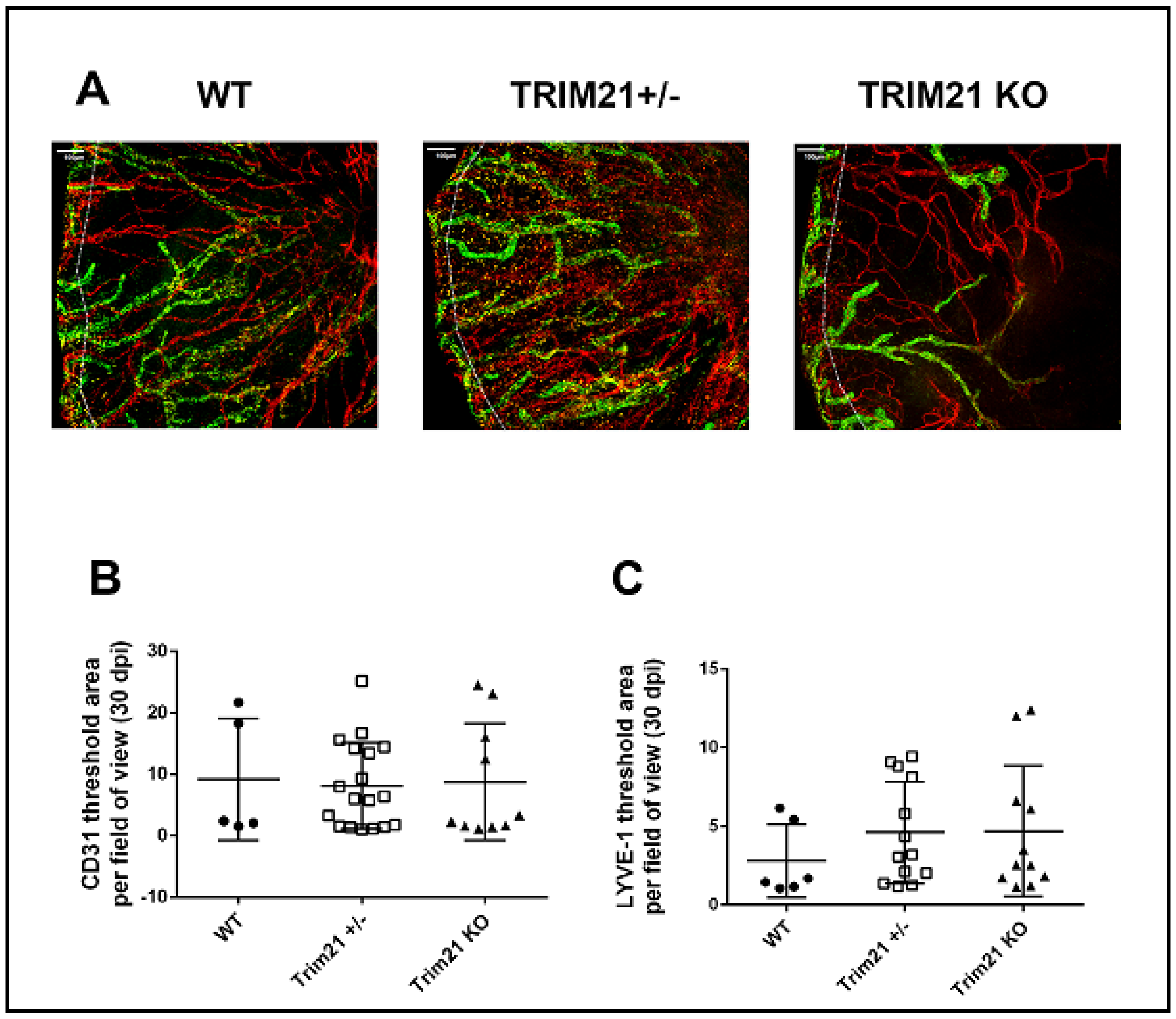
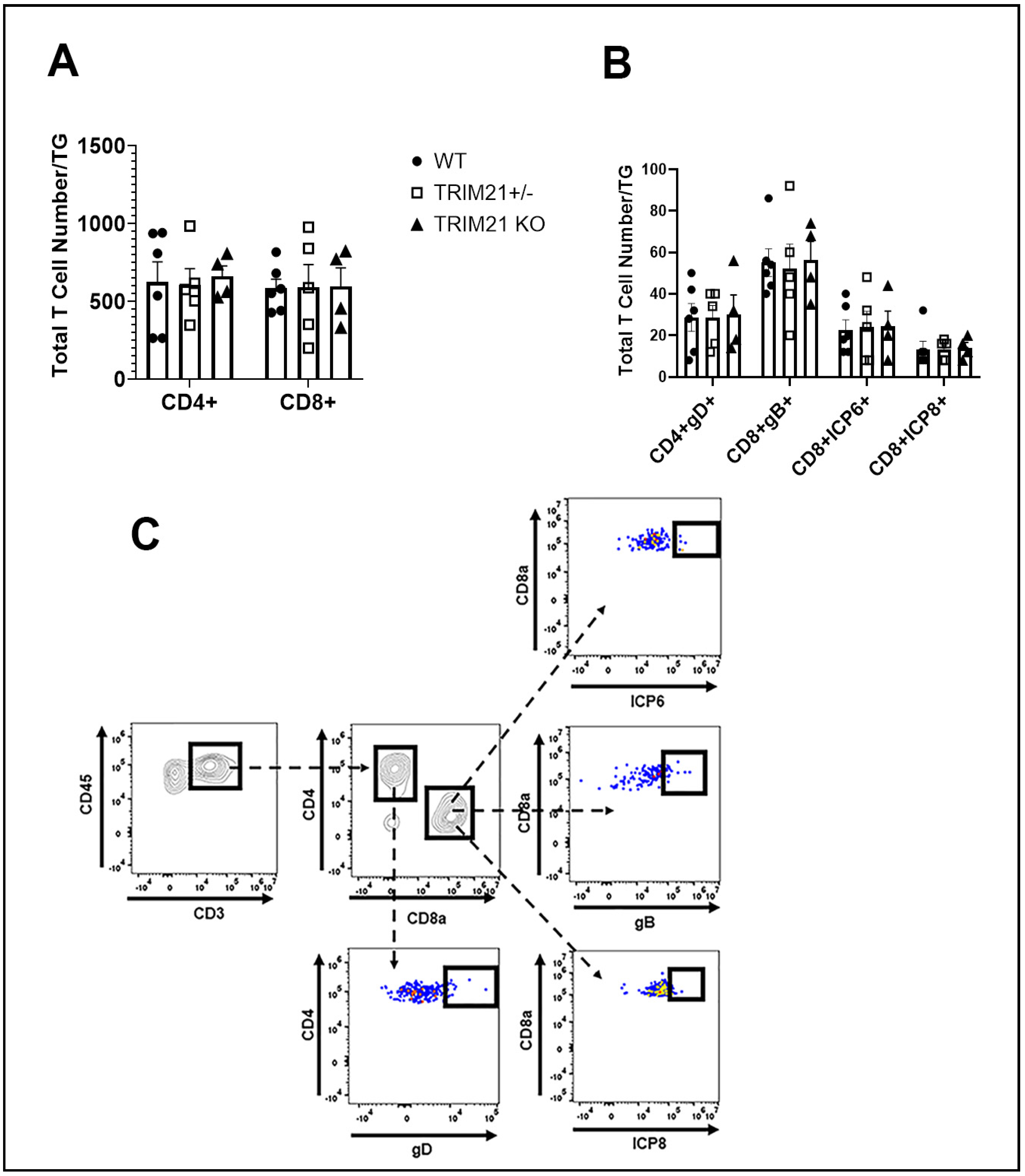
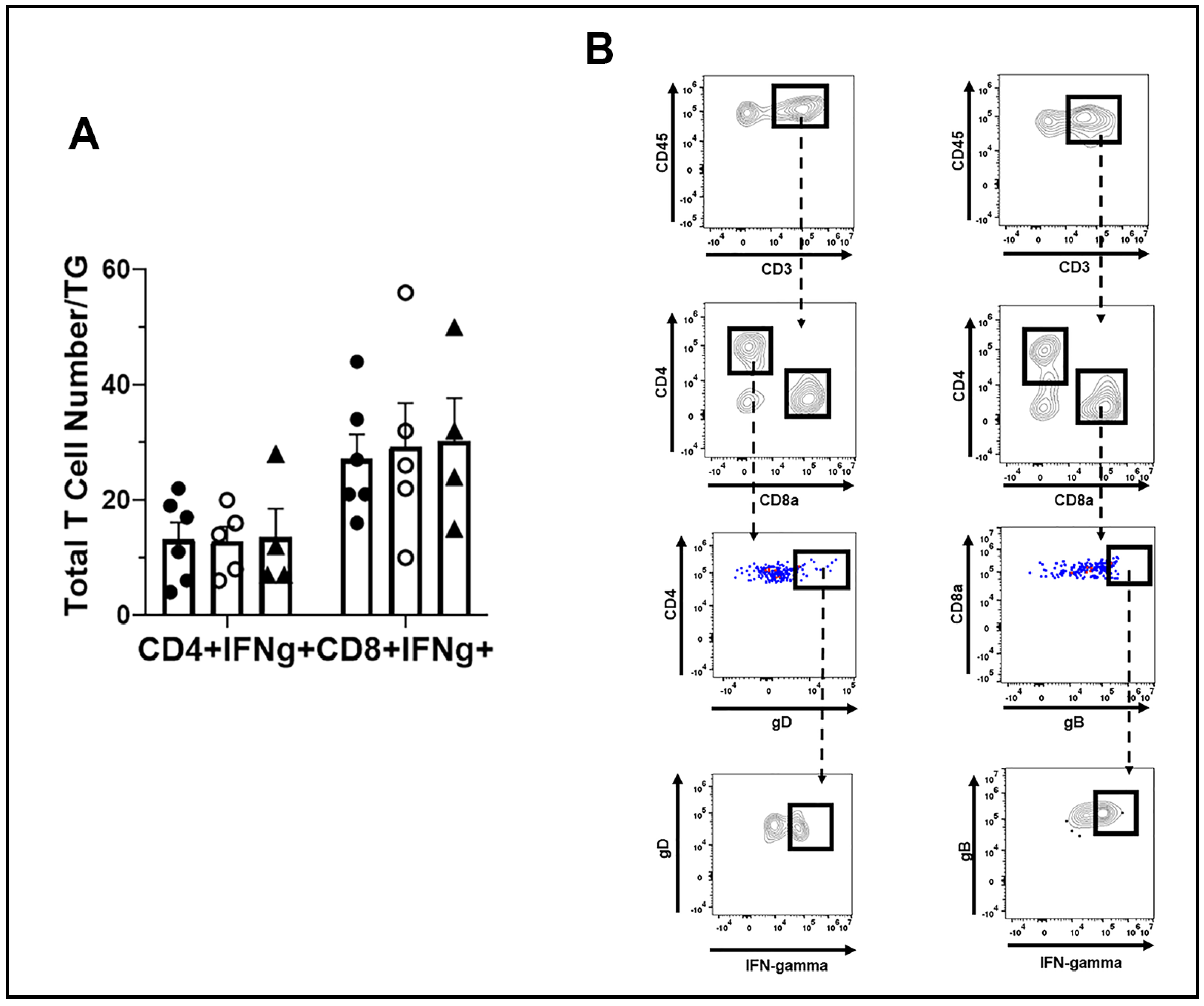
3.5. T Cell Infiltration and Function Are Similar between WT, TRIM21+/−, and TRIM21 KO Mice
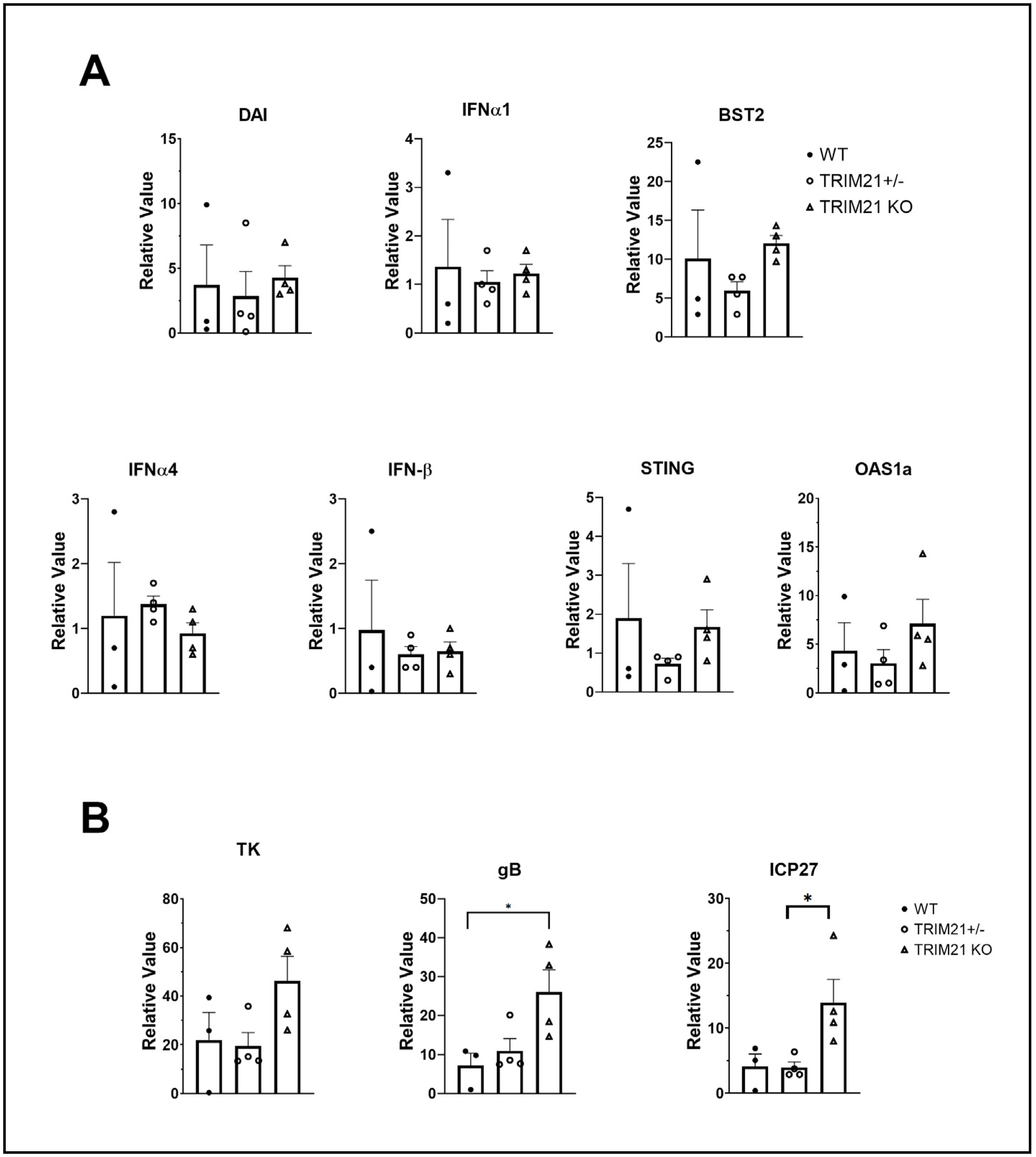
3.6. TG of TRIM21 KO Mice Display an Elevation in HSV-1 Lytic Gene Expression but Not Type I IFN or IFN-Inducible Genes Following Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aravalli, R.N.; Hu, S.; Rowen, T.N.; Palmquist, J.M.; Lokensgard, J.R. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 2005, 175, 4189–4193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez, F.; Suryawanshi, A.; Rouse, B.T. Pathogenesis of herpes stromal keratitis—A focus on corneal neovascularization. Prog Retin Eye Res. 2013, 33, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant-Hudson, K.; Conrady, C.D.; Carr, D.J. Type I interferon and lymphangiogenesis in the HSV-1 infected cornea—Are they beneficial to the host? Prog. Retin. Eye Res. 2013, 36, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemaitelly, H.; Nagelkerke, N.; Omori, R.; Abu-Raddad, L.J. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS ONE 2019, 14, e0214151. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: Model-based predictions. BMC Med. 2019, 17, 57. [Google Scholar] [CrossRef]
- Fries, L.F.; Friedman, H.M.; Cohen, G.H.; Eisenberg, R.J.; Hammer, C.H.; Frank, M.M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 1986, 137, 1636–1641. [Google Scholar]
- Lubinski, J.M.; Wang, L.; Soulika, A.M.; Burger, R.; Wetsel, R.A.; Colten, H.; Cohen, G.H.; Eisenberg, R.J.; Lambris, J.D.; Friedman, H.M. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 1998, 72, 8257–8263. [Google Scholar] [CrossRef] [Green Version]
- Dubin, G.; Socolof, E.; Frank, I.; Friedman, H.M. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 1991, 65, 7046–7050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, A.; Jugovic, P.; Russ, G.; Bennink, J.; Yewdell, J.; Ploegh, H.; Johnson, D. Herpes simplex virus turns off the TAP to evade host immunity. Nature 1995, 375, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.D.; Han, J.; Sandifer, T.K.; Stewart, M.; Hinz, A.J.; Yoon, M.; Johnson, D.C.; Spear, P.G.; Jerome, K.R. Inhibition of TCR signaling by herpes simplex virus. J. Immunol. 2006, 176, 1825–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, J.; Eis-Hübinger, A.M.; Koch, N. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J. Immunol. 2003, 171, 3075–3083. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zheng, C. The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1. Microbiol. Mol. Biol. Rev. 2020, 84, e00099-20. [Google Scholar] [CrossRef]
- Austin, B.A.; James, C.; Silverman, R.H.; Carr, D.J. Critical role for the oligoadenylate synthetase/RNase L pathway in response to IFN-beta during acute ocular herpes simplex virus type 1 infection. J. Immunol. 2005, 175, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Conrady, C.D.; Zheng, M.; Fitzgerald, K.A.; Liu, C.; Carr, D.J. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Tscharke, D.C.; Simmons, A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J. Exp. Med. 1994, 180, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Z.; Russell, T.A.; Spelman, T.; Carbone, F.R.; Tscharke, D.C. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS Pathog. 2014, 10, e1004237. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kim, T.; Bao, M.; Facchinetti, V.; Jung, S.; Ghaffari, A.; Qin, J.; Cheng, G.; Liu, Y. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 2011, 34, 866–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Rönnblom, L. The importance of the type I interferon system in autoimmunity. Clin. Exp. Rheumatol. 2016, 34, 21–24. [Google Scholar] [PubMed]
- Kretschmer, S.; Lee-Kirsch, M.A. Type I interferon-mediated autoinflammation and autoimmunity. Curr. Opin. Immunol. 2017, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.L.; Krucker, T.; Steffensen, S.; Akwa, Y.; Powell, H.C.; Lane, T.; Carr, D.J.; Gold, L.H.; Henriksen, S.J.; Siggins, G.R. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-alpha. Brain Res. 1999, 835, 46–61. [Google Scholar] [CrossRef]
- Naka, T.; Fujimoto, M.; Tsutsui, H.; Yoshimura, A. Negative regulation of cytokine and TLR signalings by SOCS and others. Adv. Immunol. 2005, 87, 61–122. [Google Scholar] [PubMed]
- Frey, K.G.; Ahmed, C.M.I.; Dabelic, R.; Jager, L.D.; Noon-Song, E.N.; Haider, S.M.; Johnson, H.M.; Bigley, N.J. HSV-1-induced SOCS-1 expression in keratinocytes: Use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J. Immunol. 2009, 183, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Yokota, S.I.; Yokosawa, N.; Okabayashi, T.; Suzutani, T.; Miura, S.; Jimbow, K.; Fujii, N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 2004, 78, 6282–6286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNab, F.W.; Rajsbaum, R.; Stoye, J.P.; O’Garra, A. Tripartite-motif proteins and innate immune regulation. Curr. Opin. Immunol. 2011, 23, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Diez-Roux, G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 2005, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.; Gabhann, J.N.; Ben Larbi, N.; Breen, E.P.; Fitzgerald, K.A.; Jefferies, C.A. The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J. Immunol. 2008, 181, 1780–1786. [Google Scholar] [CrossRef]
- Yang, K.; Shi, H.X.; Liu, X.Y.; Shan, Y.F.; Wei, B.; Chen, S.; Wang, C. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J. Immunol. 2009, 182, 3782–3792. [Google Scholar] [CrossRef] [PubMed]
- McEwan, W.A.; Tam, J.C.; Watkinson, R.E.; Bidgood, S.R.; Mallery, D.L.; James, L.C. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 2013, 14, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallery, D.L.; McEwan, W.A.; Bidgood, S.R.; Towers, G.J.; Johnson, C.M.; James, L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. USA 2010, 107, 19985–19990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiberti, A.; Gmyrek, G.B.; Montgomery, M.L.; Sallack, R.; Carr, D.J.J. Loss of Osteopontin Expression Reduces HSV-1-Induced Corneal Opacity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Gebhardt, B.M.; Carr, D.J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 1996, 157, 3542–3549. [Google Scholar] [PubMed]
- Harle, P.; Cull, V.; Agbaga, M.P.; Silverman, R.; Williams, B.R.; James, C.; Carr, D.J. Differential effect of murine alpha/beta interferon transgenes on antagonization of herpes simplex virus type 1 replication. J. Virol. 2002, 76, 6558–6567. [Google Scholar] [CrossRef] [Green Version]
- Wuest, T.R.; Carr, D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010, 207, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Xia, L. TRIM21 Aggravates Herpes Simplex Virus Epithelial Keratitis by Attenuating STING-IRF3-Mediated Type I Interferon Signaling. Front. Microbiol. 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaysburd, M.; Watkinson, R.E.; Cooper, H.; Reed, M.; O’Connell, K.; Smith, J.; Cuickshanks, J.; James, L.C. Intracellular antibody receptor TRIM21 prevents fatal viral infection. Proc. Natl. Acad. Sci. USA 2013, 110, 12397–12401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkinson, R.E.; McEwan, W.A.; Tam, J.C.; Vaysburd, M.; James, L.C. TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus. PLoS Pathog. 2015, 11, e1005253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuest, T.; Austin, B.A.; Uematsu, S.; Thapa, M.; Akira, S.; Carr, D.J. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J. Neuroimmunol. 2006, 179, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Twardy, B.S.; Channappanavar, R.; Suvas, S. Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8604–8613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Deshpande, S.; Lee, S.; Ferrara, N.; Rouse, B.T. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 2001, 75, 9828–9835. [Google Scholar] [CrossRef] [Green Version]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Thomas, J.; Gangappa, S.; Kanangat, S.; Rouse, B.T. On the essential involvement of neutrophils in the immunopathologic disease: Herpetic stromal keratitis. J. Immunol. 1997, 158, 1383–1391. [Google Scholar] [PubMed]
- O’Brien, W.J.; Guy, J.; Taylor, J.L. Pathogenesis of corneal oedema associated with herpetic eye disease. Br. J. Ophthalmol. 1990, 74, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.T.; Tumpey, T.M.; Kunkel, S.L.; Oakes, J.E.; Lausch, R.N. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1854–1862. [Google Scholar]
- Royer, D.J.; Carr, M.M.; Gurung, H.R.; Halford, W.P.; Carr, D.J.J. The Neonatal Fc Receptor and Complement Fixation Facilitate Prophylactic Vaccine-Mediated Humoral Protection against Viral Infection in the Ocular Mucosa. J. Immunol. 2017, 199, 1898–1911. [Google Scholar] [CrossRef] [Green Version]
- Hamrah, P.; Cruzat, A.; Dastjerdi, M.H.; Zheng, L.; Shahatit, B.M.; Bayhan, H.A.; Dana, R.; Pavan-Langston, D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: An in vivo confocal microscopy study. Ophthalmology 2010, 117, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.; Rowe, A.M.; Lathrop, K.L.; Harvey, S.A.; Hendricks, R.L. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J. Virol. 2014, 88, 7870–7880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chucair-Elliott, A.J.; Zheng, M.; Carr, D.J. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chucair-Elliott, A.J.; Jinkins, J.; Carr, M.M.; Carr, D.J. IL-6 Contributes to Corneal Nerve Degeneration after Herpes Simplex Virus Type I Infection. Am. J. Pathol. 2016, 186, 2665–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Douglas, R.M.; Alam, N.M.; Silver, B.D.; McGill, T.J.; Tschetter, W.W.; Prusky, G.T. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis. Neurosci. 2005, 22, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Khanna, K.M.; Chen, X.; Fink, D.J.; Hendricks, R.L. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 2000, 191, 1459–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noisakran, S.; Carr, D.J. Lymphocytes delay kinetics of HSV-1 reactivation from in vitro explants of latent infected trigeminal ganglia. J. Neuroimmunol. 1999, 95, 126–135. [Google Scholar] [CrossRef]
- Simmons, A.; Tscharke, D.; Speck, P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr. Top. Microbiol. Immunol. 1992, 179, 31–56. [Google Scholar] [PubMed]
- Royer, D.J.; Conrady, C.D.; Carr, D.J. Herpesvirus-Associated Lymphadenitis Distorts Fibroblastic Reticular Cell Microarchitecture and Attenuates CD8 T Cell Responses to Neurotropic Infection in Mice Lacking the STING-IFNα/β Defense Pathways. J. Immunol. 2016, 197, 2338–2352. [Google Scholar] [CrossRef] [Green Version]
- Sjöstrand, M.; Ambrosi, A.; Brauner, S.; Sullivan, J.; Malin, S.; Kuchroo, V.K.; Espinosa, A.; Wahren-Herlenius, M. Expression of the immune regulator tripartite-motif 21 is controlled by IFN regulatory factors. J. Immunol. 2013, 191, 3753–3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manocha, G.D.; Mishra, R.; Sharma, N.; Kumawat, K.L.; Basu, A.; Singh, S.K. Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J. NeuroInflamm. 2014, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Wu, R.; Guo, W.; Xie, L.; Qiao, Z.; Chen, S.; Zhu, J.; Huang, C.; Huang, J.; Chen, B.; et al. STING-Mediated IFI16 Degradation Negatively Controls Type I Interferon Production. Cell Rep. 2019, 29, 1249–1260.e4. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, R.L.; Weber, P.C.; Taylor, J.L.; Koumbis, A.; Tumpey, T.M.; Glorioso, J.C. Endogenously produced interferon alpha protects mice from herpes simplex virus type 1 corneal disease. J. Gen. Virol. 1991, 72 Pt 7, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Veress, L.A.; Gebhardt, B.M.; Carr, D.J. Innate and acquired immunity to herpes simplex virus type 1. Virology 1997, 236, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinert, L.S.; Lopušná, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vægter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royer, D.J.; Carr, D.J. A STING-dependent innate-sensing pathway mediates resistance to corneal HSV-1 infection via upregulation of the antiviral effector tetherin. Mucosal Immunol. 2016, 9, 1065–1075. [Google Scholar] [CrossRef] [Green Version]
- Al-khatib, K.; Williams, B.R.; Silverman, R.H.; Halford, W.; Carr, D.J. The murine double-stranded RNA-dependent protein kinase PKR and the murine 2′,5′-oligoadenylate synthetase-dependent RNase L are required for IFN-beta-mediated resistance against herpes simplex virus type 1 in primary trigeminal ganglion culture. Virology 2003, 313, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Bao, M.; Lu, N.; Weng, L.; Yuan, B.; Liu, Y.J. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat. Immunol. 2013, 14, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantin, E.M.; Hinton, D.R.; Chen, J.; Openshaw, H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 1995, 69, 4898–4905. [Google Scholar] [CrossRef] [Green Version]
- Thacore, H.R.; Mount, D.T.; Chadha, K.C. Interferon system of human cornea cells: Interferon production, characterization, and development of antiviral state. J. Interferon Res. 1982, 2, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.H.; Adachi, A. Induction of apoptosis by herpes simplex virus type 1. J. Gen. Virol. 1997, 78 Pt 11, 2909–2912. [Google Scholar] [CrossRef] [Green Version]
- Krzyzowska, M.; Kowalczyk, A.; Skulska, K.; Thörn, K.; Eriksson, K. Fas/FasL Contributes to HSV-1 Brain Infection and Neuroinflammation. Front Immunol. 2021, 12, 714821. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, S.; Liang, Y.; Zhou, X.; Chen, W.; Li, L.; Wu, J.; Zhuang, Q.; Chen, C.; Li, J.; et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 2015, 17, 229–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mélanie, S.E.; Florence, F.; Kara-Ali Ghania, H.; Mélanie, L.; Perrine, U.; Bonnet Marion, C.; Isabelle, G.; Emmanuelle, C.; Charles, P.; Michel, S.; et al. TRIM21, a New Component of the TRAIL-Induced Endogenous Necrosome Complex. Front Mol. Biosci. 2021, 8, 645134. [Google Scholar]
| Forward | Reverse | |
|---|---|---|
| IFNa4 | 5′-TTC TGC AAT GAC CTC CAT CA-3′ | 5′-GGC ACA GAG GCT GTG TTT CT-3′ |
| mSTING | 5′-CCT AGC CTC GCA CGA ACT TG-3′ | 5′-CGC ACA GCC TTC CAG TAG C-3′ |
| DAI | 5′-GGA AGA TCT ACC ACT CAC GTC-3′ | 5′-CCT TGT TGG CAG ATG ATG TTG-3′ |
| Oas1a | 5′-CTT TGA TGT CCT GGG TCA TGT-3′ | 5′-GCT CCG TGA AGC AGG TAG AG-3′ |
| ICP27 | 5’- GCA TCC TTC GTG TTT GTC AT-3’ | 5’- ACC AAG GGT CGC GTA GTC-3’ |
| TK | 5′-ATA CCG ACG ATC TGC GAC CT-3′ | 5′-TTA TTG CCG TCA TAG CGC GG-3′ |
| gB | 5′-TCT GCA CCA TGA CCA AGT G-3′ | 5′-TGG TGA AGG TGG TGG ATA TG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berube, A.; Gmyrek, G.B.; Royer, D.J.; Carr, D.J.J. Tripartite-Motif 21 (TRIM21) Deficiency Results in a Modest Loss of Herpes Simplex Virus (HSV)-1 Surveillance in the Trigeminal Ganglia Following Cornea Infection. Viruses 2022, 14, 589. https://doi.org/10.3390/v14030589
Berube A, Gmyrek GB, Royer DJ, Carr DJJ. Tripartite-Motif 21 (TRIM21) Deficiency Results in a Modest Loss of Herpes Simplex Virus (HSV)-1 Surveillance in the Trigeminal Ganglia Following Cornea Infection. Viruses. 2022; 14(3):589. https://doi.org/10.3390/v14030589
Chicago/Turabian StyleBerube, Amanda, Grzegorz B. Gmyrek, Derek J. Royer, and Daniel J. J. Carr. 2022. "Tripartite-Motif 21 (TRIM21) Deficiency Results in a Modest Loss of Herpes Simplex Virus (HSV)-1 Surveillance in the Trigeminal Ganglia Following Cornea Infection" Viruses 14, no. 3: 589. https://doi.org/10.3390/v14030589
APA StyleBerube, A., Gmyrek, G. B., Royer, D. J., & Carr, D. J. J. (2022). Tripartite-Motif 21 (TRIM21) Deficiency Results in a Modest Loss of Herpes Simplex Virus (HSV)-1 Surveillance in the Trigeminal Ganglia Following Cornea Infection. Viruses, 14(3), 589. https://doi.org/10.3390/v14030589







