A Comparative Evaluation of the Estimation of Rabies Virus Antibodies among Free-Roaming, Vaccinated Dogs in Bengaluru, India
Abstract
:1. Introduction
2. Materials and Methods
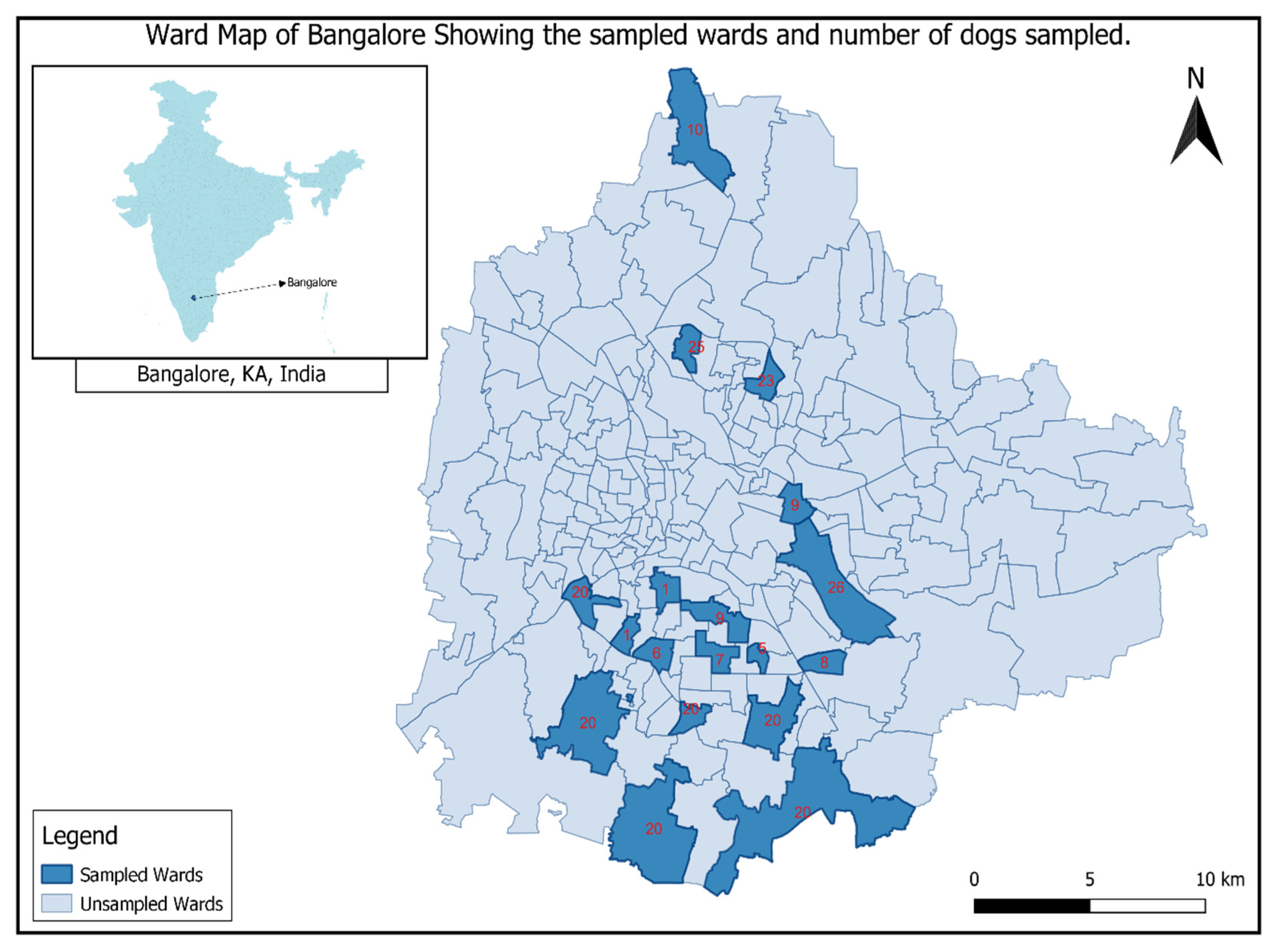
2.1. Study Area
2.2. Serum Sample Collection
2.3. Virus and BHK-21 Cells
2.4. Rapid Fluorescent Focus Inhibition Test
2.5. Indirect Enzyme-Linked Immunosorbant Assay
2.6. Statistical Analysis
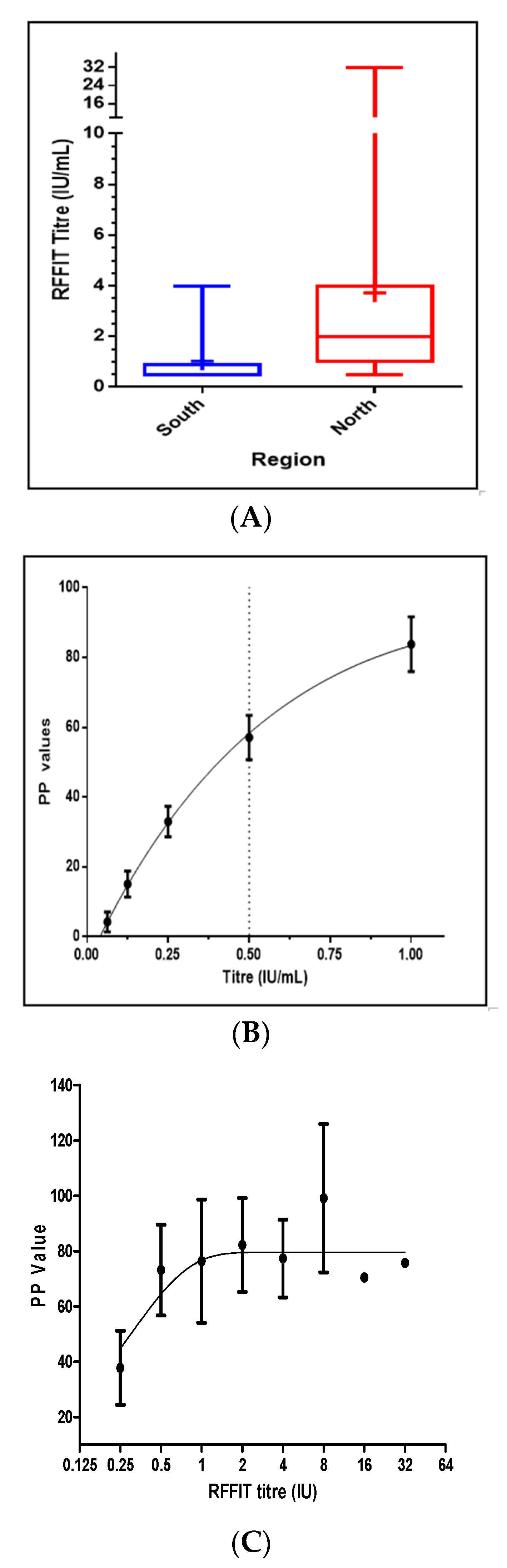
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV Virus Taxonomy: 2020 Release. Available online: https://talk.ictvonline.org/taxonomy (accessed on 31 October 2020).
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies; Third Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Gompper, M.E. Free-Ranging Dogs and Wildlife Conservation; Oxford University Press: Oxford, UK, 2013; pp. 27–29. [Google Scholar]
- Sudarshan, M.K.; Madhusudan, S.N.; Mahendra, B.J.; Rao, N.S.N.; Ashwath Narayana, D.H.; Abdul Rahman, S. Assessing the burden of human rabies in India: Results of a national multi-center epidemiological survey. Int. J. Infect. Dis. 2007, 11, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudarshan, M.K.; Mahendra, B.J.; Madhusudana, S.N.; Narayana, D.A.; Rahman, A.; Rao, N.S.N.; X-Meslin, F.; Lobo, D.; Ravikumar, K. An epidemiological study of animal bites in India: Results of a WHO sponsored national multi-centric rabies survey. J. Commun. Dis. 2006, 38, 32–39. [Google Scholar] [PubMed]
- Hiby, E.F.; Hiby, L.R. Dog population management. In The Domestic Dog. Its Evolution, Behaviour and Interactions with People, 2nd ed.; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 2017; pp. 385–403. [Google Scholar]
- Vanak, A.T.; Gompper, M.E. Dogs Canis familiaris as carnivores: Their role and function in intraguild competition. Mamm. Rev. 2009, 39, 265–283. [Google Scholar] [CrossRef]
- Smith, L.M.; Hartmann, S.; Munteanu, A.M.; Dalla Villa, P.; Quinnell, R.J.; Collins, L.M. The effectiveness of dog population management: A systematic review. Animals 2019, 9, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patronek, G.J. Free-roaming and feral cats—their impact on wildlife and human beings. J. Am. Vet. Med. Assoc. 1998, 212, 218–226. [Google Scholar]
- Katz, I.S.S.; Guedes, F.; Fernandes, E.R.; dos Ramos Silva, S. Immunological aspects of rabies: A literature review. Arch. Virol. 2017, 162, 3251–3268. [Google Scholar] [CrossRef]
- Cliquet, F.; Aubert, M.; Sagne, L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods 1998, 212, 79–87. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B.; World Health Organization. Laboratory Techniques in Rabies, 5th ed.; World Health Organization: Geneva, Switzerland, 2018; Volume 1, pp. 196–197. [Google Scholar]
- Timiryasova, T.M.; Luo, P.; Zheng, L.; Singer, A.; Zedar, R.; Garg, S.; Petit, C.; Moore, S.; Hu, B.T.; Brown, M. Rapid fluorescent focus inhibition test optimization and validation: Improved detection of neutralizing antibodies to rabies virus. J. Immunol. Methods 2019, 474, 112626. [Google Scholar] [CrossRef]
- Animal Welfare Board of India. Animal Birth Control (Dogs) Rules. 2001. Available online: http://www.awbi.in/policy_acts_rules.html (accessed on 8 January 2019).
- Belsare, A.; Vanak, A.T. Modelling the challenges of managing free-ranging dog populations. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Santosh, A.K.; Isloor, S.; Rathnamma, D.; Sharada, R.; Sunilkumar, K.M.; Balamurugan, V.; Yathiraj, S.; Satyanarayana, M.L. Assessment of Humoral Immune Response in Vaccinated Domestic Dogs and Cats Intended for Pet Travel from India by Rapid Florescent Focus Inhibition Test (RFFIT). 2017. Available online: https://krishi.icar.gov.in. (accessed on 22 December 2021).
- Neelufer, M.S. Standardisation and Application of Rabies Virus Neutralizing Antibody Assay for Assessment of Vaccinal Efficacy in Dogs. Master’s Thesis, Karnataka Veterinary and Fishery Sciences University, Bangalore, India, 2016. [Google Scholar]
- Moore, S.M.; Hanlon, C.A. Rabies-specific antibodies: Measuring surrogates of protection against a fatal disease. PloS. Negl. Trop. Dis. 2010, 44, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Kramer, B.; Schildger, H.; Nicol, H. The rapid fluorescent focus inhibition test (RFFIT) is a suitable method for batch potency testing of inactivated rabies vaccines. Biologicals 2009, 37, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Santosh, A.K. Development of ELISA and its Comparative Evaluation with RFFIT for Estimation of Anti-Rabies Vaccinal Antibodies in Dogs. Ph.D. Thesis, Karnataka Veterinary and Fishery Sciences University, Bengaluru, India, 2017. [Google Scholar]
- Singh, M.P.; Goyal, K.; Majumdar, M.; Ratho, R.K. Prevalence of rabies antibodies in street and household dogs. Trop. Anim. Health Prod. 2011, 43, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Nale, J.M.; Pharande, R.R.; Majee, S.B.; Gandge, R.S.; Sawane, M.P.; Ingle, S.A. Serosurveillance of rabies antibodies in dogs in Mumbai region by using indirect ELISA. Comp. Immunol. Microbiol. Infect. Dis. 2021, 76, 101655. [Google Scholar] [CrossRef] [PubMed]
- Yale, G.; Sudarshan, S.; Taj, S.; Patchimuthu, G.I.; Mangalanathan, B.V.; Belludi, A.Y.; Shampur, M.N.; Krishnaswamy, T.G.; Mazeri, S. Investigation of protective level of rabies antibodies in vaccinated dogs in Chennai, India. Vet. Rec. Open 2021, 8, e8. [Google Scholar] [CrossRef] [PubMed]
- Kasempimolporn, S.; Sichanasai, B.; Saengseesom, W.; Puempumpanich, S.; Chatraporn, S.; Sitprija, V. Prevalence of rabies virus infection and rabies antibody in stray dogs: A survey in Bangkok, Thailand. Prev. Vet. Med. 2007, 78, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Olugasa, O.O.; Aiyedun, J.O.; Emikpe, B.O. Prevalence of antibody against rabies among confined, free roaming and stray dogs in a transit city of Nigeria. Vet. Ital. 2011, 47, 453–460. [Google Scholar]
- Muhamuda, K.; Madhusudana, S.N.; Ravi, V. Development and evaluation of a competitive ELISA for estimation of rabies neutralizing antibodies after post-exposure rabies vaccination in humans. Int. J. Infect. Dis. 2007, 11, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Shyamsundar, K.A.; Isloor, S.; Madhusudhana, S.N.; Mahesh, V.; Yathiraj, S.; Nandini, V.M.; Rathanamma, D.; Veeregowda, B.M.; Satyanarayana, M.L.; Bhat, M.N.; et al. Comparative evaluation of RFFIT and RABV G-protein based ELISA for seromonitoring of anti-rabies vaccinal antibodies in domestic dogs in and around Bengaluru. In Proceedings of the 16th National Conference of APCRICON, Mysore, India, 4–6 July 2014. [Google Scholar]
- Feyssaguet, M.; Dacheux, L.; Audry, L.; Compoint, A.; Morize, J.L.; Blanchard, I.; Bourhy, H. Multicenter comparative study of a new ELISA, PLATELIATM RABIES II, for the detection and titration of anti-rabies glycoprotein antibodies and comparison with the rapid fluorescent focus inhibition test (RFFIT) on human samples from vaccinated and non-vaccinated people. Vaccine 2007, 25, 2244–2251. [Google Scholar]
- Zhao, R.; Yu, P.; Shan, Y.; Thirumeni, N.; Li, M.; Lv, Y.; Li, J.; Ren, W.; Huang, L.; Wei, J.; et al. Rabies virus glycoprotein serology ELISA for measurement of neutralizing antibodies in sera of vaccinated human subjects. Vaccine 2019, 37, 6060–6067. [Google Scholar] [CrossRef]
- Debnath, A.; Pathak, D.C.; Narayan Ramamurthy, G.M.; Pandey, A.B.; Upmanyu, V.; Tiwari, A.K.; Saravanan, R.; Chellappa, M.M.; Dey, S. Serological profiling of rabies antibodies by enzyme-linked immunosorbent assay and its comparative analysis with rapid fluorescent focus inhibition test in mouse model. Vet. World. 2019, 12, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manickam, R.; Basheera, M.D.; Jayakumar, R. Post-exposure prophylaxis (PEP) of rabies-infected Indian street dogs. Vaccine 2008, 26, 6564–6568. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, C.; Taieb, D.; Kaabi, B.; Diouani, M.F.; Hadjahmed, S.B.; Chtourou, Y.; B’chir, B.I.; Dellagi, K. Comparative evaluation of specific ELISA and RFFIT antibody assays in the assessment of dog immunity against rabies. Epidemiol. Infect. 2005, 133, 749–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Action Plan for Eliminating Dog Mediated Rabies from India. Available online: https://ncdc.gov.in/WriteReaData/l892s/25879243771600146411.pdf (accessed on 11 September 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, L.J.; Isloor, S.; Santosh, A.K.; Bhat, A.; Sharada, R.; Rathnamma, D.; Veeregowda, B.M.; Phaniraj, K.L.; Abhijit Kumar, N.; T. Vanak, A. A Comparative Evaluation of the Estimation of Rabies Virus Antibodies among Free-Roaming, Vaccinated Dogs in Bengaluru, India. Viruses 2022, 14, 484. https://doi.org/10.3390/v14030484
Das LJ, Isloor S, Santosh AK, Bhat A, Sharada R, Rathnamma D, Veeregowda BM, Phaniraj KL, Abhijit Kumar N, T. Vanak A. A Comparative Evaluation of the Estimation of Rabies Virus Antibodies among Free-Roaming, Vaccinated Dogs in Bengaluru, India. Viruses. 2022; 14(3):484. https://doi.org/10.3390/v14030484
Chicago/Turabian StyleDas, Lekshmi J., Shrikrishna Isloor, Alur Kotrappa Santosh, Avinash Bhat, Ramakrishnaiah Sharada, Doddamane Rathnamma, Belamaranahally Muniveerappa Veeregowda, Konanduru Lingappa Phaniraj, Nageshkumar Abhijit Kumar, and Abi T. Vanak. 2022. "A Comparative Evaluation of the Estimation of Rabies Virus Antibodies among Free-Roaming, Vaccinated Dogs in Bengaluru, India" Viruses 14, no. 3: 484. https://doi.org/10.3390/v14030484
APA StyleDas, L. J., Isloor, S., Santosh, A. K., Bhat, A., Sharada, R., Rathnamma, D., Veeregowda, B. M., Phaniraj, K. L., Abhijit Kumar, N., & T. Vanak, A. (2022). A Comparative Evaluation of the Estimation of Rabies Virus Antibodies among Free-Roaming, Vaccinated Dogs in Bengaluru, India. Viruses, 14(3), 484. https://doi.org/10.3390/v14030484






