Metagenome-Assembled Viral Genomes Analysis Reveals Diversity and Infectivity of the RNA Virome of Gerbillinae Species
Abstract
1. Introduction
2. Materials and Methods
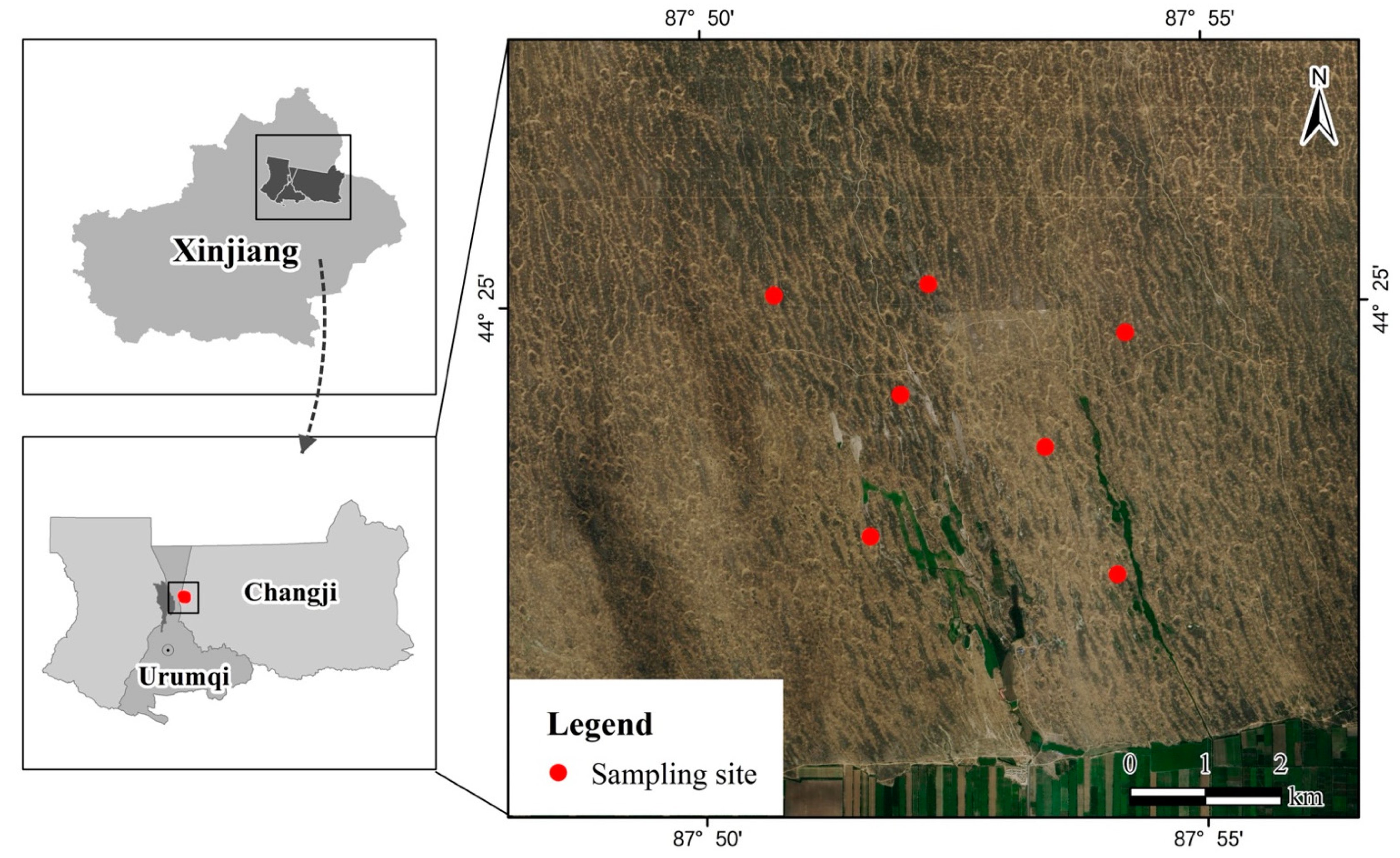
2.1. Animal Sampling
2.2. Library Preparation and Sequencing
2.3. Raw Reads Filtering and Rapid Identification of Virus Species
2.3.1. Quality Control
2.3.2. Contamination Removal
2.3.3. Virus Classification
2.4. Reads Assembly
2.5. Identification of Viruses
2.5.1. Identification of Known Viruses
2.5.2. Identification of De Novo Viruses
2.6. Abundance Statistics of Virus
2.7. Prediction of Gene
2.8. Phylogenetic Analysis
3. Results
3.1. Metagenomic Analysis and Virome Overview
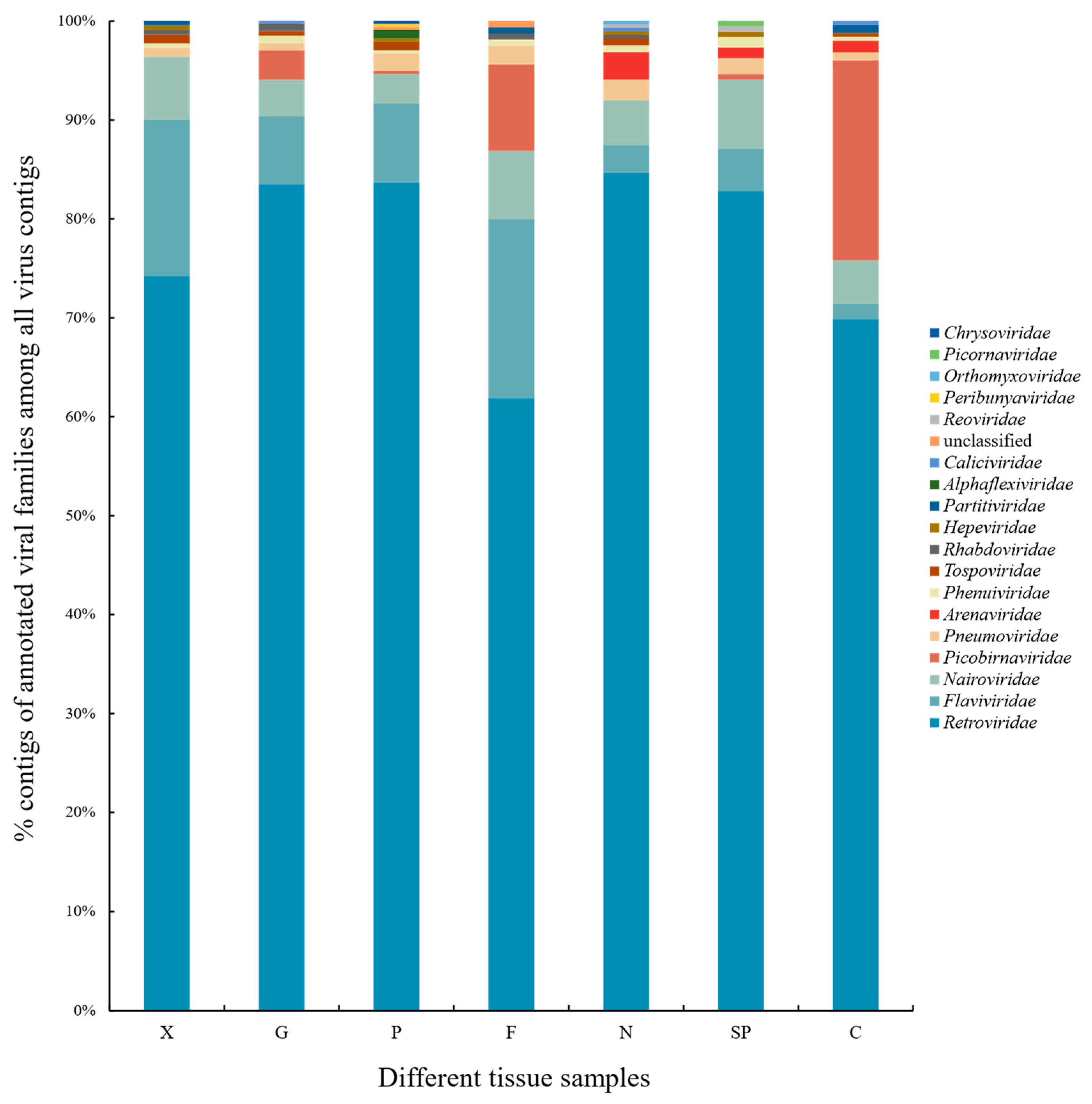
3.2. Analysis of the Results of Species Taxonomic Annotations
3.3. Analysis of Sequence Assembly and Gene Prediction
3.4. Interesting Taxonomical Families of Vertebrate Viruses due to Zoonotic Potential
3.4.1. Retroviridae
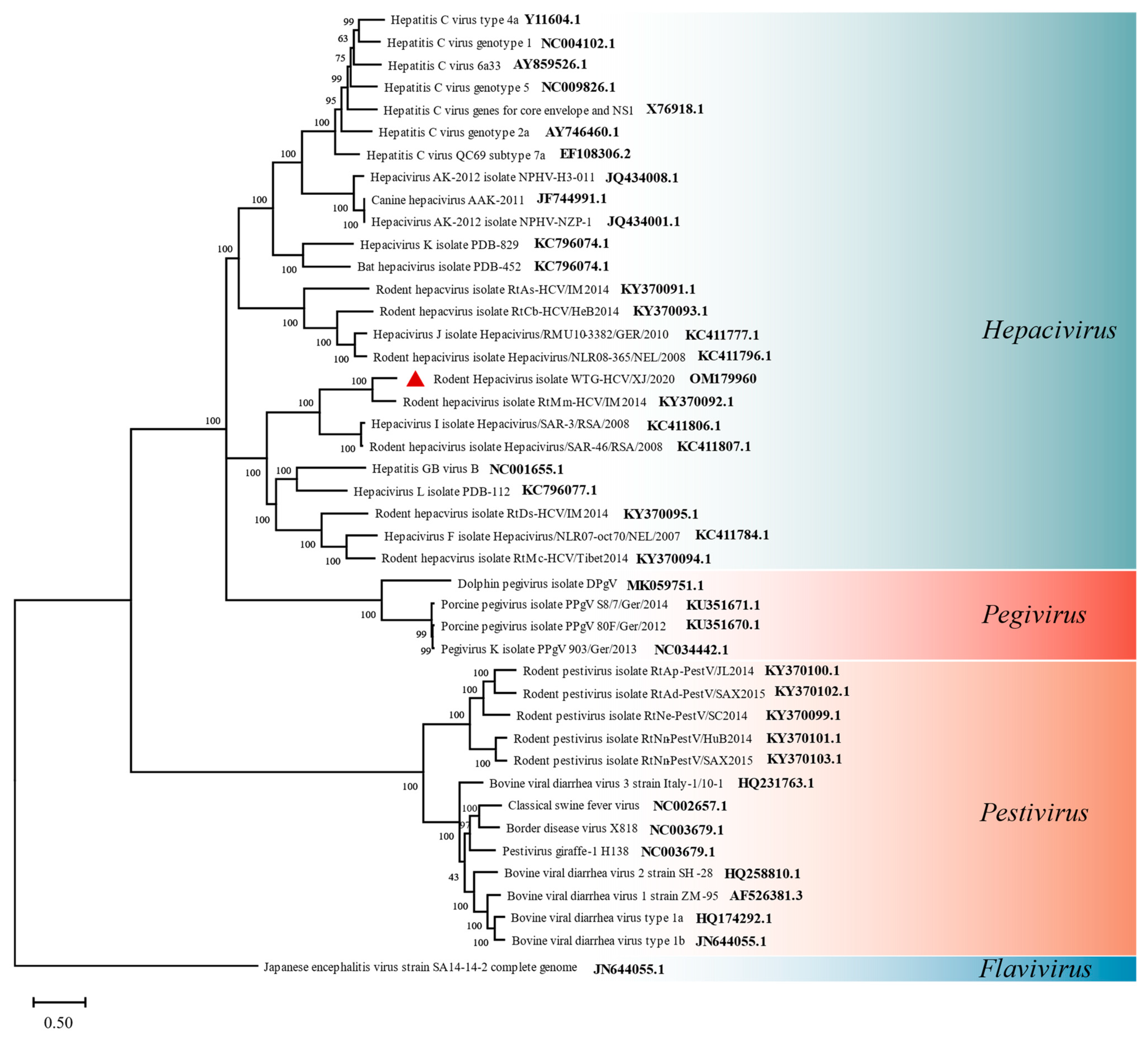
3.4.2. Flaviviridae
3.4.3. Nairoviridae
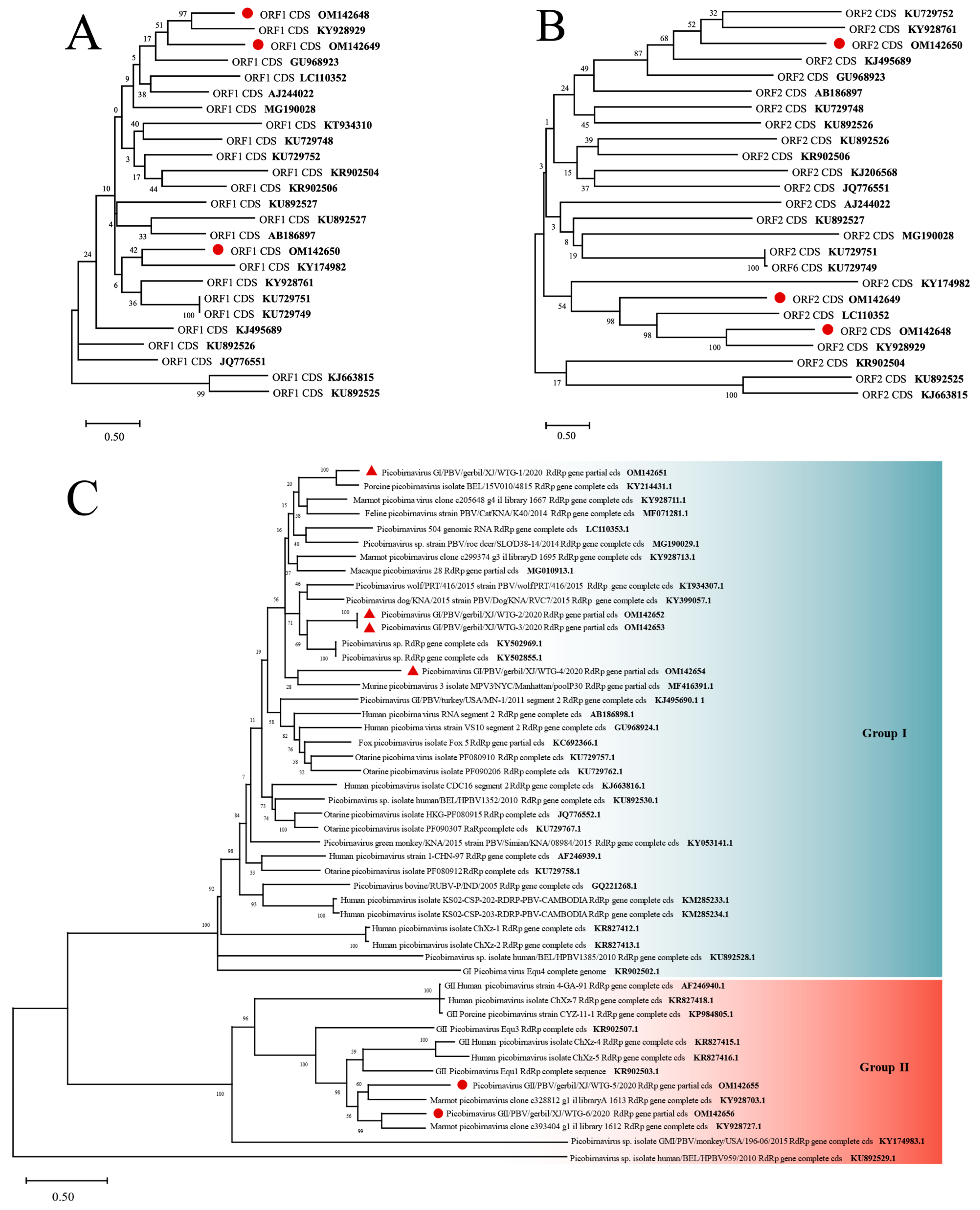
3.4.4. Picobirnaviridae
3.4.5. Low-abundance viruses
3.5. Ten Genome Sequences of Novel Viruses Were Identified
3.5.1. Flaviviridae
3.5.2. Picobirnaviridae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanga-Kanfi, S.; Miranda, H.; Penn, O.; Pupko, T.; DeBry, R.W.; Huchon, D. Rodent phylogeny revised: Analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 2009, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Solari, S.; Baker, R.J. Mammal Species of the World: A Taxonomic and Geographic Reference by D. E. Wilson; D. M. Reeder. J. Mammal. 2007, 88, 824–830. [Google Scholar] [CrossRef]
- Huchon, D.; Madsen, O.; Sibbald, M.J.; Ament, K.; Stanhope, M.J.; Catzeflis, F.; de Jong, W.W.; Douzery, E.J. Rodent phylogeny and a timescale for the evolution of Glires: Evidence from an extensive taxon sampling using three nuclear genes. Mol. Biol. Evol. 2002, 19, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Purvis, A.; MacLarnon, A.N.N.; Bininda-Emonds, O.R.P.; Simmons, N.B. A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol. Rev. 2002, 77, 223–259. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.D.; Hayman, D.T.; O’Shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.; Mills, J.N.; Timonin, M.E.; Willis, C.K.; Cunningham, A.A.; et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc. Biol. Sci. 2013, 280, 20122753. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Warimwe, G.M.; Francis, M.J.; Bowden, T.A.; Thumbi, S.M.; Charleston, B. Using cross-species vaccination approaches to counter emerging infectious diseases. Nat. Rev. Immunol. 2021, 21, 815–822. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef]
- Wu, Z.; Du, J.; Lu, L.; Yang, L.; Dong, J.; Sun, L.; Zhu, Y.; Liu, Q.; Jin, Q. Detection of Hantaviruses and Arenaviruzses in three-toed jerboas from the Inner Mongolia Autonomous Region, China. Emerg. Microbes Infect. 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.; Leung, K.H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.J.; Gilray, J.; Orton, R.J.; Baird, M.; Wilkie, G.; Filipe, A.D.S.; Johnson, N.; McInnes, C.J.; Kohl, A.; Biek, R. Population genomics of louping ill virus provide new insights into the evolution of tick-borne flaviviruses. PLoS Negl. Trop. Dis. 2020, 14, e0008133. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, A.; de Thoisy, B.; Tirera, S.; Donato, D.; Bouchier, C.; Catzeflis, F.; Lacoste, V. Identification of lymphocytic choriomeningitis mammarenavirus in house mouse (Mus musculus, Rodentia) in French Guiana. Infect. Genet. Evol. 2016, 37, 225–230. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.M.; Fumagalli, M.J.; Sabino-Santos, G., Jr.; Motta Maia, F.G.; Modha, S.; Teixeira Nunes, M.R.; Murcia, P.R.; Moraes Figueiredo, L.T. A Novel Hepacivirus in Wild Rodents from South America. Viruses 2019, 11, 297. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- Witkowski, P.T.; Perley, C.C.; Brocato, R.L.; Hooper, J.W.; Jürgensen, C.; Schulzke, J.-D.; Krüger, D.H.; Bücker, R. Gastrointestinal Tract As Entry Route for Hantavirus Infection. Front. Microbiol. 2017, 8, 1721. [Google Scholar] [CrossRef]
- Mir, M. Hantaviruses. Clin. Lab. Med. 2010, 30, 67–91. [Google Scholar] [CrossRef]
- Nichol, S.T.; Spiropoulou, C.F.; Morzunov, S.; Rollin, P.E.; Ksiazek, T.G.; Feldmann, H.; Sanchez, A.; Childs, J.; Zaki, S.; Peters, C.J. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993, 262, 914–917. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, F.X.; Wang, J.B.; Zhao, Z.W.; Li, M.H.; Chen, H.X.; Zou, Y.; Plyusnin, A. Hantaviruses in rodents and humans, Inner Mongolia Autonomous Region, China. Emerg. Infect. Dis. 2009, 15, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Khabbaz, R.F.; Armstrong, L.R.; Holman, R.C.; Bauer, S.P.; Graber, J.; Strine, T.; Miller, G.; Reef, S.; Tappero, J.; et al. Hantavirus pulmonary syndrome: The first 100 US cases. J. Infect. Dis 1996, 173, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Rivers, M.N.; Alexander, J.L.; Rohde, R.E.; Pierce, J.R., Jr. Hantavirus pulmonary syndrome in Texas: 1993–2006. South. Med. J. 2009, 102, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Scheel, T.K.; Simmonds, P.; Kapoor, A. Surveying the global virome: Identification and characterization of HCV-related animal hepaciviruses. Antivir. Res. 2015, 115, 83–93. [Google Scholar] [CrossRef]
- Andrade, K.R.; Boratto, P.P.; Rodrigues, F.P.; Silva, L.C.; Dornas, F.P.; Pilotto, M.R.; La Scola, B.; Almeida, G.M.; Kroon, E.G.; Abrahão, J.S. Oysters as hot spots for mimivirus isolation. Arch. Virol. 2015, 160, 477–482. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Scheel, T.K.; Hjelle, B.; Cullen, J.M.; Burbelo, P.D.; Chauhan, L.V.; Duraisamy, R.; Sanchez Leon, M.; Jain, K.; et al. Identification of rodent homologs of hepatitis C virus and pegiviruses. mBio 2013, 4, e00216-13. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Lukashev, A.N.; Gmyl, A.; Coutard, B.; Adam, A.; Ritz, D.; Leijten, L.M.; van Riel, D.; et al. Evidence for novel hepaciviruses in rodents. PLoS Pathog. 2013, 9, e1003438. [Google Scholar] [CrossRef]
- Quan, P.L.; Firth, C.; Conte, J.M.; Williams, S.H.; Zambrana-Torrelio, C.M.; Anthony, S.J.; Ellison, J.A.; Gilbert, A.T.; Kuzmin, I.V.; Niezgoda, M.; et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl. Acad. Sci. USA 2013, 110, 8194–8199. [Google Scholar] [CrossRef]
- Elia, G.; Caringella, F.; Lanave, G.; Martella, V.; Losurdo, M.; Tittarelli, M.; Colitti, B.; Decaro, N.; Buonavoglia, C. Genetic heterogeneity of bovine hepacivirus in Italy. Transbound Emerg. Dis. 2020, 67, 2731–2740. [Google Scholar] [CrossRef]
- Berggren, K.A.; Suzuki, S.; PLoSs, A. Animal Models Used in Hepatitis C Virus Research. Int. J. Mol. Sci. 2020, 21, 3869. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-C.; Riezu-Boj, J.-I.; Lasarte, J.-J.; Guillen, J.; Su, J.-H.; Civeira, M.-P.; Prieto, J. Transmission of hepatitis C virus infection to tree shrews. Virology 1998, 244, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Scull, M.A.; Shi, C.; de Jong, Y.P.; Gerold, G.; Ries, M.; von Schaewen, M.; Donovan, B.M.; Labitt, R.N.; Horwitz, J.A.; Gaska, J.M. Hepatitis C virus infects rhesus macaque hepatocytes and simianized mice. Hepatology 2015, 62, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Vasilakis, N.; Tian, J.H.; Li, C.X.; Chen, L.J.; Eastwood, G.; Diao, X.N.; Chen, M.H.; Chen, X.; et al. Divergent Viruses Discovered in Arthropods and Vertebrates Revise the Evolutionary History of the Flaviviridae and Related Viruses. J. Virol. 2016, 90, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.G.; Flewett, T.H.; Candeias, J.A.; Barth, O.M. A virus with a bisegmented double-stranded RNA genome in rat (Oryzomys nigripes) intestines. J. Gen. Virol. 1988, 69 Pt 11, 2749–2754. [Google Scholar] [CrossRef]
- Chandra, R. Picobirnavirus, a novel group of undescribed viruses of mammals and birds: A minireview. Acta Virol. 1997, 41, 59–62. [Google Scholar]
- Phan, T.G.; Kapusinszky, B.; Wang, C.; Rose, R.K.; Lipton, H.L.; Delwart, E.L. The fecal viral flora of wild rodents. PLoS Pathog. 2011, 7, e1002218. [Google Scholar] [CrossRef]
- Bodewes, R.; van der Giessen, J.; Haagmans, B.L.; Osterhaus, A.D.; Smits, S.L. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J. Virol. 2013, 87, 7758–7764. [Google Scholar] [CrossRef]
- Smits, S.L.; van Leeuwen, M.; Schapendonk, C.M.; Schürch, A.C.; Bodewes, R.; Haagmans, B.L.; Osterhaus, A.D. Picobirnaviruses in the human respiratory tract. Emerg. Infect. Dis. 2012, 18, 1539–1540. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Williams, M.M.; Koraka, P.; Simon, J.H.; Smits, S.L.; Osterhaus, A.D. Human picobirnaviruses identified by molecular screening of diarrhea samples. J. Clin. Microbiol. 2010, 48, 1787–1794. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351.e2. [Google Scholar] [CrossRef]
- Drewes, S.; Straková, P.; Drexler, J.F.; Jacob, J.; Ulrich, R.G. Assessing the Diversity of Rodent-Borne Viruses: Exploring of High-Throughput Sequencing and Classical Amplification/Sequencing Approaches. Adv. Virus Res. 2017, 99, 61–108. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Wei, H.Y.; Huang, S.; Yao, T.; Gao, F.; Jiang, J.Z.; Wang, J.Y. Detection of viruses in abalone tissue using metagenomics technology. Aquac. Res. 2018, 49, 2704–2713. [Google Scholar] [CrossRef]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; Kyrpides, N.C. Uncovering Earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef]
- Skewes-Cox, P.; Sharpton, T.J.; Pollard, K.S.; DeRisi, J.L. Profile Hidden Markov Models for the Detection of Viruses within Metagenomic Sequence Data. PLoS ONE 2014, 9, e105067. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, G.; Lim, E.S.; Droit, L.; Krishnamurthy, S.; Barouch, D.H.; Virgin, H.W.; Wang, D. VirusSeeker, a computational pipeline for virus discovery and virome composition analysis. Virology 2017, 503, 21–30. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wakuda, M.; Pongsuwanna, Y.; Taniguchi, K. Complete nucleotide sequences of two RNA segments of human picobirnavirus. J. Virol. Methods 2005, 126, 165–169. [Google Scholar] [CrossRef]
- Mishra, N.; Fagbo, S.F.; Alagaili, A.N.; Nitido, A.; Williams, S.H.; Ng, J.; Lee, B.; Durosinlorun, A.; Garcia, J.A.; Jain, K.; et al. A viral metagenomic survey identifies known and novel mammalian viruses in bats from Saudi Arabia. PLoS ONE 2019, 14, e0214227. [Google Scholar] [CrossRef]
- Vibin, J.; Chamings, A.; Klaassen, M.; Alexandersen, S. Metagenomic characterisation of additional and novel avian viruses from Australian wild ducks. Sci. Rep. 2020, 10, 22284. [Google Scholar] [CrossRef]
- Congying, C.; Yunyan, Z.; Hao, F.; Xinwei, X.; Shaoming, F.; Hui, J.; Jinyuan, W.; Hui, Y.; Jun, G.; Lusheng, H. Expanded catalog of microbial genes and metagenome-assembled genomes from the pig gut microbiome. Nat. Commun. 2021, 12, 1106. [Google Scholar]
- Williams, S.H.; Che, X.; Garcia, J.A.; Klena, J.D.; Lee, B.; Muller, D.; Ulrich, W.; Corrigan, R.M.; Nichol, S.; Jain, K.; et al. Viral Diversity of House Mice in New York City. mBio 2018, 9, e01354-17. [Google Scholar] [CrossRef]
- Wu, Z.; Han, Y.; Liu, B.; Li, H.; Zhu, G.; Latinne, A.; Dong, J.; Sun, L.; Su, H.; Liu, L.; et al. Decoding the RNA viromes in rodent lungs provides new insight into the origin and evolutionary patterns of rodent-borne pathogens in Mainland Southeast Asia. Microbiome 2021, 9, 18. [Google Scholar] [CrossRef]
- Tirera, S.; de Thoisy, B.; Donato, D.; Bouchier, C.; Lacoste, V.; Franc, A.; Lavergne, A. The Influence of Habitat on Viral Diversity in Neotropical Rodent Hosts. Viruses 2021, 13, 1690. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Wu, Z.; Jin, Q.; Yang, J. DRodVir: A resource for exploring the virome diversity in rodents. J. Genet. Genomics 2017, 44, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Rabiela, F.; Suzán, G.; Wiratsudakul, A.; Rico-Chávez, O. Viral metacommunities associated to bats and rodents at different spatial scales. Community Ecol. 2018, 19, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, H.; Shu, J.; Zhang, Q.; Han, M.; Liu, Z.; Jin, X.; Zhang, F.; Wu, X. Vaccines and Therapeutics Against Hantaviruses. Front. Microbiol. 2019, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kasai, H.; Yamashita, A.; Okuyama-Dobashi, K.; Yasumoto, J.; Maekawa, S.; Enomoto, N.; Okamoto, T.; Matsuura, Y.; Morimatsu, M.; et al. Hallmarks of hepatitis C virus in equine hepacivirus. J. Virol. 2014, 88, 13352–13366. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.; Masachessi, G.; Mladenova, Z. Animal picobirnavirus. Virusdisease 2014, 25, 223–238. [Google Scholar] [CrossRef]
- Bányai, K.; Jakab, F.; Reuter, G.; Bene, J.; Uj, M.; Melegh, B.; Szücs, G. Sequence heterogeneity among human picobirnaviruses detected in a gastroenteritis outbreak. Arch. Virol. 2003, 148, 2281–2291. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Sahoo, G.C.; Nayak, M.K.; Saha, D.R.; Sur, D.; Naik, T.N.; Bhattacharya, S.K.; Krishnan, T. Molecular epidemiology of human picobirnaviruses among children of a slum community in Kolkata, India. Infect. Genet. Evol. 2006, 6, 453–458. [Google Scholar] [CrossRef]
- Cascio, A.; Bosco, M.; Vizzi, E.; Giammanco, A.; Ferraro, D.; Arista, S. Identification of picobirnavirus from faeces of Italian children suffering from acute diarrhea. Eur. J. Epidemiol. 1996, 12, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.; Nataraju, S.M.; Rajendran, K.; Ramamurthy, T.; Kanungo, S.; Manna, B.; Nagashima, S.; Sur, D.; Kobayashi, N.; Krishnan, T. Detection of closely related Picobirnaviruses among diarrhoeic children in Kolkata: Evidence of zoonoses? Infect. Genet. Evol. 2010, 10, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Carruyo, G.M.; Mateu, G.; Martínez, L.C.; Pujol, F.H.; Nates, S.V.; Liprandi, F.; Ludert, J.E. Molecular characterization of porcine picobirnaviruses and development of a specific reverse transcription-PCR assay. J. Clin. Microbiol. 2008, 46, 2402–2405. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.I.; Fang, Z.Y.; Glass, R.I.; Monroe, S.S. Cloning of human picobirnavirus genomic segments and development of an RT-PCR detection assay. Virology 2000, 277, 316–329. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, J.; Guo, Y.; Sun, W.; He, Y.; Liu, K.; Yan, J.; Tao, A.; Zhong, N. SARS-CoV-2: Origin, Intermediate Host and Allergenicity Features and Hypotheses. Healthcare 2021, 9, 1132. [Google Scholar] [CrossRef]



| Samples | Raw Reads | Number of Reads Remaining after Filtering (%) | Assembly Data on Rm. rRNA Clean Reads | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clean Reads (PE) | Rm. rRNA (PE) | Virus Reads (PE) | Total No. | Max Len. | Min Len. | N50 | GC (%) | |||||
| C | 68,016,775 | 53,477,225 | 78.62 | 27,236,851 | 50.93 | 6506 | 0.01 | 411,219 | 12,701 | 300 | 610 | 45.46 |
| F | 71,127,692 | 55,001,720 | 77.33 | 52,231,458 | 94.96 | 36,444 | 0.06 | 261,682 | 4443 | 300 | 512 | 45.61 |
| G | 71,699,768 | 54,411,245 | 75.89 | 53,262,395 | 97.89 | 25,760 | 0.05 | 656,296 | 9489 | 300 | 543 | 46.26 |
| N | 71,058,840 | 51,163,121 | 72 | 50,711,357 | 99.12 | 8278 | 0.02 | 708,127 | 18,386 | 300 | 866 | 43.9 |
| P | 70,803,346 | 52,148,081 | 73.65 | 52,071,866 | 99.85 | 27,241 | 0.05 | 680,681 | 20,718 | 300 | 741 | 44.7 |
| SP | 70,587,365 | 47,041,404 | 66.64 | 46,790,577 | 99.47 | 11,248 | 0.02 | 504,349 | 8466 | 300 | 584 | 43.92 |
| X | 69,032,129 | 52,595,690 | 76.19 | 51,802,597 | 98.49 | 21,042 | 0.04 | 427,380 | 6831 | 300 | 527 | 45.61 |
| Mean | 70,332,274 | 52,262,641 | 74.33 | 47,729,586 | 91.53 | 19,503 | 0.04 | 521,391 | 11,576 | 300 | 626 | 45.07 |
| SD | 1,314,630 | 2,651,684 | 0.04 | 9,274,055 | 0.18 | 11,191 | 0.02 | 166,912 | 6037 | 0 | 131 | 0.91 |
| Query Id | Subject Id | Identity (%) | Alignment Length | e-Value | Bit Score |
|---|---|---|---|---|---|
| WTG.F|contig_243821 | LR597639.1 | 100 | 585 | 0 | 1056 |
| WTG.X|contig_22842 | HQ540595.1 | 82.765 | 586 | 2.98 × 10−169 | 602 |
| WTG.X|contig_63540 | M87581.1 | 99.592 | 1226 | 0 | 2189 |
| WTG.C|contig_132444 | MK032740.1 | 83.309 | 2037 | 0 | 2118 |
| WTG.C|contig_85266 | KY214431.1 | 82.672 | 1639 | 0 | 1629 |
| WTG.G|contig_109493 | KY214431.1 | 85.562 | 658 | 0 | 751 |
| WTG.C|contig_323805 | MH412924.1 | 84.261 | 521 | 1.33 × 10−155 | 556 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Zhang, L.; Zhang, X.; Yun, F.; Chang, Y.; Tuersun, A.; Aisaiti, K.; Ma, Z. Metagenome-Assembled Viral Genomes Analysis Reveals Diversity and Infectivity of the RNA Virome of Gerbillinae Species. Viruses 2022, 14, 356. https://doi.org/10.3390/v14020356
Du H, Zhang L, Zhang X, Yun F, Chang Y, Tuersun A, Aisaiti K, Ma Z. Metagenome-Assembled Viral Genomes Analysis Reveals Diversity and Infectivity of the RNA Virome of Gerbillinae Species. Viruses. 2022; 14(2):356. https://doi.org/10.3390/v14020356
Chicago/Turabian StyleDu, Han, Lijuan Zhang, Xinqiang Zhang, Fengze Yun, Yuhao Chang, Awaguli Tuersun, Kamila Aisaiti, and Zhenghai Ma. 2022. "Metagenome-Assembled Viral Genomes Analysis Reveals Diversity and Infectivity of the RNA Virome of Gerbillinae Species" Viruses 14, no. 2: 356. https://doi.org/10.3390/v14020356
APA StyleDu, H., Zhang, L., Zhang, X., Yun, F., Chang, Y., Tuersun, A., Aisaiti, K., & Ma, Z. (2022). Metagenome-Assembled Viral Genomes Analysis Reveals Diversity and Infectivity of the RNA Virome of Gerbillinae Species. Viruses, 14(2), 356. https://doi.org/10.3390/v14020356







