Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Volunteers and Samples
2.2. Detection of Anti-DENV IgG and Anti-ZIKV IgG Antibodies by an In-House ELISA
2.3. The Plaque Reduction Neutralization Test (PRNT)
2.4. PBMC Isolation
2.5. ZIKV CD4 MegaPool Description
2.6. In Vitro T Cell Stimulation
2.7. Extracellular and Intracellular Cytokine Staining for Flow Cytometry
2.8. Statistical Analysis
2.9. Study Approval
3. Results
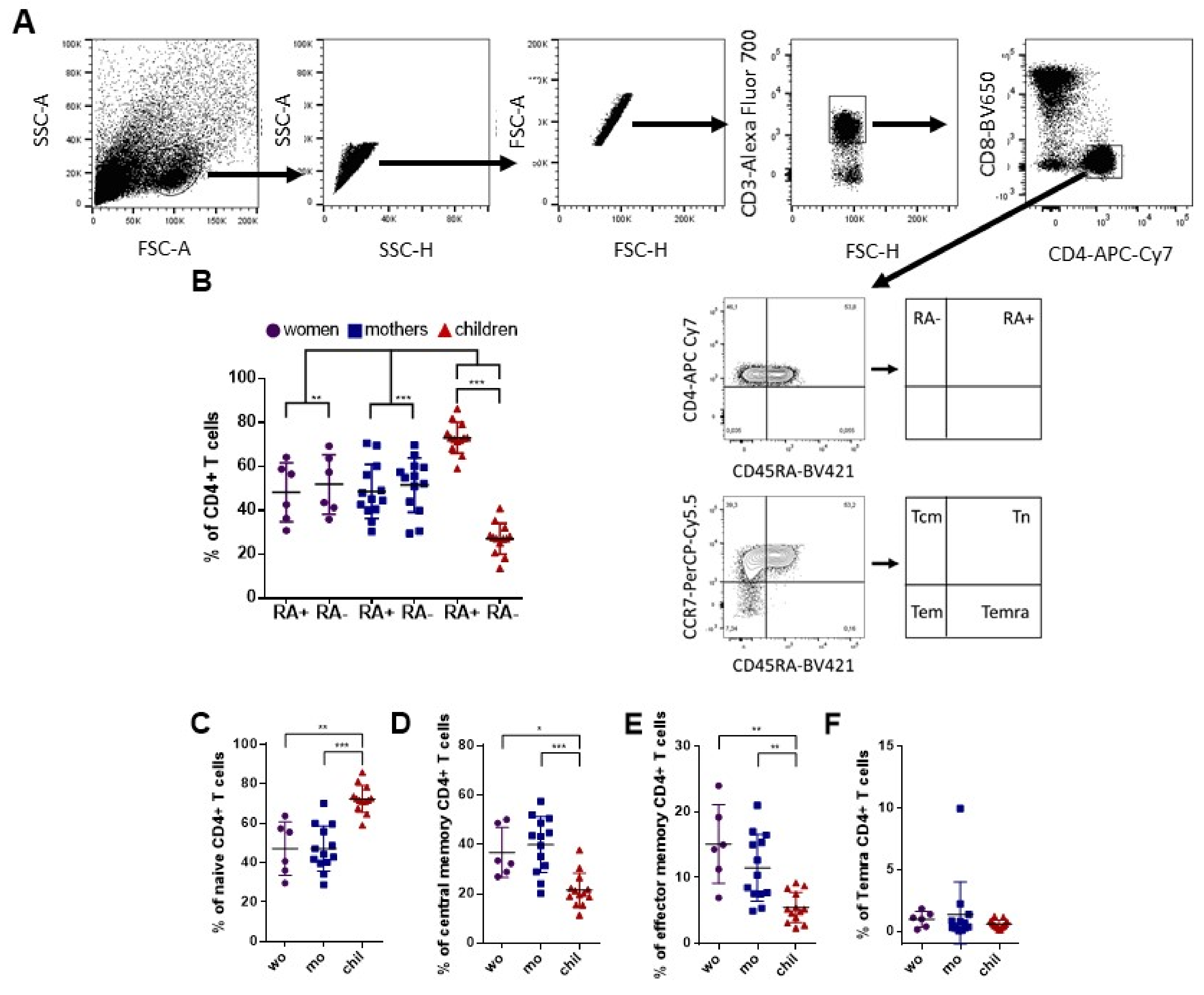
3.1. Variability of CD4+ T Cell Phenotypes among Adult Women and Children after In Vitro Stimulation with ZIKV Megapool (ZIKV MP)
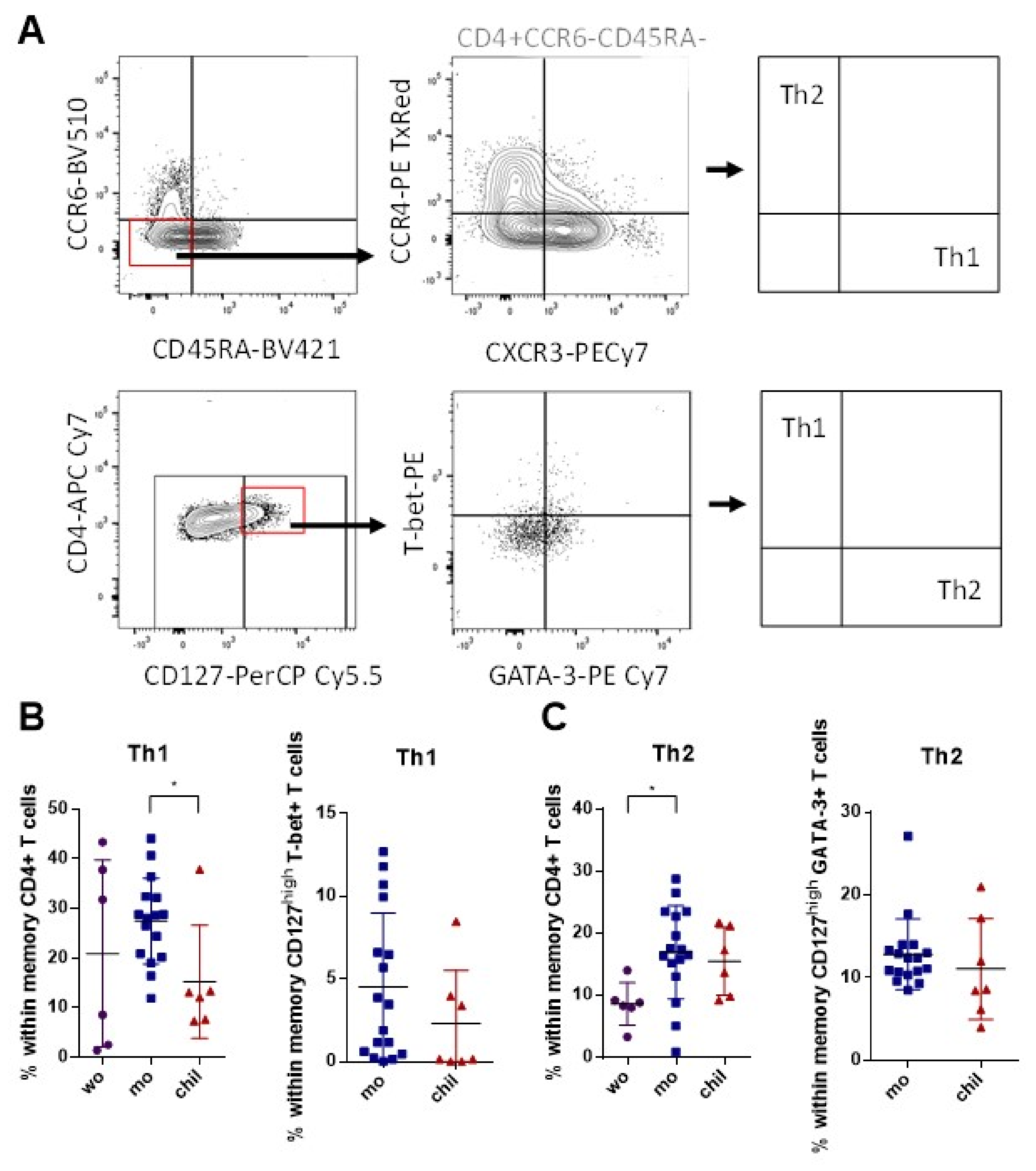
3.2. Higher Frequency of Memory Th1 Cells in Mothers Compared to Children
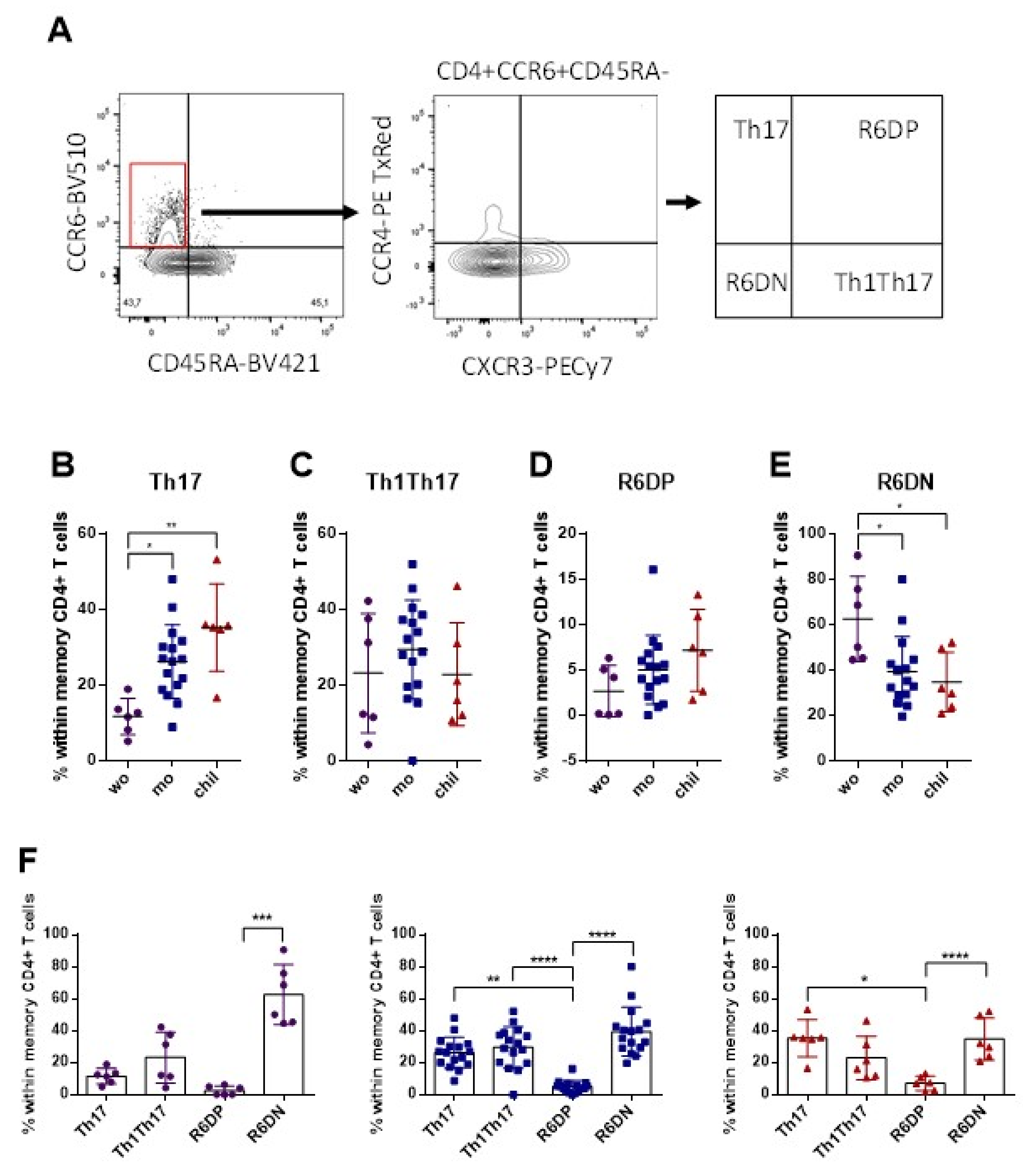
3.3. Higher Frequency of Memory Th17 Cells and Lower Frequency of R6+DN in the Mothers and Children, Compared with Women
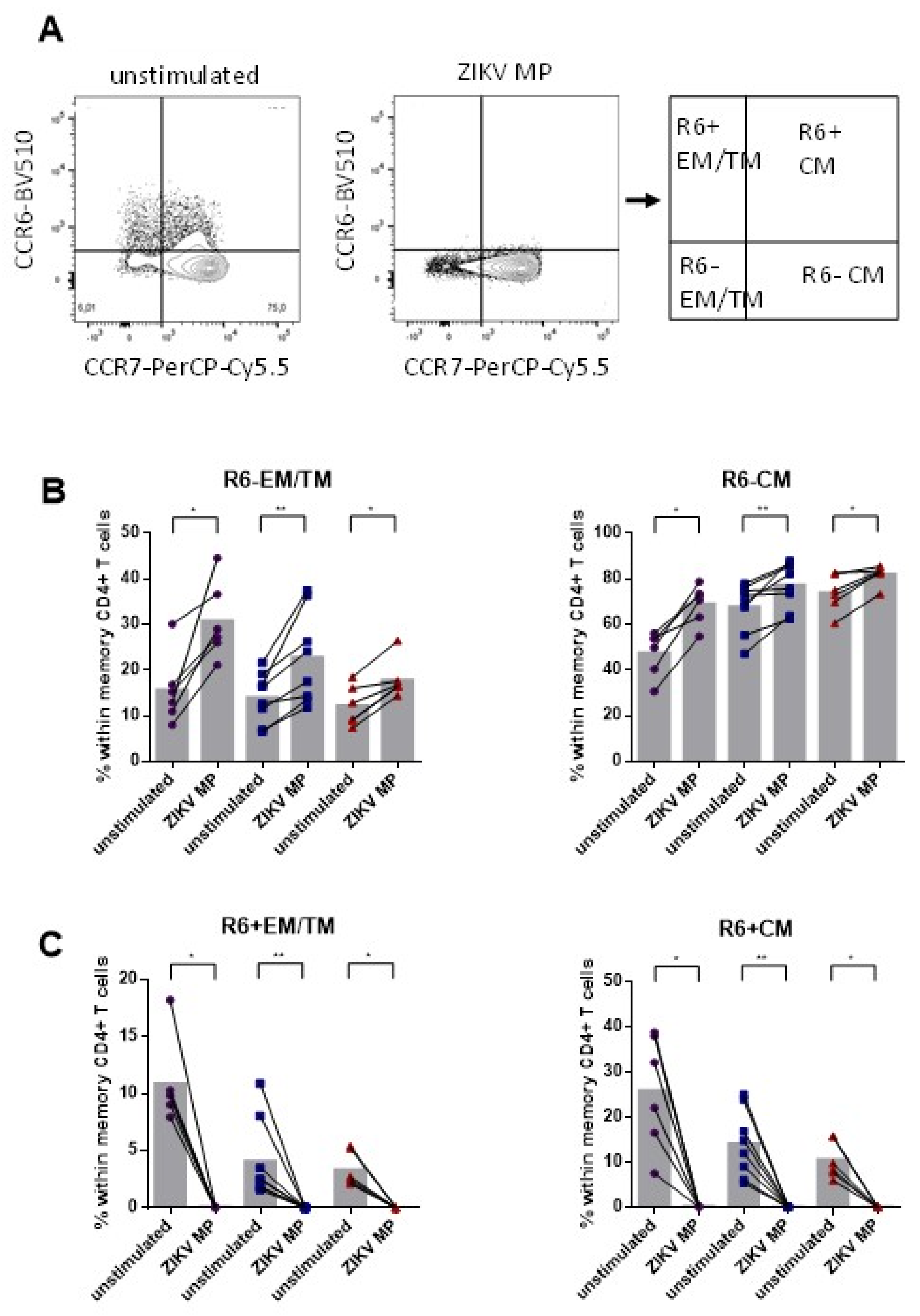
3.4. Lower Frequency of Memory CD4+ CCR6+ Cells after Stimulation with ZIKV MP in Individuals Who Had Recovered from ZIKV Infection
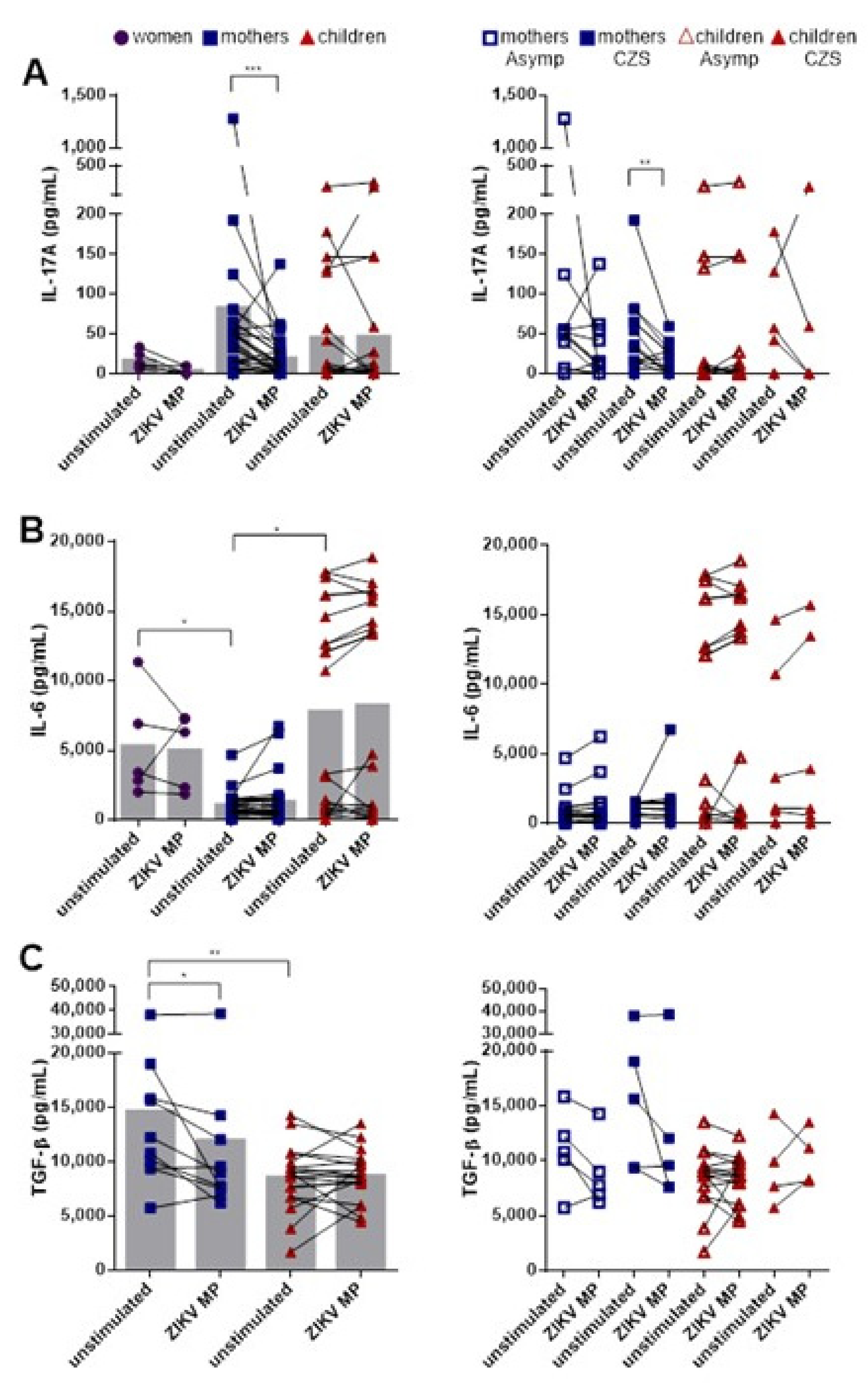
3.5. Lower Production of IL-17A after Stimulation with ZIKV MP in Mothers Who Had Children with CZS
3.6. Lower Frequency of Responding IL-17-Producing CD4+ T Cell Subsets and Higher Frequency of Responding IL-17+ IFN-γ+-Coproducing CD4+ T Cell Subsets in Individuals with Histories of ZIKV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.; Haddow, A. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization; World Health Organization. Epidemiological Update: Neurological Syndrome, Congenital Anomalies and Zika Virus Infection. Washinton, DC. 2016. Available online: https://www.paho.org/hq/dmdocuments/2016/2016-jan-17-cha-epi-update-zika-virus.pdf (accessed on 3 June 2020).
- Ministry of Health of Brazil; Health Surveillance Department. Protocolo de Vigilância e Resposta À Microcefalia Relacionada À Infecção Pelo Vírus Zika. 2015. Available online: https://pesquisa.bvsalud.org/gim/resource/en/lil-773183 (accessed on 3 June 2020).
- Heang, V.; Yasuda, C.Y.; Sovann, L.; Haddow, A.D.; da Rosa, A.P.T.; Tesh, R.B.; Kasper, M.R. Zika virus infection, Cambodia, 2010. Emerg. Infect. Dis. 2012, 18, 349–351. [Google Scholar] [CrossRef]
- De Oliveira, W.K.; Cortez-Escalante, J.; de Oliveira, W.T.G.H.; do Carmo, G.M.I.; Henriques, C.M.P.; Coelho, G.E.; de França, G.V.A. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Calvet, G.A.; Filippis, A.M.B.; Mendonça, M.C.L.; Sequeira, P.C.; Siqueira, A.M.; Veloso, V.G.; Nogueira, R.M.; Brasil, P. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J. Clin. Virol. 2016, 74, 1–3. [Google Scholar] [CrossRef]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; de Filippis, A.M.B. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obs. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Tang, J.; Xu, L.; Ye, L. Molecular basis of the differrentiation and function of virus specific follicular helper CD4+ T cells. Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Zhou, Z.; Liu, Y.; Lai, Y.; Luo, Y.; Peng, X.; Zou, W. Regulatory T cells and T helper 17 cells in viral infection. Scand. J. Immunol. 2020, 91, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Hemmat, N.; Nahand, J.S.; Shamekh, A.; Memar, M.Y.; Babaei, A.; Baghi, H.B. The role of Th17 cells in viral infections. Int. Immunopharmacol. 2021, 91, 33418239. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 lineage: From barrier surfaces homeostasis to autoimmunity, cancer, and HIV-1 pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Zhang, H.H.; Foley, J.F.; Hedrick, M.N.; Farber, J.M. Human T Cells that Are Able to Produce IL-17 Express the Chemokine Receptor CCR6. J. Immunol. 2008, 180, 214–221. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, E.J.; Campbell, D.J.; Butcher, E.C. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation 2003, 10, 313–323. [Google Scholar] [CrossRef]
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-Induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 2012, 484, 514–518. [Google Scholar] [CrossRef]
- Okada, S.; Markle, J.G.; Deenick, E.K.; Mele, F.; Averbuch, D.; Lagos, M.; Alzahrani, M.; Al-Muhsen, S.; Halwani, R.; Ma, C.S.; et al. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015, 349, 606–613. [Google Scholar] [CrossRef] [Green Version]
- Duhen, T.; Campbell, D.J. IL-1β Promotes the Differentiation of Polyfunctional Human CCR6 + CXCR3 + Th1/17 Cells that Are Specific for Pathogenic and Commensal Microbes. J. Immunol. 2014, 193, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, A.; Maggi, L.; Liotta, F.; Cosmi, L.; Annunziato, F. Biological and clinical significance of T helper 17 cell plasticity. Immunology 2019, 158, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Wacleche, V.S.; Goulet, J.P.; Gosselin, A.; Monteiro, P.; Soudeyns, H.; Fromentin, R.; Jenabian, M.A.; Vartanian, S.; Deeks, S.G.; Chomont, N.; et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology 2016, 13, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tappe, D.; Pérez-Girón, J.V.; Zammarchi, L.; Rissland, J.; Ferreira, D.F.; Jaenisch, T.; Gómez-Medina, S.; Günther, S.; Bartoloni, A.; Muñoz-Fontela, C.; et al. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med. Microbiol. Immunol. 2016, 205, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Quental, O.B.; França, E.L.; Honório-França, A.C.; Morais, T.C.; Daboin, B.E.G.; Bezerra, I.M.P.; Komninakis, S.V.; de Abreu, L.C. Zika Virus Alters the Viscosity and Cytokines Profile in Human Colostrum. J. Immunol. Res. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.S.S.; de Sousa, J.R.; Araujo, M.T.F.; Martins Filho, A.J.; de Alcantara, B.N.; Araujo, F.M.C.; Queiroz, M.G.L.; Cruz, A.C.R.; Vasconcelos, B.H.B.; Chiang, J.O.; et al. In Situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fares-Gusmao, R.; Rocha, B.C.; Sippert, E.; Lanteri, M.C.; Áñez, G.; Rios, M. Differential Pattern of Soluble Immune Markers in Asymptomatic Dengue, West Nile and Zika Virus Infections. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Bränn, E.; Edvinsson, Å.; Rostedt Punga, A.; Sundström-Poromaa, I.; Skalkidou, A. Inflammatory and anti-inflammatory markers in plasma: From late pregnancy to early postpartum. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- De Oliveira Vianna, R.A.; Lovero, K.L.; de Oliveira, S.A.; Fernandes, A.R.; dos Santos, T.C.S.; de Souza Lima, L.C.S.; Carvalho, F.R.; Quintans, M.D.S.; Bueno, A.C.; Torbey, A.F.M.; et al. Children Born to Mothers with Rash during Zika Virus Epidemic in Brazil: First 18 Months of Life. J. Trop. Pediatr. 2019, 65, 592–602. [Google Scholar] [CrossRef]
- Ministry of Healthy of Brazil. Zika Virus in Brasil. 2017. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2017/setembro/21/zika-virus-in-brazil-2017.pdf (accessed on 3 June 2020).
- Miagostovich, M.P.; Nogueira, R.M.R.; dos Santos, F.B.; Schatzmayr, H.G.; Araújo, E.S.M.; Vorndam, V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J. Clin. Virol. 1999, 14, 183–189. [Google Scholar] [CrossRef]
- Steinhagen, K.; Probst, C.; Radzimski, C.; Schmidt-Chanasit, J.; Emmerich, P.; van Esbroeck, M.; Schinkel, J.; Grobusch, M.P.; Goorhuis, A.; Warnecke, J.M.; et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: A multicohort study of assay performance, 2015 to 2016. Eurosurveillance 2016, 21, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Secretaria Estadual de Saúde do Rio de Janeiro. Boletim Epidemiológico; 005/2016; Secretaria Estadual de Saúde do Rio de Janeiro: Rio de Janeiro, Brazil, 2016.
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Russell, P.K.; Nisalak, A.; Sukhavachana, P.; Vivona, S. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 1967, 99, 285–290. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6031202 (accessed on 4 June 2020).
- Grifoni, A.; Tian, Y.; Sette, A.; Weiskopf, D. Transcriptomic immune profiles of human flavivirus-specific T-cell responses. Immunology 2020, 160, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Grifoni, A.; Voic, H.; Dhanda, S.K.; Kidd, C.K.; Brien, J.D.; Buus, S.; Stryhn, A.; Durbin, A.P.; Whitehead, S.; Diehl, S.A.; et al. T Cell Responses Induced by Attenuated Flavivirus Vaccination Are Specific and Show Limited Cross-Reactivity with Other Flavivirus Species. J. Virol. 2020, 94, e00089-20. [Google Scholar] [CrossRef]
- Xu, X.; Vaughan, K.; Weiskopf, D.; Grifoni, A.; Diamond, M.S.; Sette, A.; Peters, B. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sidney, J.; Sette, A.; Peters, B. TepiTool: A pipeline for computational prediction of T cell epitope candidates. Curr. Protoc. Immunol. 2016, 2016, 18.19.1–18.19.24. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Mahajan, S.; Paul, S.; Yan, Z.; Kim, H.; Jespersen, M.C.; Jurtz, V.; Andreatta, M.; Greenbaum, J.A.; Marcatili, P.; et al. IEDB-AR: Immune epitope database-analysis resource in 2019. Nucleic Acids Res. 2019, 47, W502–W506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Lindestam Arlehamn, C.S.; Scriba, T.J.; Dillon, M.B.C.; Oseroff, C.; Hinz, D.; McKinney, D.M.; Carrasco Pro, S.; Sidney, J.; Peters, B.; et al. Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. J. Immunol. Methods 2015, 422, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Dhanda, S.K.; Vaughan, K.; Schulten, V.; Grifoni, A.; Weiskopf, D.; Sidney, J.; Peters, B.; Sette, A. Development of a novel clustering tool for linear peptide sequences. Immunology 2018, 155, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Grifoni, A.; Costa-Ramos, P.; Pham, J.; Tian, Y.; Rosales, S.L.; Seumois, G.; Sidney, J.; de Silva, A.D.; Premkumar, L.; Collins, M.H.; et al. Cutting Edge: Transcriptional Profiling Reveals Multifunctional and Cytotoxic Antiviral Responses of Zika Virus–Specific CD8 + T Cells. J. Immunol. 2018, 201, 3487–3491. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Weiskopf, D.; Angelo, M.A.; Sidney, J.; Peters, B.; Sette, A. HLA Class I Alleles Are Associated with Peptide-Binding Repertoires of Different Size, Affinity, and Immunogenicity. J. Immunol. 2013, 191, 5831–5839. [Google Scholar] [CrossRef] [Green Version]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; de Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [Green Version]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; de Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar] [CrossRef] [Green Version]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef] [Green Version]
- Huster, K.M.; Busch, V.; Schiemann, M.; Linkemann, K.; Kerksiek, K.M.; Wagner, H.; Busch, D.H. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 2004, 101, 5610–5615. [Google Scholar] [CrossRef] [Green Version]
- Cossarizza, A.; Ortolani, C.; Paganelli, R.; Barbieri, D.; Monti, D.; Sansoni, P.; Fagiolo, U.; Castellani, G.; Bersani, F.; Londei, M.; et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: Implications for T cell memory. Mech. Ageing Dev. 1996, 86, 173–195. [Google Scholar] [CrossRef]
- Saule, P.; Trauet, J.; Dutriez, V.; Lekeux, V.; Dessaint, J.P.; Labalette, M. Accumulation of memory T cells from childhood to old age: Central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech. Ageing Dev. 2006, 127, 274–281. [Google Scholar] [CrossRef]
- Wan, Q.; Kozhaya, L.; ElHed, A.; Ramesh, R.; Carlson, T.J.; Djuretic, I.M.; Sundrud, M.S.; Unutmaz, D. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J. Exp. Med. 2011, 208, 1875–1887. [Google Scholar] [CrossRef] [Green Version]
- Acharya, D.; Wang, P.; Paul, A.M.; Dai, J.; Gate, D.; Lowery, J.E.; Stokic, D.S.; Leis, A.A.; Flavell, R.A.; Town, T.; et al. Interleukin-17A Promotes CD8+ T Cell Cytotoxicity to Facilitate West Nile Virus Clearance. J. Virol. 2017, 91, e01529-16. [Google Scholar] [CrossRef] [Green Version]
- Anipindi, V.C.; Bagri, P.; Roth, K.; Dizzell, S.E.; Nguyen, P.V.; Shaler, C.R.; Chu, D.K.; Jiménez-Saiz, R.; Liang, H.; Swift, S.; et al. Estradiol Enhances CD4+ T-Cell Anti-Viral Immunity by Priming Vaginal DCs to Induce Th17 Responses via an IL-1-Dependent Pathway. PLoS Pathog. 2016, 12, 1–27. [Google Scholar] [CrossRef]
- Wang, X.; Chan, C.C.S.; Yang, M.; Deng, J.; Poon, V.K.M.; Leung, V.H.C.; Ko, K.H.; Zhou, J.; Yung Yuen, K.; Zheng, B.J.; et al. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol. Immunol. 2011, 8, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef]
- Wu, X.; Tian, J.; Wang, S. Insight into non-pathogenic Th17 cells in autoimmune diseases. Front. Immunol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Wei, B.; Deng, Y.; Huang, Y.; Gao, X.; Wu, W. IL-10-producing B cells attenuate cardiac inflammation by regulating Th1 and Th17 cells in acute viral myocarditis induced by coxsackie virus B3. Life Sci. 2019, 235, 116838. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, M.; Lin, Q.W.; Cao, A.L.; Yu, X.; Dong, J.H.; Wang, J.P.; Zhang, J.H.; Wang, M.; Guo, H.P.; et al. Neutralization of IL-17 inhibits the production of anti-ANT autoantibodies in CVB3-induced acute viral myocarditis. Int. Immunopharmacol. 2010, 10, 272–276. [Google Scholar] [CrossRef]
- Halai, U.A.; Nielsen-Saines, K.; Moreira, M.L.; de Sequeira, P.C.; Pereira, J.P.; de Araujo Zin, A.; Cherry, J.; Gabaglia, C.R.; Gaw, S.L.; Adachi, K.; et al. Maternal Zika virus disease severity, virus load, prior dengue antibodies, and their relationship to birth outcomes. Clin. Infect. Dis. 2017, 65, 877–883. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, T.V.B.; Rodrigues, L.C.; de Alencar Ximenes, R.A.; de Barros Miranda-Filho, D.; Montarroyos, U.R.; de Melo, A.P.L.; Valongueiro, S.; de Albuquerque, M.d.F.P.M.; Souza, W.V.; Braga, C.; et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016, 16, 1356–1363. [Google Scholar] [CrossRef] [Green Version]
- Robbiani, D.F.; Olsen, P.C.; Costa, F.; Wang, Q.; Oliveira, T.Y.; Nery, N.; Aromolaran, A.; do Rosário, M.S.; Sacramento, G.A.; Cruz, J.S.; et al. Risk of Zika microcephaly correlates with features of maternal antibodies. J. Exp. Med. 2019, 216, 2302–2315. [Google Scholar] [CrossRef] [Green Version]
- Badolato-Corrêa, J.; Carvalho, F.R.; Paiva, I.A.; Familiar-Macedo, D.; Dias, H.G.; Pauvolid-Corrêa, A.; Fernandes-Santos, C.; Lima, M.d.R.Q.; Gandini, M.; Silva, A.A.; et al. Differential Longevity of Memory CD4 and CD8 T Cells in a Cohort of the Mothers with a History of ZIKV Infection and Their Children. Front. Immunol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Wong, M.T.; Ong, D.E.H.; Lim, F.S.H.; Teng, K.W.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456. [Google Scholar] [CrossRef] [Green Version]
- Sano, T.; Huang, W.; Hall, J.A.; Yang, Y.; Chen, A.; Gavzy, S.J.; Lee, J.Y.; Ziel, J.W.; Miraldi, E.R.; Domingos, A.I.; et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 2015, 163, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Naveca, F.G.; Pontes, G.S.; Chang, A.Y.H.; da Silva, G.A.V.; do Nascimento, V.A.; da Silva Monteiro, D.C.; da Silva, M.S.; Abdalla, L.F.; Santos, J.H.A.; de Almeida, T.A.P.; et al. Analysis of the immunological biomarker profile during acute zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Inst. Oswaldo Cruz 2018, 113, 1–13. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Defining the human T helper 17 cell phenotype. Trends Immunol. 2012, 33, 505–512. [Google Scholar] [CrossRef]
- Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Annunziato, F. Th17 plasticity: Pathophysiology and treatment of chronic inflammatory disorders. Curr. Opin. Pharmacol. 2014, 17, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Anderson, D.E.; Baecher-allan, C.; Hastings, W.D.; Oukka, M.; Kuchroo, V.K.; Hafler, D.A. IL-21 and TGF-β are required for differentiation of human T(H) 17cells. Nature 2008, 454, 350–352. [Google Scholar] [CrossRef]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupé, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef]
- Pereira Neto, T.A.; Gonçalves-Pereira, M.H.; de Queiroz, C.P.; Ramos, M.F.; de Oliveira, F.d.F.S.; Oliveira-Prado, R.; do Nascimento, V.A.; Abdalla, L.F.; Santos, J.H.A.; Martins-Filho, O.A.; et al. Multifunctional T cell response in convalescent patients two years after ZIKV infection. J. Leukoc. Biol. 2020, 108, 1265–1277. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Soon, M.S.; Engel, J.A.; Lee, H.J.; Haque, A. Development of circulating CD4+ T-cell memory. Immunol. Cell Biol. 2019, 97, 617–624. [Google Scholar] [CrossRef]
- Kirman, J.R.; Quinn, K.M.; Seder, R.A. Immunological memory. Immunol. Cell Biol. 2019, 97, 615–616. [Google Scholar] [CrossRef]
- Grifoni, A.; Angelo, M.A.; Lopez, B.; O’Rourke, P.H.; Sidney, J.; Cerpas, C.; Balmaseda, A.; Silveira, C.G.T.; Maestri, A.; Costa, P.R.; et al. Global Assessment of Dengue Virus-Specific CD4+ T Cell Responses in Dengue-Endemic Areas. Front. Immunol. 2017, 8, 1309. [Google Scholar] [CrossRef]
- Campbell, V.L.; Nguyen, L.; Snoey, E.; McClurkan, C.L.; Laing, K.J.; Dong, L.; Sette, A.; Lindestam Arlehamn, C.S.; Altmann, D.M.; Boyton, R.J.; et al. Proteome-Wide Zika Virus CD4 T Cell Epitope and HLA Restriction Determination. Immunohorizons 2020, 4, 444–453. [Google Scholar] [CrossRef]
- Lim, M.Q.; Kumaran, E.A.P.; Tan, H.C.; Lye, D.C.; Leo, Y.S.; Ooi, E.E.; MacAry, P.A.; Bertoletti, A.; Rivino, L. Cross-Reactivity and Anti-viral Function of Dengue Capsid and NS3-Specific Memory T Cells Toward Zika Virus. Front. Immunol. 2018, 9, 2225. [Google Scholar] [CrossRef] [Green Version]
- Abromson-Leeman, S.; Bronson, R.T.; Dorf, M.E. Encephalitogenic T cells that stably express both T-bet and RORγt consistently produce IFNγ but have a spectrum of IL-17 profiles. J. Neuroimmunol. 2009, 215, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Hirota, K.; Duarte, J.H.; Veldhoen, M.; Hornsby, E.; Li, Y.; Cua, D.J.; Ahlfors, H.; Wilhelm, C.; Tolaini, M.; Menzel, U.; et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011, 12, 255–263. [Google Scholar] [CrossRef]
- Kara, E.E.; McKenzie, D.R.; Bastow, C.R.; Gregor, C.E.; Fenix, K.A.; Ogunniyi, A.D.; Paton, J.C.; Mack, M.; Pombal, D.R.; Seillet, C.; et al. CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat. Commun. 2015, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Grifka-Walk, H.M.; Giles, D.A.; Segal, B.M. IL-12-polarized Th1 cells produce GM-CSF and induce EAE independent of IL-23. Eur. J. Immunol. 2015, 45, 2780–2786. [Google Scholar] [CrossRef] [Green Version]
- Rothhammer, V.; Heink, S.; Petermann, F.; Srivastava, R.; Claussen, M.C.; Hemmer, B.; Korn, T. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J. Exp. Med. 2011, 208, 2465–2476. [Google Scholar] [CrossRef] [Green Version]

| Group | Outcome at Birth | ID | Age a,b | Illness Tine b | Gestational Trimester at Onset Rash | RT-qPCR ZIKV | ZIKV Anti-IgG | DENV Anti-IgG | PRNT50 ZIKV | PRNT90 ZIKV | PRNT90 DENV-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | W1 | 40 | 36 | Pos | Pos | Pos | 80, Pos | <10, Neg | ≥10, Pos | ||
| W2 | 40 | 40 | Pos | Pos | Pos | ≥320, Pos | <10, Neg | <10, Neg | |||
| W3 | 23 | 38 | Pos | Pos | Pos | 80, Pos | <10, Neg | ≥10, Pos | |||
| W4 | 25 | 42 | Pos | Pos | Pos | 40, Pos | <10, Neg | ≥10, Pos | |||
| W5 | 27 | 35 | Pos | Pos | Pos | <10, Neg | <10, Neg | ≥10, Pos | |||
| W6 | 35 | 36 | Pos | Pos | Pos | - | - | - | |||
| 31 | 37 | 6/6 pos | 6/6 pos | 6/6 por | 4/5 pos | 0/5 pos | 4/5 pos | ||||
| (23–40) | (35–42) | 100% | 100% | 100% | 80% | 0% | 80% | ||||
| Mothers | Asympt. | M1 | 22 | 23 | 3rd | Pos | Pos | Neg | ≥320, Pos | 160, Pos | <10, Neg |
| M2 | 36 | 25 | 3rd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M3 | 22 | 23 | 1st | Pos | Pos | Neg | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M4 | 37 | 23 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M5 | 27 | 22 | 2nd | Pos | Pos | Neg | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M6 | 37 | 24 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M7 | 29 | 23 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M8 | 21 | 23 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M9 | 32 | 40 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M10 | 30 | 35 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| 29 | 26 | 10/10 pos | 10/10 pos | 7/10 pos | 10/10 pos | 10/10 pos | 9/10 pos | ||||
| (21–37) | (22–40) | 100% | 100% | 70% | 100% | 100% | 100% | ||||
| CZS | M1 | 21 | 19 | 1st | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | |
| M2 | 23 | 10 | 2nd | Pos | Neg | Neg | ≥320, Pos | ≥320, Pos | <10, Neg | ||
| M3 | 24 | 22 | 1st | Pos | Pos | Neg | ≥320, Pos | ≥320, Pos | <10, Neg | ||
| M4 | 42 | 20 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M5 | 25 | 37 | 3rd | Pos | Pos | Neg | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M6 | 40 | 39 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M7 | 45 | 24 | 3rd | Pos | Neg | Neg | ≥320, Pos | 160, Pos | <10, Neg | ||
| M8 | 41 | 29 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | <10, Neg | ||
| M9 | 28 | 26 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M10 | 21 | 36 | before | Pos | Neg | Neg | ≥320, Pos | ≥320, Pos | <10, Neg | ||
| M11 | 28 | 24 | 1st | Pos | Neg | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| M12 | 33 | 35 | 2nd | Pos | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| 31 | 28 | 12/12 pos | 8/12 pos | 7/12 pos | 12/12 pos | 12/12 pos | 7/12 pos | ||||
| (21–45) | (10–39) | 100% | 67% | 58% | 100% | 100% | 58% | ||||
| Children | Asympt. | C1 | 31 | 35 | x | x | Neg | Pos | |||
| C2 | 29 | 37 | x | x | Neg | Neg | <10, Neg | <10, Neg | ≥10, Pos | ||
| C3 | 34 | 36 | x | x | Pos | Pos | ≥320, Pos | ≥320, Pos | ≥10, Pos | ||
| C4 | 30 | 36 | x | x | Neg | Neg | |||||
| C5 | 41 | 45 | x | x | Neg | Neg | <10, Neg | <10, Neg | ≥10, Pos | ||
| C6 | 31 | 37 | x | x | - | - | |||||
| C7 | 32 | 35 | x | x | Neg | Neg | <10, Neg | <10, Neg | <10, Neg | ||
| C8 | 36 | 42 | x | x | - | - | |||||
| C9 | 30 | 36 | x | x | Pos | Pos | |||||
| C10 | 38 | 42 | x | x | Neg | Neg | <10, Neg | <10, Neg | <10, Neg | ||
| C11 | 24 | 33 | x | x | Neg | Neg | <10, Neg | <10, Neg | ≥10, Pos | ||
| 32 | 38 | 2/9 pos | 3/9 pos | 1/6 pos | 1/7 pos | 4/6 pos | |||||
| (24–41) | (33–45) | 22% | 33% | 14% | 17% | 67% | |||||
| CZS | C1 | 17 | 18 | x | x | Neg | Pos | <10, Neg | <10, Neg | ≥10, Pos | |
| C2 | 32 | 36 | x | x | Neg | Neg | |||||
| C3 | 22 | 28 | x | x | Neg | Pos | ≥320, Pos | 160, Pos | ≥10, Pos | ||
| C4 | 30 | 36 | x | x | - | - | <10, Neg | <10, Neg | ≥10, Pos | ||
| C5 | 32 | 36 | x | x | Neg | Neg | <10, Neg | <10, Neg | ≥10, Pos | ||
| C6 | 24 | 36 | x | x | Neg | Neg | <10, Neg | <10, Neg | ≥10, Pos | ||
| C7 | 27 | 34 | x | x | Neg | Pos | <10, Neg | <10, Neg | <10, Neg | ||
| C8 | 32 | 38 | x | x | Neg | Pos | <10, Neg | <10, Neg | ≥10, Pos | ||
| C9 | 26 | 32 | x | x | Neg | Neg | |||||
| 27 | 33 | 0/8 pos | 4/8 pos | 1/7 pos | 1/7 pos | 6/7 pos | |||||
| (17–32) | (18–38) | 0% | 50% | 17% | 17% | 86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, I.A.; Familiar-Macedo, D.; Badolato-Corrêa, J.; Carvalho, F.R.; Dias, H.G.; Pauvolid-Corrêa, A.; Santos, C.F.d.; Silva, A.A.; Azeredo, E.L.d.; Vianna, R.A.d.O.; et al. Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS). Viruses 2022, 14, 250. https://doi.org/10.3390/v14020250
Paiva IA, Familiar-Macedo D, Badolato-Corrêa J, Carvalho FR, Dias HG, Pauvolid-Corrêa A, Santos CFd, Silva AA, Azeredo ELd, Vianna RAdO, et al. Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS). Viruses. 2022; 14(2):250. https://doi.org/10.3390/v14020250
Chicago/Turabian StylePaiva, Iury Amancio, Débora Familiar-Macedo, Jéssica Badolato-Corrêa, Fabiana Rabe Carvalho, Helver Gonçalves Dias, Alex Pauvolid-Corrêa, Caroline Fernandes dos Santos, Andréa Alice Silva, Elzinandes Leal de Azeredo, Renata Artimos de Oliveira Vianna, and et al. 2022. "Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS)" Viruses 14, no. 2: 250. https://doi.org/10.3390/v14020250
APA StylePaiva, I. A., Familiar-Macedo, D., Badolato-Corrêa, J., Carvalho, F. R., Dias, H. G., Pauvolid-Corrêa, A., Santos, C. F. d., Silva, A. A., Azeredo, E. L. d., Vianna, R. A. d. O., Cardoso, C. A. A., Grifoni, A., Sette, A., Weiskopf, D., & de-Oliveira-Pinto, L. M. (2022). Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS). Viruses, 14(2), 250. https://doi.org/10.3390/v14020250









