Effectiveness of Hepatitis B Vaccination Campaign in Italy: Towards the Control of HBV Infection for the First Time in a European Country
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Incidence Rates Estimate
2.3. Case Control Study
2.4. Statistical Analysis
3. Results
3.1. Incidence Data
3.2. Main Characteristics
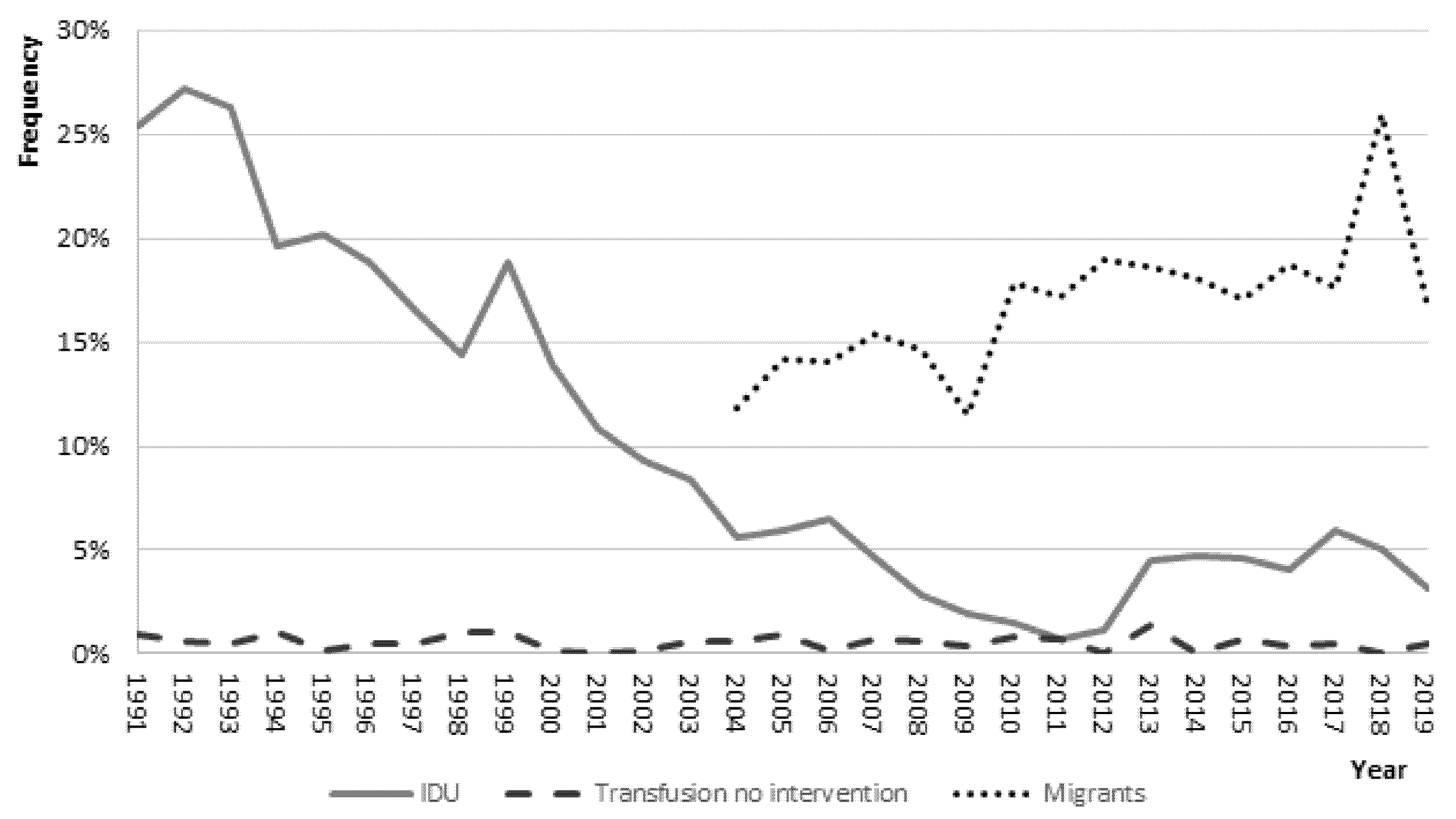
3.3. Risk Factor Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mastrodomenico, M.; Muselli, M.; Provvidenti, L.; Scatigna, M.; Bianchi, S.; Fabiani, L. Long-term immune protection against HBV: Associated factors and determinants. Hum. Vaccines Immunother. 2021, 17, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.C.; Hermann, G.E. Hindbrain astrocytes and glucose counter-regulation. Physiol. Behav. 2019, 204, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Mele, A.; Tosti, M.E.; Gallo, G.; Balocchini, E.; Ragni, P.; Santonastasi, F.; Marzolini, A.; Ciccozzi, M.; Moiraghi, A. The impact of the hepatitis B mass immunisation campaign on the incidence and risk factors of acute hepatitis B in Italy. J. Hepatol. 2000, 33, 980–985. [Google Scholar] [CrossRef]
- Mele, A.; Tosti, M.E.; Mariano, A.; Pizzuti, R.; Ferro, A.; Borrini, B.; Zotti, C.M.; Lopalco, P.L.; Curtale, F.; Balocchini, E.; et al. Acute Hepatitis B 14 Years after the Implementation of Universal Vaccination in Italy: Areas of Improvement and Emerging Challenges. Clin. Infect. Dis. 2008, 46, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Tosti, M.E.; Longhi, S.; de Waure, C.; Mele, A.; Franco, E.; Ricciardi, W.; Filia, A. Assessment of timeliness, representativeness and quality of data reported to Italy's national integrated surveillance system for acute viral hepatitis (SEIEVA). Public Health 2015, 129, 561–568. [Google Scholar] [CrossRef]
- Walter, S.D. Calculation of Attributable Risks from Epidemiological Data. Int. J. Epidemiol. 1978, 7, 175–182. [Google Scholar] [CrossRef]
- Giusti, G.; Ruggiero, G.; Galanti, B.; Piccinino, F.; Sagnelli, E. Chronic active hepatitis in Italy: A multicentric study. Hepatogastroenterology 1994, 41, 397–400. [Google Scholar]
- Stroffolini, T.; Ciancio, A.; Furlan, C.; Vinci, M.; Niro, G.A.; Russello, M.; Colloredo, G.; Morisco, F.; Coppola, N.; Babudieri, S.; et al. Chronic hepatitis B virus infection in Italy during the twenty-first century: An updated survey in 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 40, 607–614. [Google Scholar] [CrossRef]
- Stroffolini, T.; Guadagnino, V.; Rapicetta, M.; Ippolito, F.M.; Caroleo, B.; De Sarro, G.; Focà, A.; Liberto, M.C.; Giancotti, A.; Barreca, G.S.; et al. The impact of a vaccination campaign against hepatitis B on the further decrease of hepatitis B virus infection in a southern Italian town over 14 years. Eur. J. Intern. Med. 2012, 23, e190–e192. [Google Scholar] [CrossRef]
- Spada, E.; Tosti, M.E.; Zuccaro, O.; Stroffolini, T.; Mele, A. Evaluation of the compliance with the protocol for preventing perinatal hepatitis B infection in Italy. J. Infect. 2011, 62, 165–171. [Google Scholar] [CrossRef]
- Ni, Y.-H.; Chang, M.-H.; Wu, J.-F.; Hsu, H.-Y.; Chen, H.-L.; Chen, D.-S. Minimization of hepatitis B infection by a 25-year universal vaccination program. J. Hepatol. 2012, 57, 730–735. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Jan, C.-F.; Kuo, H.-S.; Chen, C.-J. Nationwide Hepatitis B Vaccination Program in Taiwan: Effectiveness in the 20 Years After It Was Launched. Epidemiologic Rev. 2006, 28, 126–135. [Google Scholar] [CrossRef]
- McMahon, B.J.; Bulkow, L.R.; Singleton, R.J.; Williams, J.; Snowball, M.; Homan, C.; Parkinson, A.J. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 2011, 54, 801–807. [Google Scholar] [CrossRef]
- Peto, T.J.; Mendy, M.E.; Lowe, Y.; Webb, E.L.; Whittle, H.C.; Hall, A.J. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986–90) and in the nationwide immunisation program. BMC Infect. Dis. 2014, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, K.; Klevens, M.; Kainer, M.A.; Baumgartner, J.; Gerard, K.; Poissant, T.; Sweet, K.; Vonderwahl, C.; Knickerbocker, T.; Khudyakov, Y.; et al. Epidemiology of acute hepatitis B in the United States from population-based sur-veillance, 2006–2011. Clin. Infect. Dis. 2015, 61, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Ly, K.N.; Xing, J.; Spradling, P.R. Trends in Prevalence and Characteristics of Resolved and Current Hepatitis B Among US-Born Persons: National Health and Nutrition Examination Survey, 2001–2018. J. Infect. Dis. 2021, 224, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; So, S.; Gane, E.; Cheung, R.C.; Nguyen, M.H. Prevalence of Hepatitis B Vaccination Coverage and Serologic Evidence of Immunity Among US-Born Children and Adolescents From 1999 to 2016. JAMA Netw. Open 2020, 3, e2022388. [Google Scholar] [CrossRef]
- Velati, C.; Romanò, L.; Prati, E.; Marano, G.; Piccinini, V.; Catalano, L.; Pupella, S.; Vaglio, S.; Veropalumbo, E.; Masiello, F.; et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis B virus in-fection in Italy from 2009 to 2018. Blood Transfus. 2019, 17, 409–417. [Google Scholar]
- Smellie, M.K.R.; Carman, W.F.; Elder, S.; Walker, D.; Lobidel, D.; Hardie, R.; Downie, G.; McMenamin, J.; Cameron, S.; Morrison, D.; et al. Hospital transmission of hepatitis B virus in the absence of exposure prone procedures. Epidemiology Infect. 2005, 134, 259–263. [Google Scholar] [CrossRef]
- Ndumbi, P.; Freidl, G.S.; Williams, C.J.; Mårdh, O.; Varela, C.; Avellón, A.; Friesema, I.; Vennema, H.; Beebeejaun, K.; Ngui, S.L.; et al. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018, 33, 1700641. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Regional Office for Europe (WHO-EURO). Health of Refugees and Migrants Regional Situation Analysis, Practices, Experiences, Lessons Learned and Ways Forward, WHO European Region (2018). Available online: https://www.euro.who.int/en/publications/abstracts/report-on-the-health-of-refugees-and-migrants-in-the-who-european-region-no-public-health-without-refugee-and-migrant-health-2018 (accessed on 15 December 2021).
- Tosti, M.E.; Marceca, M.; Eugeni, E.; D’Angelo, F.; Geraci, S.; Declich, S.; Della Seta, M.; Ferrigno, L.; Marrone, R.; Pajno, C.; et al. Health assessment for migrants and asylum seekers upon arrival and while hosted in reception centres: Italian guidelines. Health Policy 2020, 125, 393–405. [Google Scholar] [CrossRef] [PubMed]

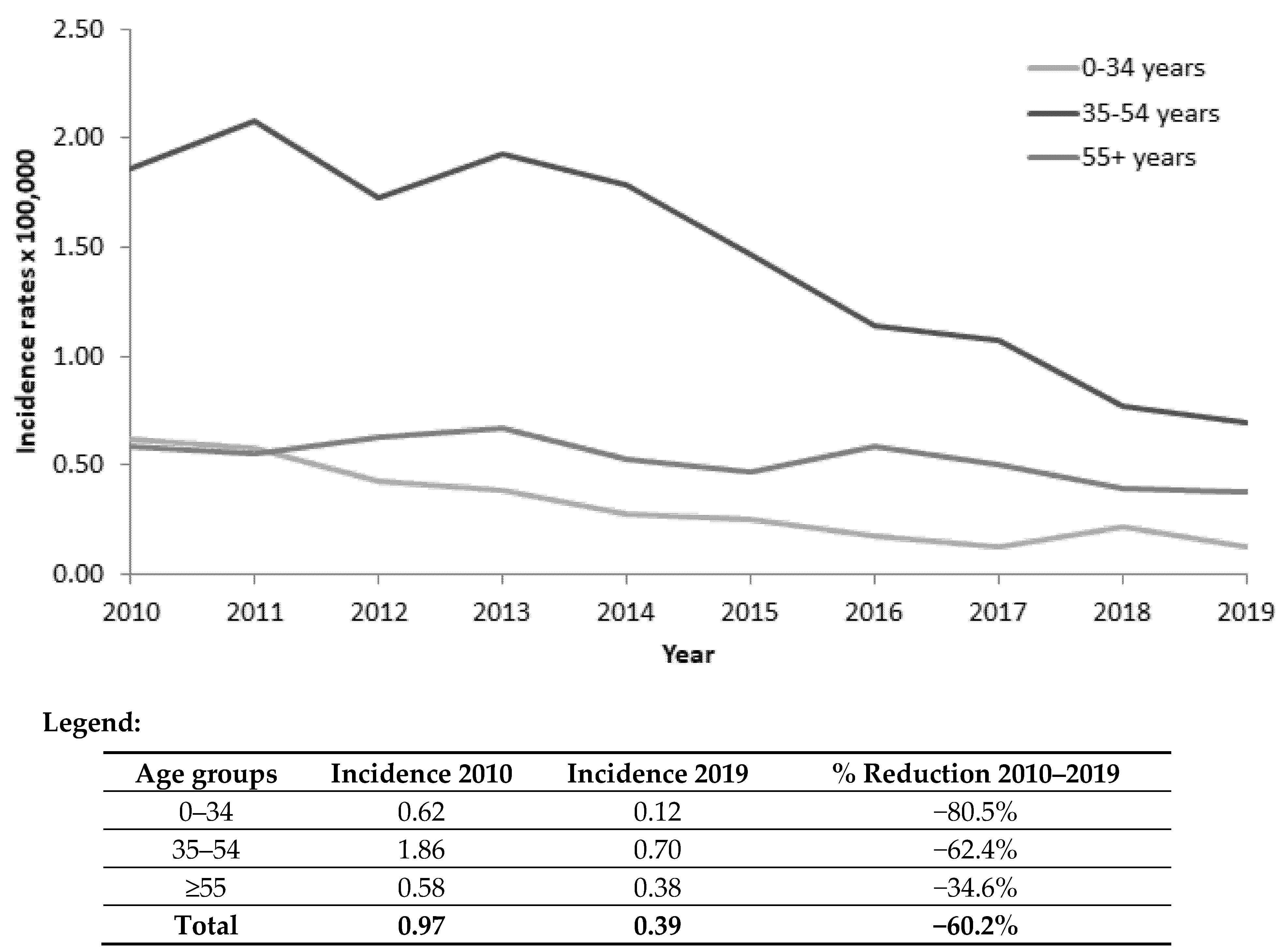
| Characteristics | 1991–1999 N = 6443 n (%) | 2000–2009 N = 5526 n (%) | 2010–2019 N = 3148 n (%) | p-Value for Trend |
|---|---|---|---|---|
| Sex ratio (M/F) | 2.8 | 3.1 | 3.0 | 0.044 |
| Age distribution: | ||||
| ≤25 | 2354 (36.7) | 596 (10.9) | 174 (5.5) | <0.001 |
| 26–40 | 2483 (38.7) | 2743 (50.3) | 909 (28.9) | |
| 41–60 | 1063 (16.6) | 1616 (29.6) | 1617 (51.5) | |
| ≥61 | 516 (8.0) | 502 (9.2) | 443 (14.1) | |
| Median age (range) | 29 (1–98) | 37 (0–100) | 45 (0–98) | <0.001 |
| Years of education: | ||||
| ≤8 | 3489 (71.2) | 1977 (53.9) | 737 (48.4) | <0.001 |
| ≥9 | 1414 (28.8) | 1691 (46.1) | 784 (51.6) | |
| Area of diagnosis: | ||||
| North/central | 5295 (82.2) | 4915 (88.9) | 2918 (92.7) | <0.001 |
| South/islands | 1148 (17.8) | 611 (11.1) | 230 (7.3) | |
| Area of birth *: | ||||
| Italy | 2541 (80.7) | - | ||
| Abroad | 607 (19.3) | |||
| Escaped mandatory vaccination | 55 (0.9) | 279 (5.2) | 280 (9.2) | <0.001 |
| Icterus | 5170 (83.5) | 4588 (85.4) | 2530 (82.7) | 0.869 |
| Hospitalization rate | 5893 (93.1) | 5084 (93.7) | 2853 (91.7) | 0.050 |
| Median days spent in hospital (range) | 16 (1–98) | 13 (1–118) | 10 (1–84) | <0.001 |
| Death | 30 (0.5) | 25 (0.4) | 33 (1.1) | 0.002 |
| Risk Factors | 1991–1999 N = 6443 n (%) | 2000–2009 N = 5526 n (%) | 2010–2019 N = 3148 n (%) | p-Value for Trend |
|---|---|---|---|---|
| Blood transfusion without surgical intervention | 43 (0.7) | 22 (0.4) | 16 (0.6) | |
| Surgical intervention without blood transfusion | 623 (10.4) | 622 (12.0) | 291 (10.0) | 0.013 |
| Surgical intervention and blood transfusion | 107 (1.8) | 86 (1.7) | 26 (0.9) | |
| Intravenous drug use | 1267 (20.8) | 378 (7.2) | 96 (3.3) | <0.001 |
| Unvaccinated among IDU * | 1137 (95.7) | 301 (89.9) | 72 (91.1) | <0.001 |
| Beauty treatments ** | 1827 (29.9) | 1662 (31.5) | 949 (31.8) | 0.044 |
| Sexual exposure *** | 1601 (30.4) | 1642 (32.5) | 818 (28.3) | 0.174 |
| Household contact of an HBsAg+ carrier | 598 (12.9) | 421 (10.4) | 201 (8.7) | <0.001 |
| Aware of the positivity of the household | 181 (47.8) | 160 (43.7) | 77 (41.8) | 0.153 |
| Characteristics | Italians N = 2541 n (%) | Foreigners N = 607 n (%) | p-Value |
|---|---|---|---|
| Sex ratio (M/F): | 3.4 | 2.0 | <0.001 |
| Age: | |||
| ≤40 | 631 (24.9) | 452 (74.7) | <0.001 |
| 41–60 | 1474 (58.1) | 143 (23.6) | |
| ≥61 | 433 (17.0) | 10 (1.6) | |
| Median age (range) | 47 (0–98) | 32 (2–80) | <0.001 |
| Area of diagnosis: | |||
| North/Central | 2343 (92.2) | 575 (94.7) | 0.032 |
| South/Islands | 198 (7.8) | 32 (5.3) | |
| I.V. Drug use | 64 (2.7) | 32 (6.0) | <0.001 |
| Household contact of an HBsAg+ carrier | 144 (7.4) | 57 (15.5) | <0.001 |
| Sexual exposure * | 698 (29.5) | 120 (22.9) | 0.003 |
| Characteristics | HBV N = 3148 n (%) | HAV N = 7517 n (%) | OR (95% CI) |
|---|---|---|---|
| Sex: | |||
| Females | 780 (24.8) | 2240 (29.9) | 1 |
| Males | 2360 (75.2) | 5258 (70.1) | 1.42 (1.24–1.63) |
| Age: | |||
| ≤40 | 1083 (34.5) | 4917 (65.5) | 1 |
| ≥41 | 2060 (65.5) | 2591 (34.5) | 5.33 (4.70–6.05) |
| Area of diagnosis: | |||
| South/islands | 230 (7.3) | 903 (12.0) | 1 |
| North/central | 2918 (92.7) | 6614 (88.0) | 1.59 (1.28–1.92) |
| Area of birth: | |||
| Italy | 2541 (80.7) | 6416 (85.4) | 1 |
| Abroad | 607 (19.3) | 1101 (14.6) | 3.02 (2.54–3.60) |
| Blood transfusion without surgical intervention | 16 (0.6) | 7 (0.1) | 5.08(1.81–14.3) |
| Surgical intervention without blood transfusion | 291 (10.0) | 300 (4.5) | 2.25 (1.83–2.76) |
| Surgical intervention and blood transfusion | 26 (0.9) | 10 (0.1) | 4.76 (2.01–11.3) |
| Intravenous drug use | 96 (3.3) | 230 (3.5) | 0.72 (0.51–1.02) |
| Beauty treatments ** | 949 (31.8) | 1581 (23.4) | 1.62 (1.43–1.83) |
| Sexual exposure *** | 818 (28.3) | 983 (15.4) | 2.38 (2.06–2.74) |
| Household contact of an HBsAg+ carrier | 201 (8.7) | 71 (1.2) | 10.8 (7.88–14.9) |
| Characteristics | PAR |
|---|---|
| Blood transfusion without surgical intervention | 1.1% |
| Surgical intervention without blood transfusion | 5.2% |
| Surgical intervention and blood transfusion | 1.8% |
| Intravenous drug use | - |
| Beauty treatments ** | 10.2% |
| Sexual exposure *** | 9.1% |
| Household contact of an HBsAg+ carrier | 7.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroffolini, T.; Morisco, F.; Ferrigno, L.; Pontillo, G.; Iantosca, G.; Cossiga, V.; Crateri, S.; Tosti, M.E.; the SEIEVA Collaborating Group. Effectiveness of Hepatitis B Vaccination Campaign in Italy: Towards the Control of HBV Infection for the First Time in a European Country. Viruses 2022, 14, 245. https://doi.org/10.3390/v14020245
Stroffolini T, Morisco F, Ferrigno L, Pontillo G, Iantosca G, Cossiga V, Crateri S, Tosti ME, the SEIEVA Collaborating Group. Effectiveness of Hepatitis B Vaccination Campaign in Italy: Towards the Control of HBV Infection for the First Time in a European Country. Viruses. 2022; 14(2):245. https://doi.org/10.3390/v14020245
Chicago/Turabian StyleStroffolini, Tommaso, Filomena Morisco, Luigina Ferrigno, Giuseppina Pontillo, Giuseppina Iantosca, Valentina Cossiga, Simonetta Crateri, Maria Elena Tosti, and the SEIEVA Collaborating Group. 2022. "Effectiveness of Hepatitis B Vaccination Campaign in Italy: Towards the Control of HBV Infection for the First Time in a European Country" Viruses 14, no. 2: 245. https://doi.org/10.3390/v14020245
APA StyleStroffolini, T., Morisco, F., Ferrigno, L., Pontillo, G., Iantosca, G., Cossiga, V., Crateri, S., Tosti, M. E., & the SEIEVA Collaborating Group. (2022). Effectiveness of Hepatitis B Vaccination Campaign in Italy: Towards the Control of HBV Infection for the First Time in a European Country. Viruses, 14(2), 245. https://doi.org/10.3390/v14020245








