β-Glucans from Trametes versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, C. versicolor Extracts
2.2. Animals and Ethics Statement
2.3. Induction of BMDCs
2.4. Real-Time Quantitative RT-PCR
2.5. Virus Challenge in Mice and Chicks
2.6. Histopathology
2.7. Statistical Analysis
3. Results
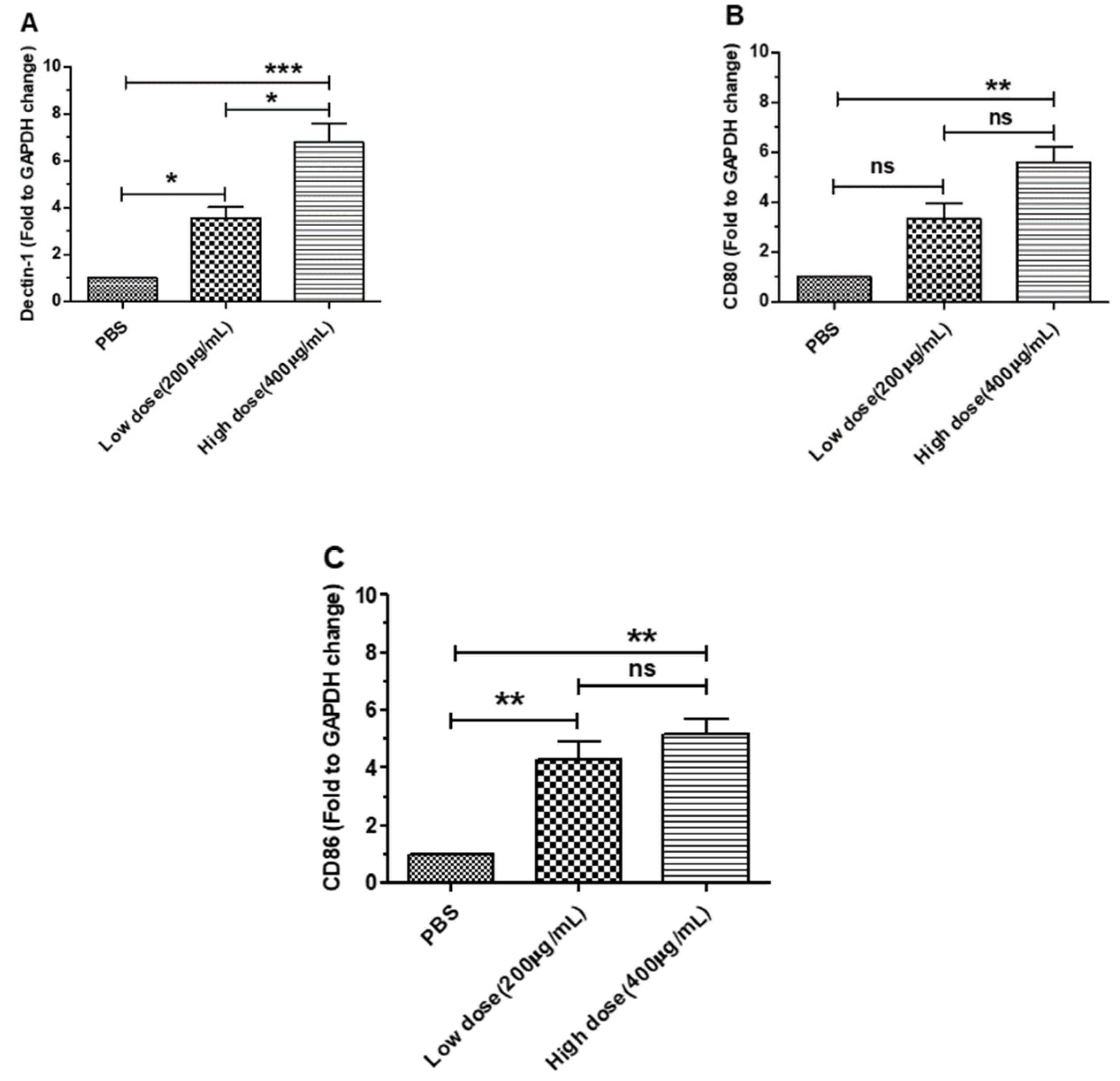
3.1. β-Glucans Induced the Expression of Dectin-1 and Costimulatory Factor Genes in BMDCs
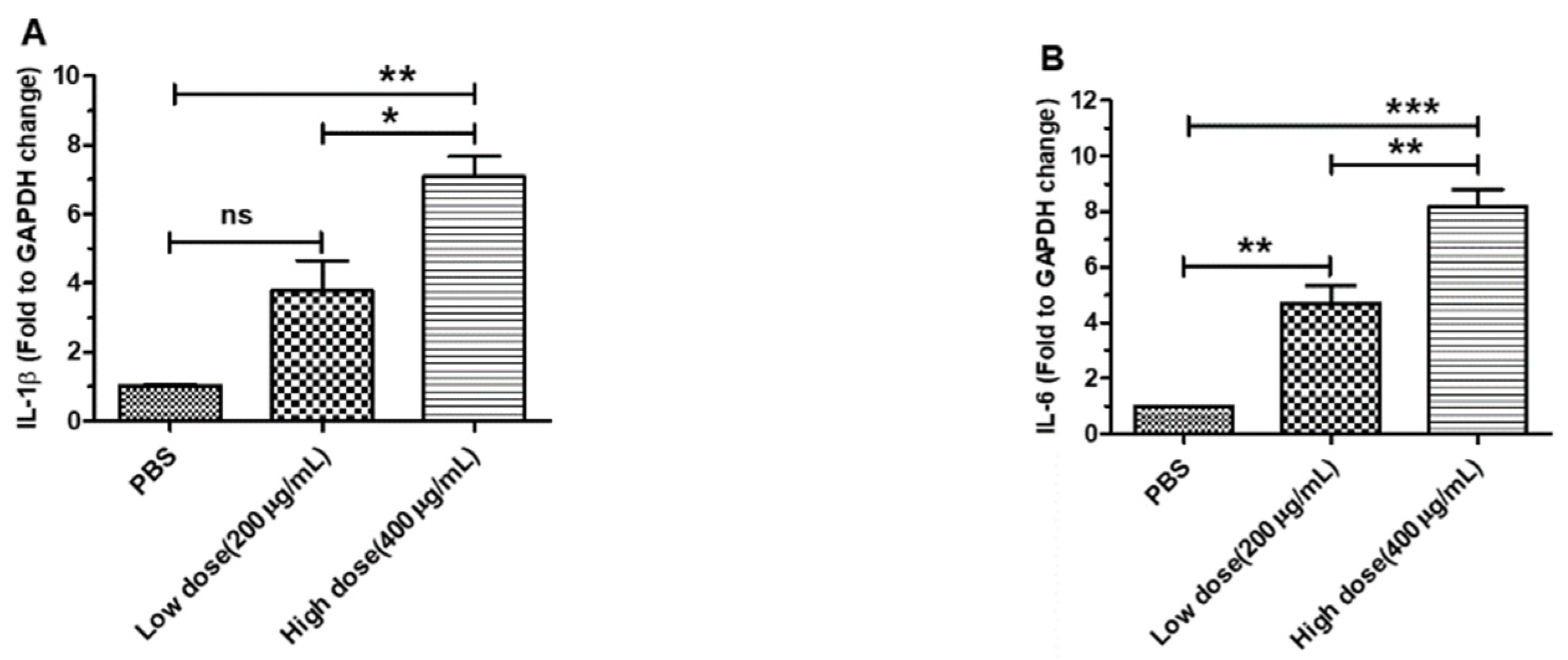
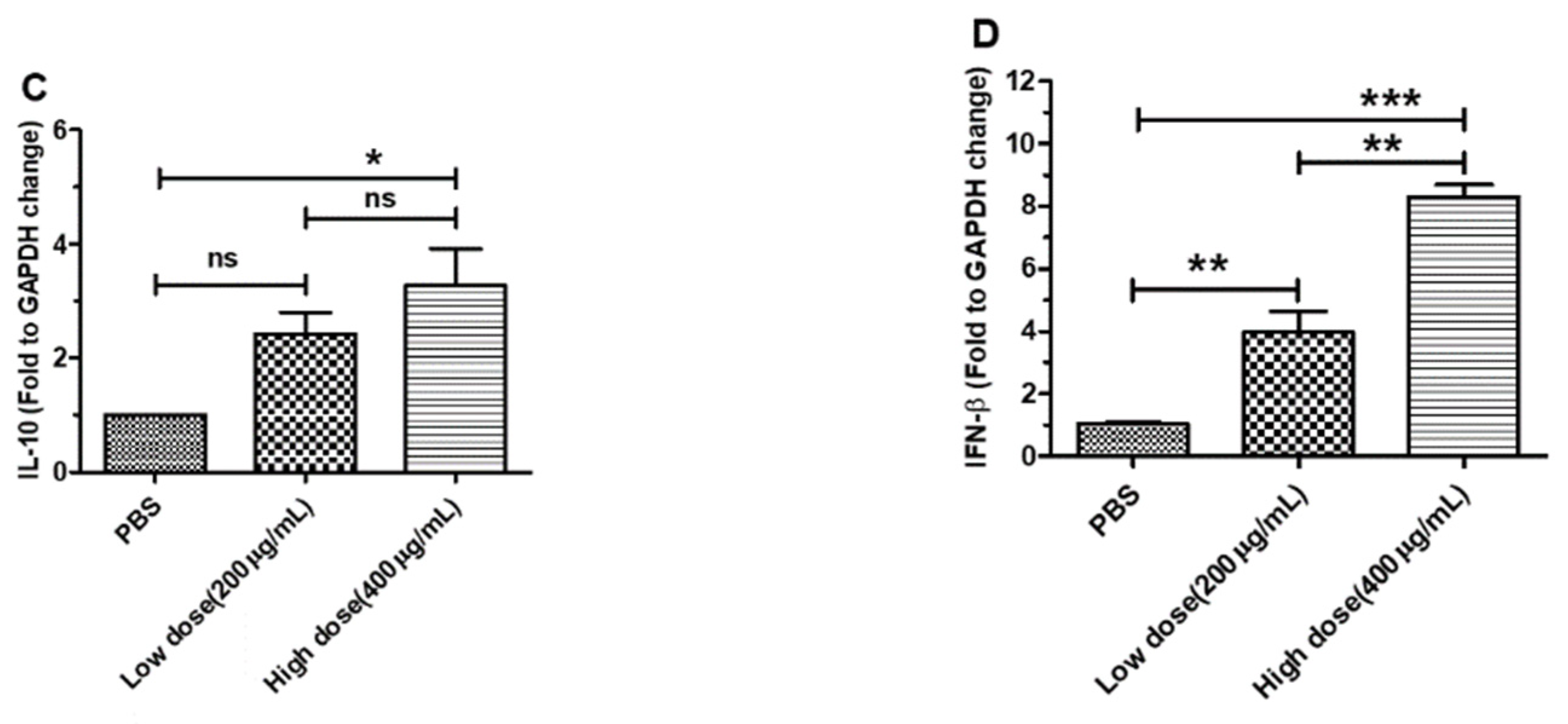
3.2. β-Glucans Promoted IL-1β, IL-6, IL-10 and IFN-β Gene Expression in BMDCs
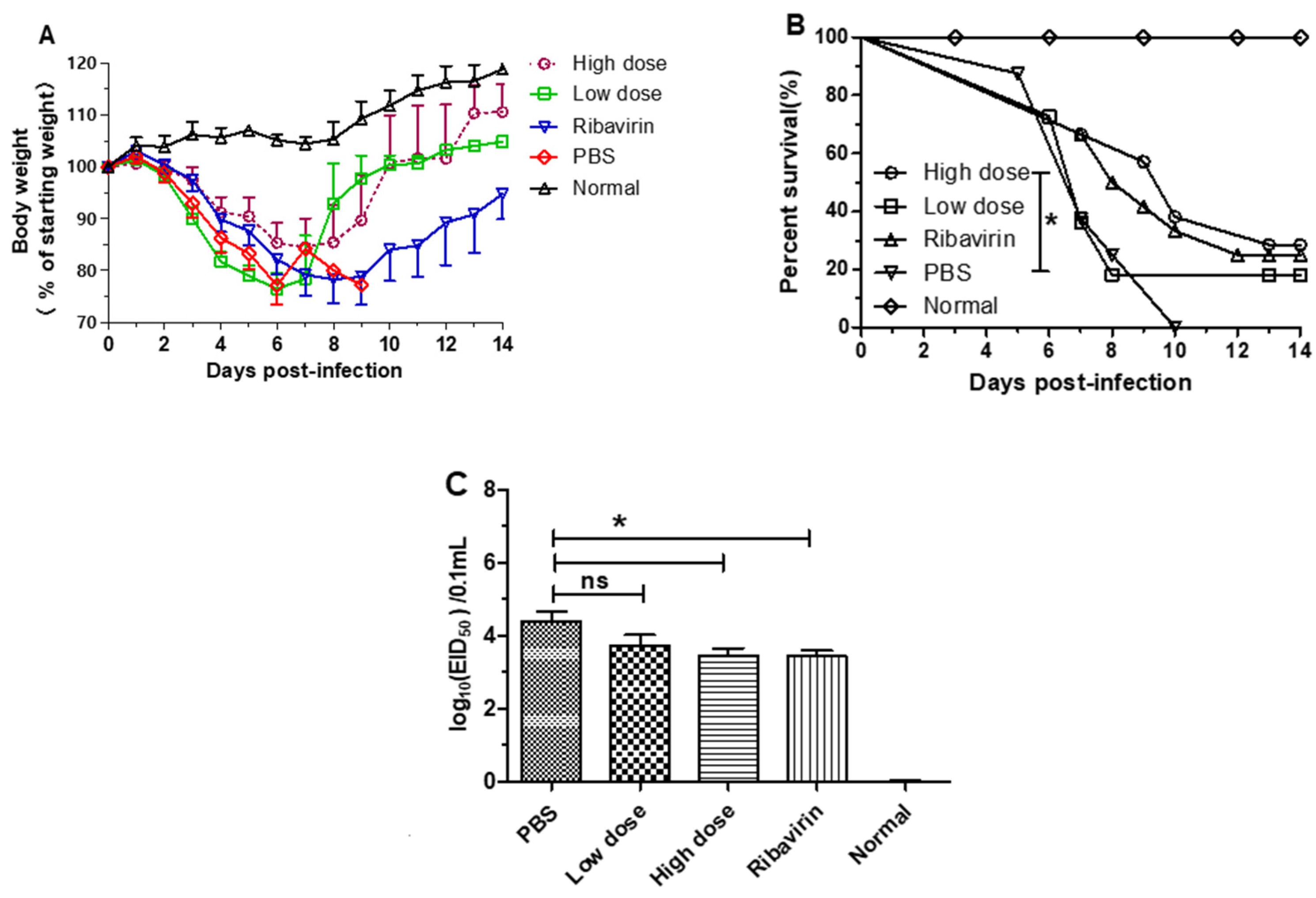
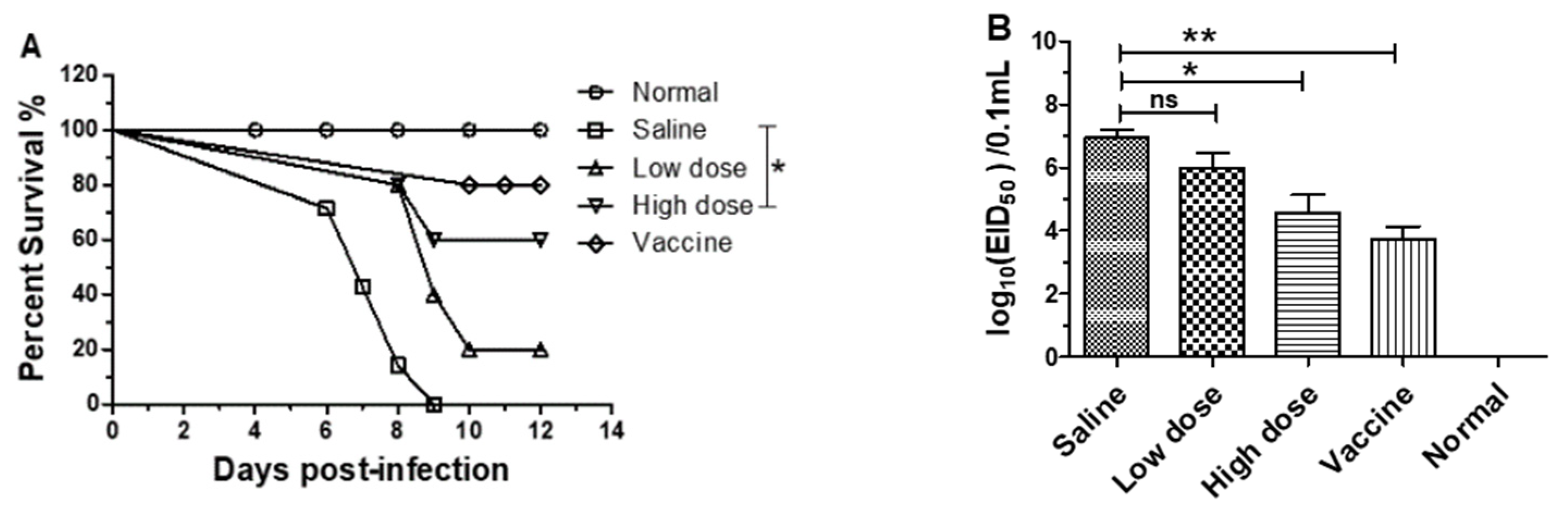
3.3. β-Glucans Provided Protection against H1N1 Influenza Virus Infection in Mice
3.4. β-Glucans Alleviated the Lung Pathological Changes in Mice
3.5. β-Glucans Provided Protection against H9N2 AIV Infection in Chicks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, W.; Qin, K. Human-infecting influenza A (H9N2) virus: A forgotten potential pandemic strain? Zoonoses Public Health 2020, 67, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.T.; Duong, B.T.; Nguyen, A.T.V.; Tuong, H.T.; Hoang, V.T.; Than, D.D.; Nam, S.J.; Sung, H.W.; Yun, K.J.; Yeo, S.J.; et al. Emergence of Novel Reassortant H1N1 Avian Influenza Viruses in Korean Wild Ducks in 2018 and 2019. Viruses 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Koçer, Z.A.; Krauss, S.; Zanin, M.; Danner, A.; Gulati, S.; Jones, J.C.; Friedman, K.; Graham, A.; Forrest, H.; Seiler, J.; et al. Possible basis for the emergence of H1N1 viruses with pandemic potential from avian hosts. Emerg. Microbes Infec. 2015, 4, e40. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. The use of mushroom glucans and proteoglycans in cancer treatment. Altern. Med. Rev. 2000, 5, 4–27. [Google Scholar]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Li, B.; Xi, P.; Wang, Z.; Han, X.; Xu, Y.; Zhang, Y.; Miao, J. PI3K/Akt/mTOR signaling pathway participates in Streptococcus uberis-induced inflammation in mammary epithelial cells in concert with the classical TLRs/NF-kB pathway. Vet. Microbiol. 2018, 227, 103–111. [Google Scholar] [CrossRef]
- Dou, H.; Chang, Y.; Zhang, L. Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 361–381. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Huang, K.Y.; Jiang, Y.L.; Yang, G.L.; Wang, C.F.; Li, Y. β-glucans from Coriolus versicolor protect mice against S. typhimurium challenge by activation of macrophages. Int. J. Biol. Macromol. 2016, 86, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Felicioli, A.; Forzan, M.; Sagona, S.; D’Agostino, P.; Baido, D.; Fronte, B.; Mazzei, M. Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.). Vet. Sci. 2020, 7, 52. [Google Scholar] [CrossRef]
- Medina-Gali, R.M.; Ortega-Villaizan, M.D.M.; Mercado, L.; Novoa, B.; Coll, J.; Perez, L. Beta-glucan enhances the response to SVCV infection in zebrafish. Dev. Comp. Immunol. 2018, 84, 307–314. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Yang, R.; Fei, C.; Wang, X.; Zhang, K.; Wang, C.; Zheng, W.; Xue, F. Improvement of immune responses to influenza vaccine (H5N1) by sulfated yeast beta-glucan. Int. J. Biol. Macromol. 2016, 93, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, D.; Iwai, A.; Aoki, S.; Uchiyama, H.; Kawata, K.; Nakayama, Y.; Nikawa, Y.; Kusano, K.; Okabe, M.; Miyazaki, T. β-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 2012, 7, e41399. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiang, Y.; Yang, G.; Yang, W.; Hu, J.; Cui, Y.; Shi, C.; Liu, J.; Wang, C. Murine bone marrow-derived DCs activated by porcine rotavirus stimulate the Th1 subtype response in vitro. Microb. Pathog. 2017, 110, 325–334. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Cong, Y.L.; Huang, H.B.; Wang, Q.; Cai, R.P.; Ye, L.P.; Hu, J.T.; Zhou, J.Y.; et al. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarumNC8 expressing hemagglutinin in BALB/c mice. Virology 2014, 464–465, 166–176. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Zhang, X.K.; Liu, Y.Y.; Zhang, L.J.; Ye, L.P.; Hu, J.T.; Xing, X.; Qi, C.; et al. Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res. 2016, 211, 46–57. [Google Scholar] [CrossRef]
- Gong, W.; Huang, K.; Zhang, Y.; He, X.; Li, C.; Mao, H.; Wei, Y.; Zou, Z.; Jin, M. Transcriptome Profiles of Highly Pathogenic Pure Avian H7N9 Virus-Infected Lungs of BALB/c Mice. Front. Vet. Sci. 2020, 7, 603584. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma lucidum: A potential source to surmount viral infections through beta-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. The role of dectin-1 in health and disease. Immunobiology 2021, 226, 152071. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Jiang, Y.; Gao, S.; Wang, Y.; Wang, D.; Wang, A.; Yi, H.; Gu, R.; Yi, Q.; et al. Interleukin-33 Contributes to the Induction of Th9 Cells and Antitumor Efficacy by Dectin-1-Activated Dendritic Cells. Front. Immunol. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Dinh, T.T.H.; Tummamunkong, P.; Padungros, P.; Ponpakdee, P.; Boonprakong, L.; Saisorn, W.; Leelahavanichkul, A.; Kueanjinda, P.; Ritprajak, P. Interaction Between Dendritic Cells and Candida krusei β-Glucan Partially Depends on Dectin-1 and It Promotes High IL-10 Production by T Cells. Front. Cell. Infect. Microbiol. 2020, 10, 566661. [Google Scholar] [CrossRef] [PubMed]
- Geervliet, M.; Lute, L.C.P.; Jansen, C.A.; Rutten, V.; Savelkoul, H.F.J.; Tijhaar, E. Differential immunomodulation of porcine bone marrow derived dendritic cells by E. coli Nissle 1917 and β-glucans. PLoS ONE 2020, 15, e0233773. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.M.; Dokter-Fokkens, J.; de Vos, P. Particulate β-glucans synergistically activate TLR4 and Dectin-1 in human dendritic cells. Mol. Nutr. Food Res. 2016, 60, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Kiabi, N.; Yáñez, A.; Dang, I.; Martins, G.A.; Underhill, D.M.; Goodridge, H.S. Autocrine Type I IFN Signaling in Dendritic Cells Stimulated with Fungal β-Glucans or Lipopolysaccharide Promotes CD8 T Cell Activation. J. Immunol. 2017, 198, 375–382. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. Could the Induction of Trained Immunity by β-Glucan Serve as a Defense Against COVID-19? Front. Immunol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Ji, L.; Sun, G.; Li, J.; Wang, Y.; Du, Y.; Li, X.; Liu, Y. Effect of dietary β-glucan on growth, survival and regulation of immune processes in rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida. Fish Shellfish Immunol. 2017, 64, 56–67. [Google Scholar] [CrossRef]
- Yun, C.H.; Estrada, A.; Van Kessel, A.; Park, B.C.; Laarveld, B. Beta-glucan, extracted from oat, enhances disease resistance against bacterial and parasitic infections. FEMS Immunol. Med. Microbiol. 2003, 35, 67–75. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, D.; Shi, B.; Ren, G.; Peng, X.; Fang, Z.; Kozlowski, M.; Zhou, X.; Zhang, X.; Wu, M.; et al. Oral administered particulate yeast-derived glucan promotes hepatitis B virus clearance in a hydrodynamic injection mouse model. PLoS ONE 2015, 10, e0123559. [Google Scholar] [CrossRef]
- Mehraj, V.; Ramendra, R.; Isnard, S.; Dupuy, F.P.; Ponte, R.; Chen, J.; Kema, I.; Jenabian, M.A.; Costinuik, C.T.; Lebouché, B.; et al. Circulating (1→3)-β-D-glucan Is Associated with Immune Activation During Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2020, 70, 232–241. [Google Scholar] [CrossRef]
- Kaya, K.; Ciftci, O.; Aydın, M.; Cetin, A.; Basak, N. Favourable effect of β-glucan treatment against cisplatin-induced reproductive system damage in male rats. Andrologia 2019, 51, e13342. [Google Scholar] [CrossRef]
- Erkol, H.; Kahramansoy, N.; Kordon, Ö.; Büyükaşık, O.; Serin, E.; Ulaş, N. Effects of beta-glucan on hepatic damage caused by obstructive jaundice. Ulus Travma Acil Cerrahi Derg. 2011, 17, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nie, Q.; Xie, M.; Yao, H.; Zhang, K.; Yin, J.; Nie, S. Protective effects of β-glucan isolated from highland barley on ethanol-induced gastric damage in rats and its benefits to mice gut conditions. Food Res Int. 2019, 122, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hermans, L.; De Pelsmaeker, S.; Denaeghel, S.; Cox, E.; Favoreel, H.W.; Devriendt, B. β-Glucan-Induced IL-10 Secretion by Monocytes Triggers Porcine NK Cell Cytotoxicity. Front. Immunol. 2021, 12, 634402. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Le, T.; Doan, T.H.; Quyen, D.; Le, K.X.; Pham, V.; Nagataki, M.; Nomura, H.; Ikeue, Y.; Watanabe, Y.; et al. The adjuvant effect of Sophy β-glucan to the antibody response in poultry immunized by the avian influenza A H5N1 and H5N2 vaccines. J. Microbiol. Biotechnol. 2011, 21, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Horst, G.; Levine, R.; Chick, R.; Hofacre, C. Effects of beta-1,3-glucan (AletaTM) on vaccination response in broiler chickens. Poult. Sci. 2019, 98, 1643–1647. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Genbank Accession No. | Forward Primer (5-3) | Reverse Primer (5-3) | Amplicon/bp |
|---|---|---|---|---|
| CD80 | AF065894.1 | CCCCAGAAGACCCTCCTGATAG | CCGAAGGTAAGGCTGTTGTTTG | 172 |
| CD86 | NM_019388.3 | GCCGTGCCCATTTACAAAGG | GTTCCTGTCAAAGCTCGTGC | 160 |
| Dectin-1 | AY534909.1 | TGGTAGTAGTGGTTGCTGCAGTGCTGGG | GTAGTTTGGGATGCCTTGGAGGGAGCCA | 169 |
| IL-10 | NM_010548.2 | GCCAGAGCCACATGCTCCTA | GATAAGGCTTGGCAACCCAAGTAA | 145 |
| IL-1β | NM_008361.4 | GTTCCCATTAGACAACTGCACTACAG | GTCGTTGCTTGGTTCTCCTTGTA | 162 |
| IL-6 | X54542.1 | CGGCCTTCCCTACTTCACAAGTCCG | CAGGTCTGTTGGGAGTGGTATCC | 142 |
| IFN-β | X14455.1 | CTGGCTTCCATCATGAACAA | CATTTCCGAATGTTCGTCCT | 125 |
| GAPDH | AA661116.1 | CACTCACGGCAAATTCAACGGCAC | GACTCCACGACATACTCAGCAC | 152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Yin, L.; Shen, X.; Dai, Y.; Wang, J.; Yin, D.; Zhang, D.; Pan, X. β-Glucans from Trametes versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection. Viruses 2022, 14, 237. https://doi.org/10.3390/v14020237
Shi S, Yin L, Shen X, Dai Y, Wang J, Yin D, Zhang D, Pan X. β-Glucans from Trametes versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection. Viruses. 2022; 14(2):237. https://doi.org/10.3390/v14020237
Chicago/Turabian StyleShi, Shaohua, Lei Yin, Xuehuai Shen, Yin Dai, Jieru Wang, Dongdong Yin, Danjun Zhang, and Xiaocheng Pan. 2022. "β-Glucans from Trametes versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection" Viruses 14, no. 2: 237. https://doi.org/10.3390/v14020237
APA StyleShi, S., Yin, L., Shen, X., Dai, Y., Wang, J., Yin, D., Zhang, D., & Pan, X. (2022). β-Glucans from Trametes versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection. Viruses, 14(2), 237. https://doi.org/10.3390/v14020237






