Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathology Investigation
2.2. Virological Investigation
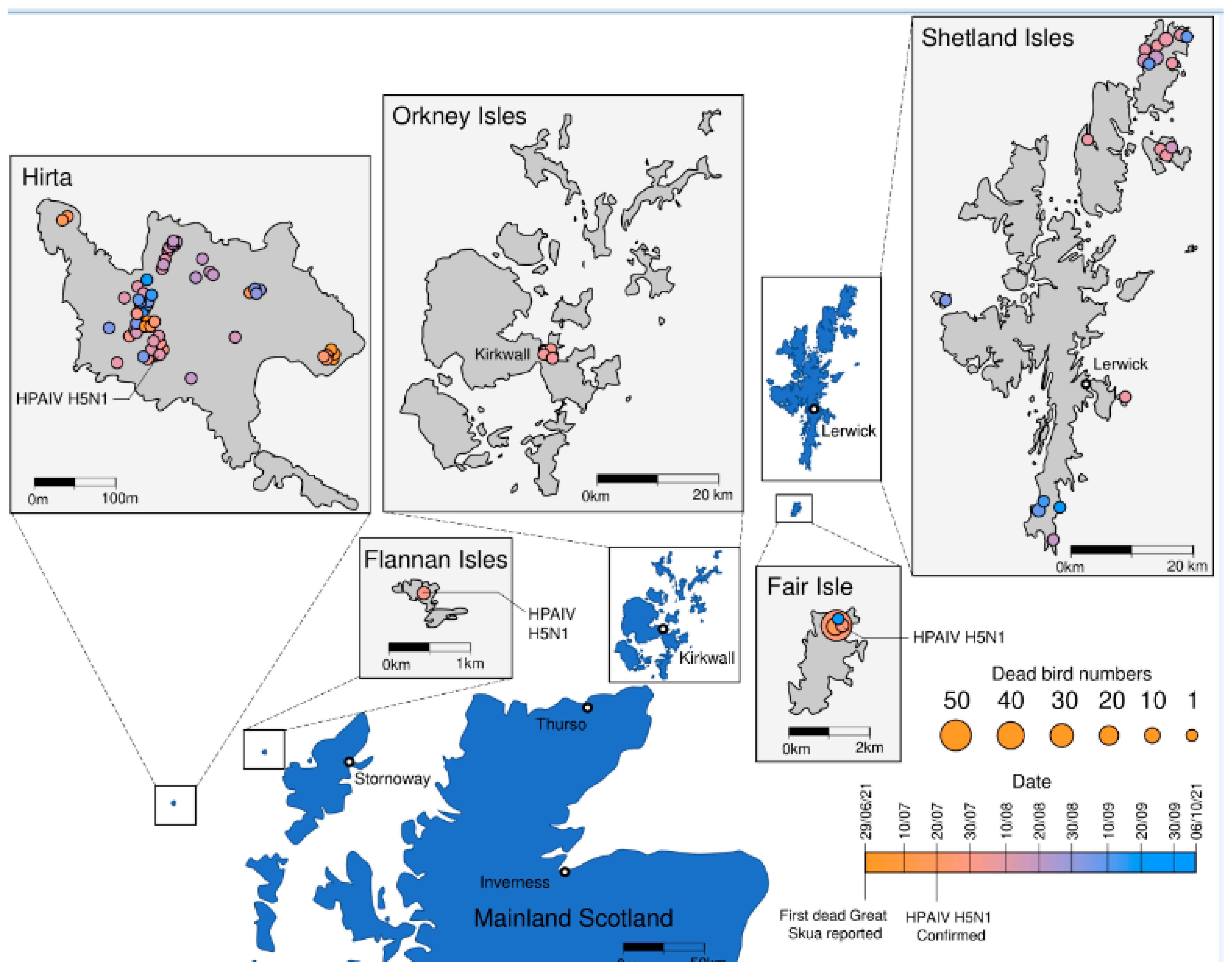
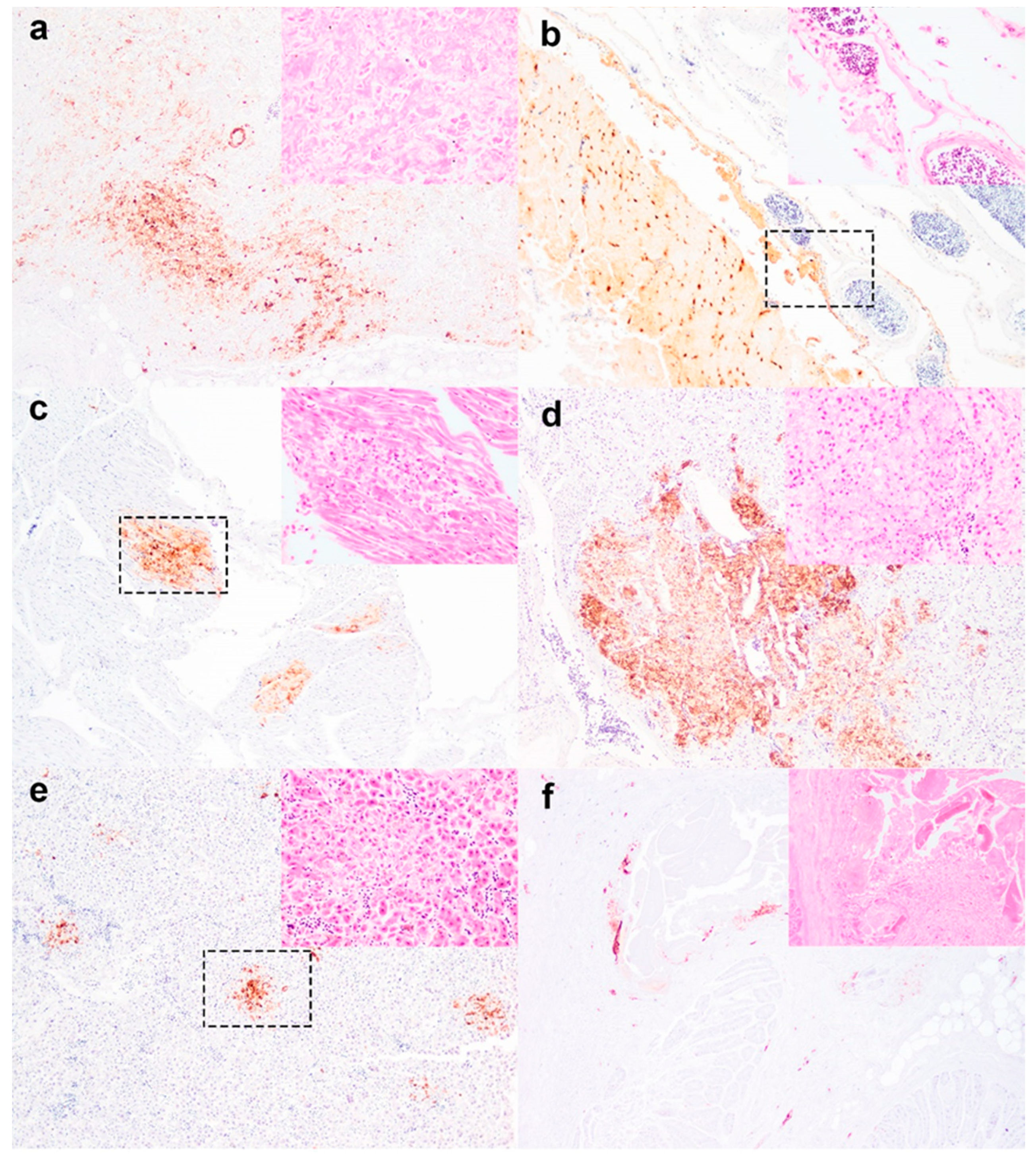
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Ethical Statement
References
- European Food Safety Authority; European Centre for Disease Prevention and Control and European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; et al. Avian influenza overview December 2020–February 2021. EFSA J 2021, 19, e06497. [Google Scholar] [CrossRef] [PubMed]
- Lean, F.Z.X.; Núñez, A.; Banyard, A.C.; Reid, S.M.; Brown, I.H.; Hansen, R.D.E. Gross pathology associated with highly pathogenic avian influenza H5N8 and H5N1 in naturally infected birds in the UK (2020–2021). Vet. Rec. 2021, e731. [Google Scholar] [CrossRef] [PubMed]
- The Animal and Plant Health Agency. GB Avian Quarterly Report: Disease Surveillance and Emerging Threats: Q1—January to March 2021. Available online: https://www.gov.uk/government/collections/animal-disease-surveillance-reports (accessed on 26 November 2019).
- The Animal and Plant Health Agency. High Pathogenicity Avian Influenza H5N8 and H5N1 Outbreaks in Great Britain: November 2020 to April 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1011519/aiv-hpai-h5n8-h5n1-epi-rept-1120-0421.pdf (accessed on 26 November 2019).
- Jakubas, D.; Iliszko, L.M.; Strøm, H.; Helgason, H.H.; Stempniewicz, L. Flexibility of foraging strategies of the great skua Stercorarius skua breeding in the largest colony in the Barents Sea region. Front. Zool. 2018, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearhop, S.; Thompson, D.R.; Phillips, R.A.; Waldron, S.; Hamer, K.C.; Gray, C.M.; Votier, S.C.; Ross, B.P.; Furness, R.W. Annual Variation in Great Skua Diets: The Importance of Commercial Fisheries and Predation on Seabirds Revealed by Combining Dietary Analyses. Condor 2001, 103, 802–809. [Google Scholar] [CrossRef]
- Votier, S.C.; Furness, R.W.; Bearhop, S.; Crane, J.E.; Caldow, R.W.G.; Catry, P.; Ensor, K.; Hamer, K.C.; Hudson, A.V.; Kalmbach, E.; et al. Changes in fisheries discard rates and seabird communities. Nature 2004, 427, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Nager, R.G.; Johnson, P.C.D.; Furness, R.W.; Provencher, J.F. Plastic debris in great skua (Stercorarius skua) pellets corresponds to seabird prey species. Mar. Pollut. Bull. 2016, 103, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.I.; Newton, S.F.; Ratcliffe, N.; Dunn, T.E. Seabird populations of Britain and Ireland; T. & AD Poyser: London, UK, 2004. [Google Scholar]
- Anker-Nilssen, T.; Bakken, V.; Strøm, H.; Golovkin, A.N.; Bianki, V.V.; Tatarinkova, I.P. The Status of Marine Birds Breeding in the Barents Sea Region; Norsk Polarinstitutt: Tromsø, Norway, 2000; ISBN 9788276661767. [Google Scholar]
- Furness, R.W. Non-breeding season populations of seabirds in UK waters: Population sizes for Biologically Defined Minimum Population Scales (BDMPS). In Natural England Commissioned Reports; Natural England: York, UK, 2015. [Google Scholar]
- Eaton, M.; Aebischer, N.; Brown, A.; Hearn, R.; Lock, L.; Musgrove, A.; Noble, D.; Stroud, D.; Gregory, R. Birds of Conservation Concern 4: The population status of birds in the UK, Channel Islands and Isle of Man. Br. Birds 2015, 108, 708–746. [Google Scholar]
- Núñez, A.; Brookes, S.M.; Reid, S.M.; Garcia-Rueda, C.; Hicks, D.J.; Seekings, J.M.; Spencer, Y.I.; Brown, I.H. Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4 Virus: Equivocal Pathogenicity and Implications for Surveillance Following Natural Infection in Breeder Ducks in the United Kingdom. Transbound. Emerg. Dis. 2016, 63, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Vostinakova, V.; Pirchanova, Z.; Cernikova, L.; Dirbakova, Z.; Mojzis, M.; Jirincova, H.; Havlickova, M.; Dan, A.; Ursu, K.; et al. Development and evaluation of a one-step real-time RT-PCR assay for universal detection of influenza A viruses from avian and mammal species. Arch. Virol. 2010, 155, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Payungporn, S.; Chutinimitkul, S.; Chaisingh, A.; Damrongwantanapokin, S.; Buranathai, C.; Amonsin, A.; Theamboonlers, A.; Poovorawan, Y. Single step multiplex real-time RT-PCR for H5N1 influenza A virus detection. J. Virol. Methods 2006, 131, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Pavlidis, T.; Banks, J.; Shell, W.; McNally, A.; Essen, S.; Brown, I.H. Validated H5 Eurasian real-time reverse transcriptase-polymerase chain reaction and its application in H5N1 outbreaks in 2005–2006. Avian Dis. 2007, 51, 373–377. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Slomka, M.J.; Reid, S.M.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Cooper, J.; Russell, C.; Mollett, B.C.; Agyeman-Dua, E.; et al. Development and Application of Real-Time PCR Assays for Specific Detection of Contemporary Avian Influenza Virus Subtypes N5, N6, N7, N8, and N9. Avian Dis. 2019, 63, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. European Commision Decision 2006/437/EC of 4 August 2006 approving Diagnostic Manual for avian influenza as provided for in Council Directive 2005/94/EC of 4 August 2006 approving Diagnostic Manual for avian influenza as provided for in Council Directive 2005/94/EC. Off. J. Eur. Union 2006, 237, 1–27. [Google Scholar]
- OIE World Organisation for Animal Health. Terrestrial Manual: Avian Influenza (Infection with Avian Influenza Viruses). Available online: https://www.oie.int/fileadmin/home/eng/health_standards/tahm/3.03.04_ai.pdf (accessed on 26 November 2019).
- Seekings, A.; Warren, C.J.; Thomas, S.S.; Mahmood, S.; James, J.; Byrne, A.M.P.; Watson, S.; Bianco, C.; Nunez, A.; Brown, I.H.; et al. Highly pathogenic avian influenza virus H5N6 (clade 2.3.4.4b) has a preferable host tropism for waterfowl reflected in its inefficient transmission to terrestrial poultry. Virology 2021, 559, 74–85. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Seekings, A.H.; Skinner, P.; Purchase, K.; Mahmood, S.; Brown, I.H.; Hansen, R.D.E.; Banyard, A.C.; Reid, S.M. Rapid sensitive detection and pathotyping of emergent Eurasian clade 2.3.4.4b viruses in wild birds. J. Virol. Methods 2022, 301, 114454. [Google Scholar] [CrossRef] [PubMed]
| Sample Location (and Collection Date) | Species | Sample ID or Bird Number | Sample Type | Real-Time RT-PCR | Interpretation (and GISAID Reference) | |||
|---|---|---|---|---|---|---|---|---|
| M Gene a | H5 HA2 b | H5 HP c | N1 d | |||||
| Fair Isle, Shetland 20 July 2021 | Great Skua (Bird 1) | AV-21-040939 | OP swab | +ve | +ve | +ve | +ve | HPAIV H5N1 (EPI_ISL_5530613) |
| 97670002 | Liver | +ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| 97670002 | Spleen | −ve | +ve | +ve | −ve | HPAIV H5Nx | ||
| Great Skua (Bird 2) | AV-21-040942 | C swab | +ve | +ve | +ve | −ve | HPAIV H5Nx | |
| 97670003 | Liver | +ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| Great Skua (Bird 4) | AV-21-040945 | OP swab | +ve | +ve | +ve | −ve | HPAIV H5Nx | |
| 97670005 | Liver | +ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| Flannan Isles, Outer Hebrides 22 July 2021 | Great Skua | AV-21-042505 | OP swab | +ve | +ve | +ve | +ve | HPAIV H5N1 (EPI_ISL_6029360) |
| AV-21-042506 | C swab | +ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| 50625976 | Bulk viscera | +ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| Black-Backed Gull | AV-21-042507 | OP swab | −ve | −ve | −ve | −ve | AIV-negative | |
| AV-21-042508 | C swab | −ve | −ve | −ve | −ve | AIV-negative | ||
| 50625977 | Bulk viscera | −ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| St. Kilda, Outer Hebrides 29 July 2021 | Great Skua | 50627155 | OP | +ve | +ve | +ve | +ve | HPAIV H5N1 |
| 50627155 | C | +ve | +ve | +ve | +ve | HPAIV H5N1 | ||
| 50627155 | Bulk viscera | +ve | +ve | +ve | +ve | HPAIV H5N1 (EPI_ISL_5804789) | ||
| St. Kilda, Outer Hebrides | Great Skua (Received and tested on 19 August 2021) | Bird 1 | OP carcass swab | +ve | +ve | +ve | +ve | HPAIV H5N1 |
| C carcass swab | −ve | −ve | −ve | −ve | ||||
| Carcass swab real-time RT-PCR performed on 20 August 2021 | Bird 2 | OP carcass swab | +ve | +ve | +ve | +ve | HPAIV H5N1 | |
| C carcass swab | +ve | +ve | +ve | −ve | ||||
| Bird 3 | OP carcass swab | −ve | +ve | +ve | −ve | HPAIV H5Nx | ||
| C carcass swab | −ve | −ve | −ve | −ve | ||||
| Bird 4 | OP carcass swab | −ve | −ve | +ve | −ve | HPAIV H5Nx | ||
| C carcass swab | −ve | −ve | −ve | −ve | ||||
| Pooled tissue (Test performed on 24 August 2021) | Birds 1–4 | Brain | +ve | +ve | +ve | +ve | HPAIV H5Nx-positive pooled carcasses | |
| Lung and trachea | +ve | +ve | +ve | +ve | ||||
| Intestinal contents | +ve | +ve | +ve | +ve | ||||
| Pooled viscera | +ve | +ve | +ve | +ve | ||||
| Tissue | Number Examined | Histopathology a | Viral Immunohistochemistry b | ||
|---|---|---|---|---|---|
| Percent (Number of Cases) | Grade | Percent (Number of Cases) | Grade | ||
| Heart | 9 | 11(1) | + | 67 (6) | + to +++ |
| Skeletal muscle | 6 | 0 (0) | − | 50 (3) | +, ++ |
| Skin | 5 | 0 (0) | − | 0 (0) | − |
| Feather | 5 | 0 (0) | − | 20 (1) | ++ |
| Brain | 6 | 50 (3) | +, ++ | 83 (5) | ++ to ++++ |
| Kidney | 7 | 0 (0) | − | 71 (5) | +, ++ |
| Spleen | 3 | 0 (0) | − | 100 (3) | +, ++, ++++ |
| Adrenal | 2 | 100 (2) | ++, +++ | 100 (2) | ++, +++ |
| Trachea | 8 | 0 (0) | − | 0 (0) | − |
| Syrinx | 4 | 0 (0) | − | 25 (1) | + |
| Lung | 9 | 0 (0) | − | 78 (7) | +, ++, ++++ |
| Air sac | 6 | 0 (0) | − | 50 (3) | +, ++ |
| Proventriculus | 8 | 13 (1) | + | 38 (3) | +, ++ |
| Gizzard | 6 | 0 (0) | + | 17 (1) | +, ++ |
| Liver | 8 | 13 (1) | ++ | 63 (5) | +, ++ |
| Pancreas | 4 | 100 (4) | ++++ | 100 (4) | ++ to ++++ |
| Duodenum | 7 | 0 (0) | − | 29 (2) | +, ++ |
| Colon | 5 | 0 (0) | − | 0 (0) | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banyard, A.C.; Lean, F.Z.X.; Robinson, C.; Howie, F.; Tyler, G.; Nisbet, C.; Seekings, J.; Meyer, S.; Whittard, E.; Ashpitel, H.F.; et al. Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses 2022, 14, 212. https://doi.org/10.3390/v14020212
Banyard AC, Lean FZX, Robinson C, Howie F, Tyler G, Nisbet C, Seekings J, Meyer S, Whittard E, Ashpitel HF, et al. Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses. 2022; 14(2):212. https://doi.org/10.3390/v14020212
Chicago/Turabian StyleBanyard, Ashley C., Fabian Z. X. Lean, Caroline Robinson, Fiona Howie, Glen Tyler, Craig Nisbet, James Seekings, Stephanie Meyer, Elliot Whittard, Henry F. Ashpitel, and et al. 2022. "Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain" Viruses 14, no. 2: 212. https://doi.org/10.3390/v14020212
APA StyleBanyard, A. C., Lean, F. Z. X., Robinson, C., Howie, F., Tyler, G., Nisbet, C., Seekings, J., Meyer, S., Whittard, E., Ashpitel, H. F., Bas, M., Byrne, A. M. P., Lewis, T., James, J., Stephan, L., Lewis, N. S., Brown, I. H., Hansen, R. D. E., & Reid, S. M. (2022). Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses, 14(2), 212. https://doi.org/10.3390/v14020212







