Abstract
Background: Comparative data on COVID-19 among health care workers (HCWs) in different health care settings are scarce. This study investigated the rates of previous COVID-19 among HCWs in nursing homes, hospitals and a municipal emergency room (ER). Methods: We prospectively included 747 HCWs: 313 from nursing homes, 394 from hospitals and 40 from the ER. The diagnosis of COVID-19 was based on serological evidence of SARS-CoV-2 antibody positivity and self-reported RT-PCR positivity prior to inclusion. Information regarding age, sex and exposure to SARS-CoV-2 infection was collected. Results: A total of 4% (11/313) of nursing home HCWs and 6% (28/434) of HCWs in hospitals/the ER tested positive by serology and/or RT-PCR (p = 0.095). Fewer HCWs in nursing homes had occupational exposure to SARS-CoV-2 compared to those in hospitals/the ER (16% vs. 48%, p < 0, 001), but nursing homes had a higher proportion of HCWs with occupational exposure using partial/no PPE (56% vs. 19%, p < 0.001). Nevertheless, no significant differences in the risk for COVID-19 were found in relation to the rate of occupational exposure (p = 0.755) or use of inadequate PPE (p = 0.631). Conclusions: Despite a small sample size, the risk for COVID-19 among HCWs did not appear to be related to the type of health care facility, rates of occupational exposure or use of PPE.
1. Introduction
Occupational exposure to Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) among HCWs in nursing homes and other primary care facilities, as well as in hospitals, has been substantial, particularly in areas with a high incidence of infection [1,2]. In the early phases of the pandemic, there was a lack of personal protective equipment (PPE) and a staffing shortage, especially in nursing homes [3,4,5]. This, along with the often-atypical clinical presentation of Coronavirus disease 19 (COVID-19) among nursing home residents [6] and difficulties in maintaining infection control measures among wandering and/or agitated persons with dementia, might put HCWs in nursing homes at a higher risk for occupational exposure to COVID-19 than those in hospitals. Most studies on the seroprevalence of SARS-CoV-2 among HCWs have been performed in hospitals, and comparative serological data on HCWs in primary care facilities and hospitals are relatively scarce [1,7,8,9]. A study from Iraq showed a significant association between the seroprevalence of SARS-CoV-2 and PPE training in primary care HCWs [10]. However, this potential relationship has to date primarily been investigated in hospitals and university-based health care systems [11,12,13].
The main aim of the present study was to explore the rates of COVID-19 among HCWs, in relation to type of health care facility, exposure to SARS-CoV-2 and the use of PPE.
2. Materials and Methods
2.1. Study Population and Clinical Data
In the period from 28 September 2020 to 11 January 2021, the study participants were recruited from four nursing homes, two hospitals and an emergency room (ER) in the city of Bergen, Norway. Nursing home HCWs (n = 313) were consecutively enrolled from three long-term institutions and one primary care facility with a mixed population of short-term and long-term residents. Two of the nursing homes had outbreaks of COVID-19 before inclusion, and were defined as high-risk institutions, and two institutions without prior outbreaks as low-risk facilities. HCWs from hospitals (n = 394) were consecutively enrolled from the tertiary care center Haukeland University Hospital and the local hospital Haraldsplass Deaconess Hospital, in the Bergen Health Region. High-risk departments treated patients with COVID-19, and low-risk departments were not involved in the clinical care of such patients. All participants from the ER (n = 40) were working in a SARS-CoV-2 testing facility. A flow chart of the study population is depicted in Figure 1. Preventive measures in wards treating patients with COVID-19 included the isolation of patients/residents with a suspected or confirmed SARS-CoV-2 infection and the use of PPE upon testing and patient/resident care during the infection course. Furthermore, HCWs with symptoms compatible with COVID-19, along with their close contacts, were tested and home-isolated awaiting test results.
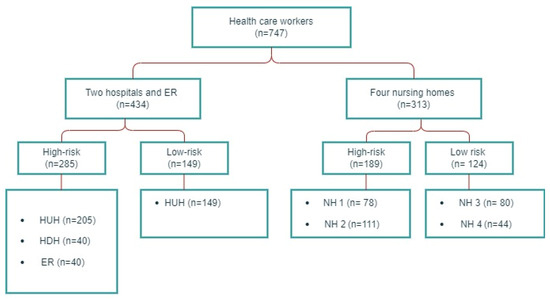
Figure 1.
Study population flow chart. HUH: Haukeland University Hospital, HDH: Haraldsplass Deaconess Hospital, ER: Bergen Municipality Emergency Room, NH: nursing home.
All HCWs included in the study provided a peripheral venous blood sample for serology at the time of inclusion, and were invited to complete a questionnaire including age, sex, and non-occupational and occupational exposure to SARS-CoV-2. Participants with positive serology, regardless of any results of prior RT-PCR-testing, along with seronegative participants who gave anamnestic information of a previous positive RT-PCR result, were defined as having been infected with SARS-CoV-2.
2.2. Laboratory Methods
The diagnosis of COVID-19 was based on self-reported SARS-CoV-2 RT-PCR positivity on samples from nasopharyngeal swabs as part of the national testing program where HCWs were prioritized, and on serological evidence of SARS-CoV-2 antibody positivity. Serum samples were allocated a unique identification number and frozen before running in the ELISA. A two-step ELISA was used to confirm SARS-CoV-2-specific antibodies, firstly through screening for antibodies to the SARS-CoV-2 receptor-binding domain (RBD) antigens from the Wuhan-1 virus and then a confirmatory SARS-CoV-2 Wuhan-1 virus spike IgG ELISA as previously described [14].
2.3. Statistical Analysis
Dichotomized variables were presented as percentages, and differences were assessed by a χ-square test, and presented with odds ratios (ORs), 95% confidence intervals (CI) and p-values. For variables with a low number of observations (<5), a Fisher’s exact test was used. Statistical analysis was performed in R version 4.2.1 (The R Foundation for Statistical Computing, http://www.R-project.org, accessed on 4 September 2022, using RStudio version 2022.07.1 (Boston, MA, USA).
3. Results
A total of 747 HCWs were included in the study, of which 394 (52, 7%) were working in hospitals, 313 (41, 9%) in nursing homes and 40 (5.4%) in the ER. The majority of the participants were female (81%). We observed a varying incidence of COVID-19 in the Municipality of Bergen during the study period (Figure 2). As shown in Table 1, there was no significant difference in non-occupational exposure to SARS-CoV-2 between HCWs working in nursing homes compared to those in hospitals/the ER (9% vs. 8%). Slightly more HCWs in hospitals/the ER (66%) had direct or indirect occupational exposure in terms of working in wards caring for COVID-19 patients, compared to nursing home HCWs (60%).
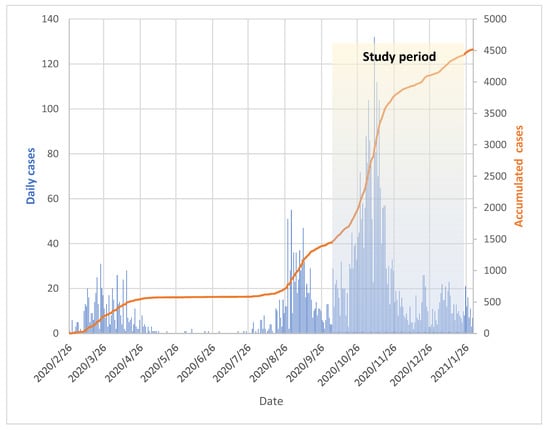
Figure 2.
Total COVID-19 cases in the Municipality of Bergen from 26 February 2020 to 26 January 2021. The shaded area depicts the study period from 28 September 2020 to 11 January 2021. The number of daily cases is shown in blue bars. The orange line graph represents accumulated cases.

Table 1.
Demographic characteristics of HCWs, by institution.
Table 2 shows the total incidence of SARS-CoV-2 positivity, and its relationship to, direct occupational exposure and type of institution (nursing home versus hospital/ER). In total, 4% (29/747) tested positive by serology. A total of 16 participants reported having tested positive for SARS-CoV-2 by RT-PCR before inclusion, of which 10 had a subsequent negative serological test. Consequently, 5% (39/747) of the participants had evidence for a prior SARS-CoV-2 infection. There was no significant difference in the rates of SARS-CoV-2 positivity in nursing homes versus hospital/ER (4% vs. 6%, p = 0.095), including those exposed with partial/no PPE (4% vs. 8%, p = 0.631).

Table 2.
Direct occupational exposure and SARS-CoV-2 positivity among HCWs, by institution.
In nursing homes, HCWs had less frequent occupational exposure to SARS-CoV-2 compared to those in hospital (16% vs. 48%, p < 0, 001), but, when exposed, they were less likely to use full PPE (44% vs. 81%, p< 0.001).
HCWs working in high-risk departments were not significantly more often infected with SARS CoV-2 than those without occupational exposure (6% vs. 3%, p = 0.087). Reanalyzing the subset of participants without non-occupational exposure, there was still no significant difference between those two groups (5% vs. 3%, p = 0.226). Stratifying for the use of PPE did not reveal significant differences in the risk of infection. Furthermore, nursing home HCWs working in high-risk settings did not contract COVID-19 significantly more often than those in hospitals/the ER did (4% vs. 8%, p = 0.177).
4. Discussion
The results from this prospective, cross-sectional study revealed that the total number of previous SARS-CoV-2 infections among nursing home HCWs was comparable to that of HCWs in hospitals/the ER, despite a significantly lower direct occupational exposure to this virus. However, it is conceivable that this similarity was at least partly a result of equal non-occupational and total occupational exposure (direct and indirect). More than 50% of the nursing home HCWs exposed to COVID-19 patients did not use full PPE, a risk factor also observed in other countries [15,16]. This finding may have several causes. Firstly, nursing home residents with COVID-19 may shed SARS-CoV-2 in their home environment in an asymptomatic or pre-symptomatic phase, or accompanied by atypical symptoms, potentially reducing the awareness of COVID-19 among HCWs [6,17,18]. Secondly, staff shortages, crowded facilities and difficulties in maintaining adequate infection control measures among patients with cognitive failure might have paved the way for a rapid and extensive transmission of SARS-CoV-2 among nursing home residents and HCWs [4,19,20]. Thirdly, the availability of PPE was lower in nursing homes than in hospitals/ERs in our region during the first phases of the pandemic, as also documented from other countries [3,4,19]. In fact, early in the pandemic, the Norwegian Ministry of Health allocated only 20% of the national stockpile of PPE to nursing homes and other primary care facilities.
A substantial variation in pooled prevalence rates of SARS-CoV-2 antibodies among HCWs has been documented in different geographical regions during the first pandemic phase; they were higher in the United States (12.4%) than in several European countries (7.7%) and East Asia (4.8%) [21]. The relatively low total seroprevalence among our HCWs probably reflects a limited transmission of SARS-CoV-2 in our community due to rigorous testing and an early lockdown, implemented on 12 March 2020.
Although nursing home HCWs were exposed to COVID-19 patients without using adequate PPE, nearly three times more often than those in hospitals/the ER, the rates of previous SARS-CoV-2 infection in this subgroup was similar. Clearly, the small numbers do not allow firm conclusions. However, we might speculate that nursing home HCWs regularly were in contact with residents who were either asymptomatic or had minimal respiratory symptoms before the diagnosis was confirmed [18]. The majority of patients in hospitals/ERs had prominent symptoms and were referred with a presumptive diagnosis of COVID-19, often during the first week of the infection, when viral load appears to be highest [22].
Although not statistically significant, we observed a higher percentage of anti-SARS-CoV-2-positive nursing home HCWs using full PPE, compared to the same group in hospitals/the ER. Again, the small numbers preclude conclusions, but it might be conceivable that nursing home personnel had received less training in the correct use of PPE than HCWs in hospitals/the ER during the first phases of the pandemic. Other potential explanations could be that only surgical face masks were available at nursing homes, which offer less protection against the transmission of SARS-CoV-2 than N95 respirators [23], or that HCWs were infected with SARS-CoV-2 through non-occupational exposure.
Among the 16 study participants who reported an RT-PCR-confirmed SARS-CoV-2 infection, 10 were negative by serology, constituting 25% of those with evidence for previous COVID-19. Data from our region during the first pandemic wave have demonstrated higher proportions of seropositivity than RT-PCR positivity among both HCWs and the general population [14,24]. Although we do not have corroborating data, participants with previous COVID-19 and negative serology might have been infected early in the first pandemic wave, more than six months prior to inclusion. Hence, as antibody titers are known to decline after infection, a prior positive PCR is indicative of true infection, despite negative serological testing. However, since PCR positivity is dependent on both the timing and technique of sampling, a prior negative PCR should not negate the interpretation of a later positive serological test, particularly as serology is more sensitive at detecting previous infection [25].
A strength of this study is that we included HCWs from both nursing homes, an ER and hospitals in a well-defined geographical region, and retrieved data on occupational exposure to COVID-19 patients/residents. Moreover, the nursing homes involved were quite similar, both regarding size, resident population, staffing and the quality of the facilities, and followed equal guidelines for infection control measures and the use of PPE.
However, our study has several limitations. The sample size was relatively small, as compared to many other SARS-CoV-2 serosurveillance studies [1,7,8], reducing the statistical power of subgroup analyses. Moreover, around 9% of the participants had non-occupational exposure to SARS-CoV-2, which might have obscured the interpretation of our data, particularly in the group of HCWs with direct exposure to COVID-19 patients. The study participants were consecutively enrolled in a period with varying incidence of COVID-19, potentially influencing the overall risk of exposure prior to serological testing. However, the level of SARS-CoV-2 transmission in our community was low-to-moderate both before and during the study period, and non-occupational exposure was reportedly similar across subgroups.
5. Conclusions
Our study shows that the rates of SARS-CoV-2 infection among HCWs in nursing homes and hospitals/ERs were comparable, although higher percentages of HCWs in the latter group had direct occupational exposure to SARS-CoV-2. Despite the fact that HCWs in nursing homes lacked sufficient PPE during the early phase of the pandemic, we did not find significant differences in the risk for COVID-19 of HCWs exposed to SARS-CoV-2 without full PPE between the two groups. These findings might be related to several factors, including varying patient/resident attributes, similar non-occupational exposure in the two HCW groups and a relatively small sample size.
Author Contributions
Conceptualization, B.R.K., N.L. and R.J.C.; data collection, N.L., R.J.C. and M.S.; laboratory assays R.J.C. and J.S.O.; data curation B.R.K., N.L., R.J.C., B.B. and M.S.; statistical analyses, B.B.; resources, N.L. and R.J.C.; project administration, N.L. and R.J.C.; funding acquisition, N.L. and R.J.C.; original draft preparation, B.R.K.; review and editing, B.R.K., N.L., R.J.C., B.B., M.S. and J.S.O. Members of the Bergen COVID-19 Research Group contributed to data collection and laboratory assays. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Trond Mohn Stiftelse (TMS2020TMT05) and Helse Vest (F-11628, F-12167, F-12621).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Ethics Committee of western Norway (no. 118664).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The small subgroups of participants make the risk of the identification of sensitive data of individual persons possible; therefore, the data are not openly accessible.
Acknowledgments
We thank all the HCWs who joined this study. We acknowledge the following collaborators/membersof the Bergen COVID-19 Research Group for help with the study: Kanika Kuwelker, Helene Heitmann Sandnes, Hanne Søyland, Mai Chi Trieu, Anders Madsen, Amit Bansal, Kristin Greve Isdahl Mohn, Camilla Tøndel and Karl Albert Brokstad. We also acknowledge Mads Munkejord and Kjell Haug for sharing data on the total burden of COVID-19 in the Municipality of Bergen from 26 November 2020 to 26 January 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grant, J.J.; Wilmore, S.M.S.; McCann, N.S.; Donnelly, O.; Lai, R.W.L.; Kinsella, M.J.; Rochford, H.L.; Patel, T.; Kelsey, M.C.; Andrews, J.A. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect. Control Hosp. Epidemiol. 2021, 42, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Rudberg, A.S.; Havervall, S.; Månberg, A.; Jernbom Falk, A.; Aguilera, K.; Ng, H.; Gabrielsson, L.; Salomonsson, A.C.; Hanke, L.; Murrell, B.; et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat. Commun. 2020, 11, 5064. [Google Scholar] [PubMed]
- McGarry, B.E.; Grabowski, D.C.; Barnett, M.L. Severe Staffing and Personal Protective Equipment Shortages Faced by Nursing Homes during the COVID-19 Pandemic. Health Aff. 2020, 39, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- White, E.M.; Wetle, T.F.; Reddy, A.; Baier, R.R. Front-line Nursing Home Staff Experiences during the COVID-19 Pandemic. J. Am. Med. Dir. Assoc. 2021, 22, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Charland, K.; Quach, C.; Nguyen, Q.D.; Zinszer, K. Institutional, therapeutic, and individual factors associated with 30-day mortality after COVID-19 diagnosis in Canadian long-term care facilities. J. Am. Geriatr. Soc. 2022, 70, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Blain, H.; Rolland, Y.; Benetos, A.; Giacosa, N.; Albrand, M.; Miot, S.; Bousquet, J. Atypical clinical presentation of COVID-19 infection in residents of a long-term care facility. Eur. Geriatr. Med. 2020, 11, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Moscola, J.; Sembajwe, G.; Jarrett, M.; Farber, B.; Chang, T.; McGinn, T.; Davidson, K.W. Prevalence of SARS-CoV-2 Antibodies in Health Care Personnel in the New York City Area. JAMA 2020, 324, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Padoan, A.; Fedeli, U.; Schievano, E.; Vecchiato, E.; Lippi, G.; Lo Cascio, G.; Porru, S.; Palù, G. SARS-CoV-2 serosurvey in health care workers of the Veneto Region. Clin. Chem. Lab. Med. 2020, 58, 2107–2111. [Google Scholar] [CrossRef]
- Lackermair, K.; William, F.; Grzanna, N.; Lehmann, E.; Fichtner, S.; Kucher, H.B.; Wilhelm, K.; Estner, H. Infection with SARS-CoV-2 in primary care health care workers assessed by antibody testing. Fam. Pract. 2021, 38, 76–79. [Google Scholar] [PubMed]
- Hussein, R.; Lami, F. Seroprevalence of COVID-19 Among Health Care Workers in Primary Health Care Centers in Al-Sader City District, Baghdad, Iraq. JMIR Publ. 2022, 8, e36473. [Google Scholar] [CrossRef]
- Baker, J.M.; Nelson, K.N.; Overton, E.; Lopman, B.A.; Lash, T.L.; Photakis, M.; Jacob, J.T.; Roback, J.D.; Fridkin, S.K.; Steinberg, J.P. Quantification of Occupational and Community Risk Factors for SARS-CoV-2 Seropositivity among Health Care Workers in a Large U.S. Health Care System. Ann. Intern. Med. 2021, 174, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Zangoue, M.; Safari, H.; Royce, S.G.; Zangooie, A.; Rezapour, H.; Zangouei, A.; Fereidouni, M. The high level of adherence to personal protective equipment in health care workers efficiently protects them from COVID-19 infection. Work 2021, 69, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hayakawa, K.; Ainai, A.; Iwata-Yoshikawa, N.; Sano, K.; Nagata, N.; Suzuki, T.; Wakimoto, Y.; Akiyama, Y.; Miyazato, Y.; et al. Effectiveness of personal protective equipment in preventing severe acute respiratory syndrome coronavirus 2 infection among healthcare workers. J. Infect. Chemother. 2021, 27, 120–122. [Google Scholar] [CrossRef]
- Trieu, M.C.; Bansal, A.; Madsen, A.; Zhou, F.; Sævik, M.; Vahokoski, J.; Brokstad, K.A.; Krammer, F.; Tøndel, C.; Mohn, K.G.I.; et al. SARS-CoV-2-Specific Neutralizing Antibody Responses in Norwegian Health Care Workers after the First Wave of COVID-19 Pandemic: A Prospective Cohort Study. J. Infect Dis. 2021, 223, 589–599. [Google Scholar] [CrossRef]
- Brainard, J.; Rushton, S.; Winters, T.; Hunter, P.R. Introduction to and spread of COVID-19-like illness in care homes in Norfolk, UK. J. Public Health 2021, 43, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: A systematic review and meta-analysis. J. Hosp. Infect. 2021, 108, 120–134. [Google Scholar] [CrossRef]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Kittang, B.R.; Hofacker, S.V.; Solheim, S.P.; Krüger, K.; Løland, K.K.; Jansen, K. Outbreak of COVID-19 at three nursing homes in Bergen. Tidsskr. Den Nor. Legeforening 2020, 140. [Google Scholar] [CrossRef]
- LeRose, J.J.; Merlo, C.; Duong, P.; Harden, K.; Rush, R.; Artzberger, A.; Sidhu, N.; Sandhu, A.; Chopra, T. The role of the social vulnerability index in personal protective equipment shortages, number of cases, and associated mortality during the coronavirus disease 2019 (COVID-19) pandemic in Michigan skilled nursing facilities. Infect. Control Hosp. Epidemiol. 2021, 42, 877–880. [Google Scholar] [CrossRef]
- McMichael, T.M.; Currie, D.W.; Clark, S.; Pogosjans, S.; Kay, M.; Schwartz, N.G.; Lewis, J.; Baer, A.; Kawakami, V.; Lukoff, M.D.; et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020, 382, 2005–2011. [Google Scholar] [CrossRef]
- Hossain, A.; Nasrullah, S.M.; Tasnim, Z.; Hasan, M.K.; Hasan, M.M. Seroprevalence of SARS-CoV-2 IgG antibodies among health care workers prior to vaccine administration in Europe, the USA and East Asia: A systematic review and meta-analysis. EClinicalMedicine 2021, 33, 100770. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.P.; Service, B.C.; Gupta, S.; Mubarak, N.; Zeini, I.M.; Osbahr, D.C.; Romeo, A.A. N95 respirator and surgical mask effectiveness against respiratory viral illnesses in the healthcare setting: A systematic review and meta-analysis. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12582. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Kuwelker, K.; Zhou, F.; Blomberg, B.; Lartey, S.; Brokstad, K.A.; Trieu, M.C.; Bansal, A.; Madsen, A.; Krammer, F.; Mohn, K.G.; et al. Attack rates amongst household members of outpatients with confirmed COVID-19 in Bergen, Norway: A case-ascertained study. Lancet Reg. Health Eur. 2021, 3, 100014. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).