Vector Competence of Mosquitoes from Germany for Sindbis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Mosquitoes
2.2. Experimental Infection of Mosquitoes
2.3. Analysis of Infection and Saliva Titration
2.4. Survival of Infected/Uninfected Culex pipiens biotype molestus at Four Temperatures
3. Results
3.1. Vector Competence Studies
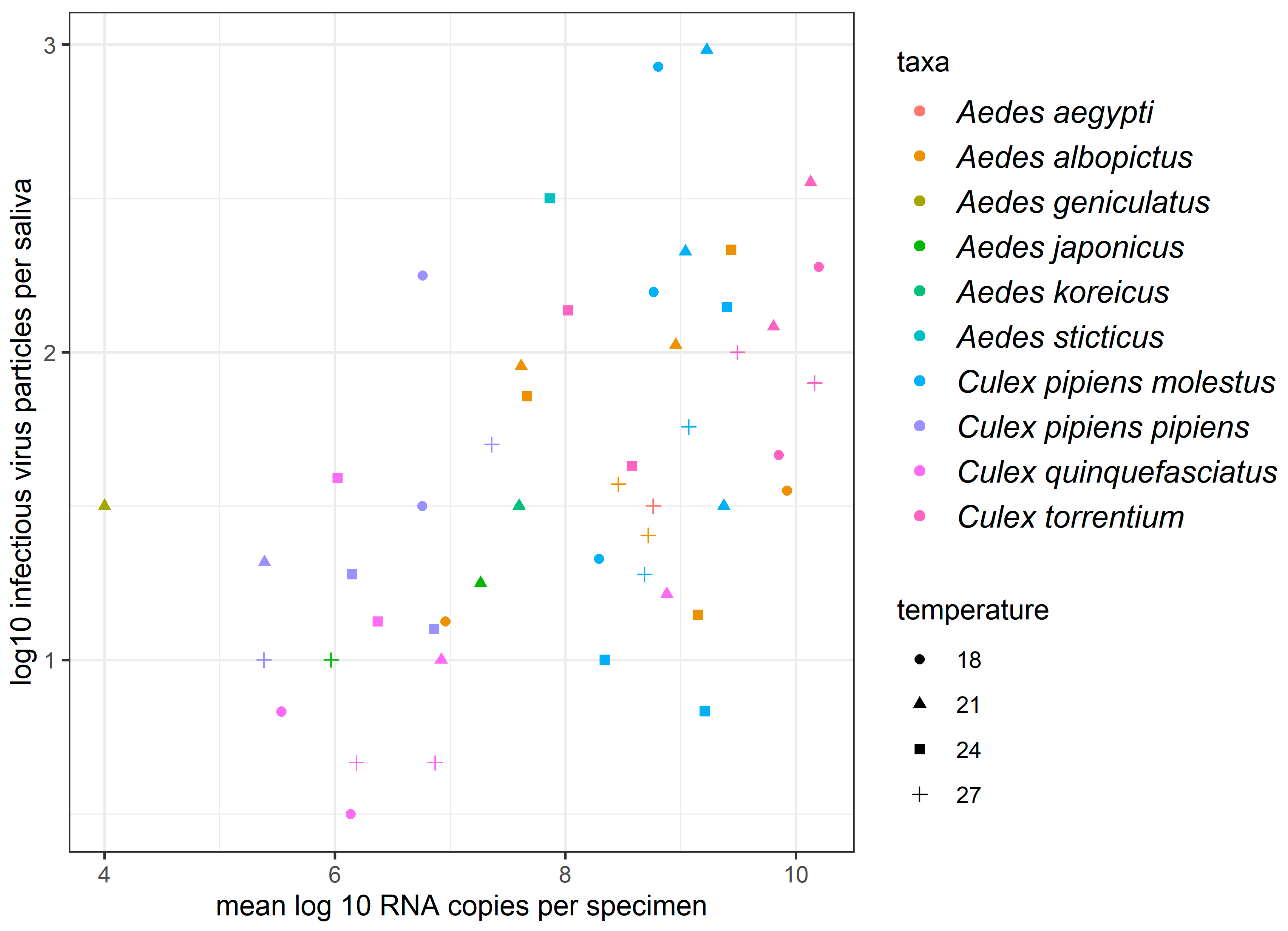
3.2. Determination of the Number of Infectious Virus Particles per Saliva Sample
3.3. Investigation of Clearance of SINV Infection on the Example of Culex pipiens biotype molestus
3.3.1. Survival of Culex pipiens biotype molestus for 28 Days
3.3.2. Vector Competence of Culex pip. biotype molestus 28 dpi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Shope, R.E.; Brandt, W.; Casals, J.; Karabatsos, N.; Murphy, F.A.; Tesh, R.B.; Wiebe, M.E. Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. Intervirology 1980, 14, 229–232. [Google Scholar] [CrossRef]
- Taylor, R.M.; Hurlbut, H.S.; Work, T.H.; Kingston, J.R.; Frothingham, T.E. Sindbis virus: A newly recognized arthropodtransmitted virus. Am. J. Trop. Med. Hyg. 1955, 4, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.Y.; Balasuriya, U.B.; Lee, C.K. Zoonotic encephalitides caused by arboviruses: Transmission and epidemiology of alphaviruses and flaviviruses. Clin. Exp. Vaccine Res. 2014, 3, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.; Luukkainen, R.; Toivanen, A. Sindbis viruses and other alphaviruses as cause of human arthritic disease. J. Intern. Med. 2004, 256, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Brummer-Korvenkontio, M.; Vapalahti, O.; Kuusisto, P.; Saikku, P.; Manni, T.; Koskela, P.; Nygren, T.; Brummer-Korvenkontio, H.; Vaheri, A. Epidemiology of Sindbis virus infections in Finland 1981–96: Possible factors explaining a peculiar disease pattern. Epidemiol. Infect. 2002, 129, 335–345. [Google Scholar] [CrossRef]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, O. Sindbis virus as a Human Pathogen—Epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef]
- Gylfe, Å.; Ribers, Å.; Forsman, O.; Bucht, G.; Alenius, G.-M.; Wållberg-Jonsson, S.; Ahlm, C.; Evander, M. Mosquito borne Sindbis Virus Infection and Long-Term Illness. Emerg. Infect. Dis. 2018, 24, 1141–1142. [Google Scholar] [CrossRef]
- Lundstrom, J.O.; Vene, S.; Saluzzo, J.F.; Niklasson, B. Antigenic comparison of Ockelbo virus isolates from Sweden and Russia with Sindbis virus isolates from Europe, Africa, and Australia: Further evidence for variation among alphaviruses. Am. J. Trop. Med. Hyg. 1993, 49, 531–537. [Google Scholar] [CrossRef]
- Suvanto, M.T.; Uusitalo, R.; Kampe, E.O.I.; Vuorinen, T.; Kurkela, S.; Vapalahti, O.; Dub, T.; Huhtamo, E.; Korhonen, E.M. Sindbis virus outbreak and evidence for geographical expansion in Finland, 2021. Euro. Surveill. 2022, 27, 2200580. [Google Scholar] [CrossRef]
- Meno, K.; Yah, C.; Mendes, A.; Venter, M. Incidence of Sindbis Virus in Hospitalized Patients with Acute Fevers of Unknown Cause in South Africa, 2019–2020. Front Microbiol. 2022, 12, 798810. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, S.; Human, S.; Williams, J.; van Wilpe, E.; Pretorius, M.; Swanepoel, R.; Venter, M. Old World Alphaviruses Associated with Neurologic Disease in Horses, South Africa. Emerg. Infect. Dis. 2015, 21, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Steyn, J.; Fourie, I.; Steyl, J.; Williams, J.; Stivaktas, V.; Botha, E.; van Niekerk, S.; Reininghaus, B.; Venter, M. Zoonotic Alphaviruses in Fatal and Neurologic Infections in Wildlife and Nonequine Domestic Animals, South Africa. Emerg. Infect. Dis. 2020, 26, 1182–1191. [Google Scholar] [CrossRef]
- Björnström, A.; Blomström, A.-L.; Singh, M.C.; Hesson, J.C. Sindbis virus neutralising antibodies detected in Swedish horses. One Health 2021, 12, 100242. [Google Scholar] [CrossRef]
- Lundström, J.O.; Pfeffer, M. Phylogeographic Structure and Evolutionary History of Sindbis Virus. Vector Borne Zoonotic Dis. 2010, 10, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Smura, T.; Lundström, J.O.; Petterson, J.H.-O.; Sironen, T.; Vapalahti, O.; Lundkvist, Å.; Hesson, J.C. Introduction and Dispersal of Sindbis Virus from Central Africa to Europe. J. Virol. 2019, 93, e00620-19. [Google Scholar] [CrossRef]
- Koren, R.; Bassal, R.; Shoat, T.; Cohen, D.; Mor, O.; Mendelson, E.; Lustig, Y. Presence of Antibodies against Sindbis Virus in the Israeli Population: A Nationwide Cross-Sectional Study. Viruses 2019, 11, 542. [Google Scholar] [CrossRef]
- Jöst, H.; Bialonski, A.; Storch, V.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J. Clin. Microbiol. 2010, 48, 1900–1903. [Google Scholar] [CrossRef]
- Eiden, M.; Ziegler, U.; Keller, M.; Müller, K.; Granzow, H.; Jöst, H.; Schmidt-Chanasit, J.; Groschup, M.H. Isolation of sindbis virus from a hooded crow in Germany. Vector Borne Zoonotic Dis. 2014, 14, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, D.E.; Schäfer, M.; Eiden, M.; Heym, E.C.; Ziegler, U.; Walter, D.; Schmidt-Chanasit, J.; Keller, M.; Groschup, M.H.; Kampen, H. Detection of Usutu, Sindbis, and Batai Viruses in Mosquitoes (Diptera: Culicidae) Collected in Germany, 2011–2016. Viruses 2018, 10, 389. [Google Scholar] [CrossRef]
- Heym, E.C.; Kampen, H.; Krone, O.; Schäfer, M.; Werner, D. Molecular detection of vector-borne pathogens from mosquitoes collected in two zoological gardens in Germany. Parasitol. Res. 2019, 118, 2097–2105. [Google Scholar] [CrossRef]
- Ziegler, U.; Fischer, D.; Eiden, M.; Reuschel, M.; Rinder, M.; Müller, K.; Schwehn, R.; Schmidt, V.; Groschup, M.H.; Keller, M. Sindbis virus—A wild bird associated zoonotic arbovirus circulates in Germany. Vet. Microbiol. 2019, 239, 108453. [Google Scholar] [CrossRef] [PubMed]
- Mossel, E.C.; Crabtree, M.B.; Mutebi, J.-P.; Lutwama, J.J.; Borland, E.M.; Powers, A.N.; Miller, B.R. Arboviruses Isolated from Mosquitoes Collected in Uganda, 2008–2012. J. Med. Entomol. 2017, 54, 1403–1409. [Google Scholar] [CrossRef]
- Hesson, J.C.; Verner-Carlsson, J.; Larsson, A.; Ahmed, R.; Lundkvist, Å.; Lundström, J.O. Culex torrentium Mosquito role as major enzootic vector defined by rate of Sindbis virus infection, Sweden, 2009. Emerg. Infect. Dis. 2015, 21, 875–878. [Google Scholar] [CrossRef]
- Korhonen, E.M.; Suvanto, M.T.; Uusitalo, R.; Faolotto, G.; Smura, T.; Sane, J.; Vapalahti, O.; Huhtamo, E. Sindbis Virus Strains of Divergent Origin Isolated from Humans and Mosquitoes During a Recent Outbreak in Finland. Vector Borne Zoonotic Dis. 2020, 20, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Dohm, D.J.; Logan, T.M.; Barth, J.F.; Turell, M.J. Laboratory transmission of Sindbis virus by Aedes albopictus, Ae. aegypti, and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 1995, 32, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Näslund, J.; Lundmark, E.; Ahlm, K.; Ahlm, C.; Bucht, G.; Evander, M. Experimental Infection and Transmission Competence of Sindbis Virus in Culex torrentium and Culex pipiens Mosquitoes from Northern Sweden. Vector Borne Zoonotic Dis. 2019, 19, 128–133. [Google Scholar] [CrossRef]
- Modlmaier, M.; Kuhn, R.; Kaaden, O.-R.; Pfeffer, M. Transmission studies of a European Sindbis virus in the floodwater mosquito Aedes vexans (Diptera: Culicidae). Int. J. Med. Microbiol. 2002, 33, 164–170. [Google Scholar] [CrossRef]
- Samuel, G.H.; Adelman, Z.N.; Myles, K.M. Temperature-dependent effects on the replication and transmission of arthropod-borne viruses in their insect hosts. Curr. Opin. Insect. Sci. 2016, 16, 108–113. [Google Scholar] [CrossRef]
- Adelman, Z.N.; Anderson, M.A.E.; Wiley, M.R.; Murreddu, M.G.; Samuel, G.H.; Morazzani, E.M.; Myles, K.M. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl. Trop. Dis. 2013, 7, e2239. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Petric’, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; Part I; pp. 206–208. [Google Scholar]
- Pfitzner, W.P.; Lehner, A.; Hoffmann, D.; Czajka, C.; Becker, N. First record and morphological characterization of an established population of Aedes (Hulecoeteomyia) koreicus (Dipter: Culicidae) in Germany. Parasit. Vectors 2018, 11, 662. [Google Scholar] [CrossRef]
- Rudolf, M.; Czajika, C.; Börstler, J.; Melaun, C.; Jöst, H.; von Thien, H.; Badusche, M.; Becker, N.; Schmidt-Chanasit, J.; Krüger, A.; et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE 2013, 8, e71832. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.-Y.; Davis, B.S.; Chang, G.-J.J. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medical important flaviviruses in mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Whitehouse, C.A.; Zoll, S.T.; Massire, C.; Pennella, T.-T.D.; Blyn, L.B.; Sampath, R.; Hall, T.A.; Ecker, J.A.; Desai, A.; et al. Direct broad-range detection of alphaviruses in mosquito extracts. Virology 2007, 368, 286–295. [Google Scholar] [CrossRef]
- Lambert, A.J.; Lanciotti, R.S. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 2009, 47, 2398–2404. [Google Scholar] [CrossRef]
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Schmidt-Chanasit, J.; Tannich, E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018, 138, e57980. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical ## Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 13 October 2022).
- Vogels, C.B.F.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J.M. Vector competence of northern and southern European Culex pipiens pipiens mosquitoes for West Nil virus across a gradient of temperatures. Med. Vet. Entomol. 2017, 31, 358–364. [Google Scholar] [CrossRef]
- Jansen, S.; Heitmann, A.; Lühken, R.; Leggewie, M.; Helms, M.; Badusche, M.; Rossini, G.; Schmidt-Chanasit, J.; Tannich, E. Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses 2019, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Badusche, M.; Pluskota, B.; Becker, N.; Vapalahti, O.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of Zika virus by mosquitoes from Central Europe. Euro. Surveill. 2017, 22, 30437. [Google Scholar] [CrossRef] [PubMed]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Ackroyd, S.; Vidana, B.; Lean, F.Z.X.; Hicks, D.; Nuñez, A.; Johnson, N. Temperate conditions restrict Japanese encephalitis virus infection to the mid-gut and prevents systemic dissemination in Culex pipiens mosquitoes. Sci. Rep. 2021, 11, 6133. [Google Scholar] [CrossRef]
- Ciocchetta, S.; Prow, N.A.; Darbro, J.M.; Frentiu, F.D.; Savino, S.; Montarsi, F.; Capelli, G.; Aaskov, J.G.; Devine, G.J. The new European invader Aedes (Finlaya) koreicus: A potential vector of chikungunya virus. Pathog. Glob. Health 2018, 112, 107–114. [Google Scholar] [CrossRef]
- Jansen, S.; Cadar, D.; Lühken, R.; Pfitzner, W.P.; Jöst, H.; Oerther, S.; Helms, M.; Zibrat, B.; Kliemke, K.; Becker, N.; et al. Vector competence of the invasive mosquito species Aedes koreicus for Arboviruses and interference with a novel insect specific virus. Viruses 2021, 13, 2507. [Google Scholar] [CrossRef]
- Heitmann, A.; Jansen, S.; Lühken, R.; Helms, M.; Pluskota, B.; Becker, N.; Kuhn, C.; Schmidt-Chanasit, J.; Tannich, E. Experimental risk assessment for chikungunya virus transmission based on vector competence, distribution and temperature suitability in Europe, 2018. Eurosurveillance 2018, 23, 1800033. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Marconcini, M.; Madec, Y.; Manni, M.; Carraretto, D.; Gomulski, L.M.; Gasperi, G.; Failloux, A.-B.; Malacrida, A.R. Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun. Biol. 2020, 3, 326. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Câmara, D.C.P.; Wiggins, K.; Eastmond, B.; Alto, B.W. High-Throughput method for detection of Arbovirus infection of saliva in mosquitoes Aedes aegypti and Ae. albopictus. Viruses 2020, 12, 1343. [Google Scholar] [CrossRef]
- Ou, T.P.; Auerswald, H.; In, S.; Peng, B.; Pang, S.; Boyer, S.; Choeung, R.; Dupont-Rouzeyrol, M.; Dussart, P.; Duong, V. Replication variance of African and Asian lineage Zika virus strains in different cell lines, mosquitoes and mice. Microorganisms 2021, 9, 1250. [Google Scholar] [CrossRef]
- Dubbrulle, M.; Mousson, L.; Moutailler, S.; Vazeille, M.; Failoux, A.-B. Chikungunya Virus and Aedes mosquitoes: Saliva is infectious as soon as two days after oral infection. PLoS ONE 2009, 4, e5895. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Aguilar, P.V.; Coffey, L.L.; Gromowski, G.D.; Wang, E.; Weaver, S.C. Venezuelan equine encephalitis virus transmission and effect on pathogenesis. Emerg. Infect. Dis. 2006, 12, 1190–1196. [Google Scholar] [CrossRef]
- Mercier, A.; Obadia, T.; Carraretto, D.; Velo, E.; Gabiane, G.; Bino, S.; Vazeille, M.; Gasperi, G.; Dauga, C.; Malacrida, A.R.; et al. Impact of temperature on dengue and chikungunya transmission by the mosquito Aedes albopictus. Sci. Rep. 2022, 12, 6973. [Google Scholar] [CrossRef]
- Turell, M.J.; Tammariello, R.F.; Spielmann, T.A. Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J. Med. Entomol. 1995, 32, 563–568. [Google Scholar] [CrossRef]
- Abbo, S.R.; Visser, T.M.; Koenraadt, C.J.M.; Pijlman, G.P.; Wang, H. Effect of blood source on vector competence of Culex pipiens biotypes for Usutu virus. Parasit. Vectors 2021, 14, 194. [Google Scholar] [CrossRef]
- Davis, N.L.; Fuller, F.J.; Dougherty, R.A.; Olmstedt, R.A.; Johnston, R.E. A single nucleotide change in the E2 glycoprotein gene of Sindbis virus affects penetration rate in cell culture and virulence in neonatal mice. Proc. Natl. Acad. Sci. USA 1989, 83, 6771–6775. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef]
- Moncayo, A.C.; Edman, J.D.; Turell, M.J. Effect of eastern equine encephalomyelitis virus on the survival of Aedes albopictus, Anopheles quadrimaculatus, and Coquillettidia pertubans (Diptera: Culicidae). J. Med. Entomol. 2000, 37, 701–706. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Westbrook, C.J.; Lounibos, L.P. Exposure to chikungunya virus and adult longevity in Aedes aegypti (L.) and Aedes albopictus (Skuse). J. Vector Ecol. 2010, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Folly, A.J.; Barrero, E.; Lumley, S.; del Mar, M.; de Marco, F.; Sewgobind, S.; McElhinney, L.M.; Fooks, A.R.; Johnson, N. Oral susceptibility of aedine and culicine mosquitoes (Diptera: Culicidae) to Batai Orthobunyavirus. Parasit. Vectors 2021, 14, 566. [Google Scholar] [CrossRef]
- Khoo, C.C.H.; Piper, J.; Sanchez-Vargas, I.; Olson, K.E.; Franz, A.W.E. The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in Aedes aegypti. BMC Microbiol. 2010, 10, 130. [Google Scholar] [CrossRef]
- Bergmann, A.; Dahl, E.; Lundkvist, Å.; Hesson, J.C. Sindbis virus infection in non-blood-fed hibernating Cule pipiens mosquitoes in Sweden. Viruses 2020, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
| Species | Temperature | dpi | n | IR (%) | Mean Body Titer log10 Copies/Mosquito (95% Confidence Interval) | TR (%) | TE (%) | Mean Saliva Titer log10 Infectious Virus Particle/Mosquito (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|---|
| Culex pipiens biotype molestus | 18 +/− 5 °C | 5 | 30 | 100 | 8.77 (8.70–8.83) | 77 | 77 | 2.20 (1.46–2.93) |
| 14 | 35 | 100 | 8.80 (8.59–9.02) | 100 | 100 | 2.93 (2.50–3.36) | ||
| 21 +/− 5 °C | 5 | 30 | 100 | 9.23 (8.94–9.51) | 97 | 97 | 2.98 (2.38–3.59) | |
| 14 | 31 | 100 | 9.04 (8.74–9.35) | 94 | 94 | 2.33 (1.91–2.75) | ||
| 24 +/− 5 °C | 5 | 55 | 100 | 9.40 (9.12–9.69) | 96 | 96 | 2.15 (1.61–2.68) * | |
| 14 | 35 | 100 | 8.34 (8.12–8.56) | 51 | 51 | 1.00 (0.61–1.39) | ||
| 27 +/− 5 °C | 5 | 36 | 100 | 9.07 (8.94–9.20) | 97 | 97 | 1.76 (1.41–2.10) | |
| 14 | 30 | 100 | 8.69 (8.40–8.98) | 60 | 60 | 1.28 (0.70–1.86) | ||
| Culex quinque-fasciatus | 18 +/− 5 °C | 5 | 30 | 57 | 6.14 (5.24–7.03) | 6 | 3 | 0.5 (n.a.) |
| 14 | 30 | 40 | 5.54 (4.29–6.78) | 25 | 10 | 0.83 (0.00–2.27) | ||
| 21 +/− 5 °C | 5 | 30 | 40 | 6.92 (5.18–8.66) | 50 | 20 | 1.00 (0–43–1.57) | |
| 14 | 30 | 43 | 8.88 (7.50–10.26) | 54 | 23 | 1.21 (0.33–2.09) | ||
| 24 +/− 5 °C | 5 | 50 | 82 | 6.37 (5.80–6.95) | 51 | 42 | 1.13 (0.65–1.60) ** | |
| 14 | 40 | 65 | 6.02 (5.30–6.75) | 42 | 28 | 1.59 (0.96–2.23) | ||
| 27 +/− 5 °C | 5 | 30 | 60 | 6.87 (5.69–8.05) | 33 | 20 | 0.67 (0.24–1.10) | |
| 14 | 30 | 53 | 6.19 (4.94–7.44) | 38 | 20 | 0.67 (0.24–1.10) | ||
| Culex torrentium | 18 +/− 5 °C | 5 | 30 | 100 | 9.85 (9.24–10.46) | 20 | 20 | 1.67 (0.12–3.21) |
| 14 | 31 | 97 | 10.20 (9.46–10.94) | 87 | 84 | 2.28 (1.43–3.13) | ||
| 21 +/− 5 °C | 5 | 36 | 97 | 9.81 (9.02–10.60) | 41 | 40 | 2.08 (0.95–3.22) | |
| 14 | 40 | 100 | 10.13 (9.90–10.36) | 93 | 93 | 2.55 (2.03–3.07) | ||
| 24 +/− 5 °C | 5 | 30 | 97 | 8.02 (7.47–8.58) | 76 | 73 | 2.14 (1.56–2.71) | |
| 14 | 30 | 93 | 8.58 (7.95–9.21) | 85 | 79 | 1.63 (1.14–2.12) | ||
| 27 +/− 5 °C | 5 | 30 | 97 | 10.17 (9.43–10.89) | 86 | 83 | 1.90 (1.29–2.51) | |
| 14 | 31 | 94 | 9.49 (9.14–9.85) | 69 | 65 | 2.00 (1.30–2.70) | ||
| Culex pipiens biotype pipiens | 18 +/− 5 °C | 5 | 30 | 33 | 6.76 (4.95–8.57) | 20 | 7 | 1.50 (0.00–14.21) |
| 14 | 30 | 63 | 6.76 (5.23–8.29) | 21 | 13 | 2.25 (0.00–5.53) | ||
| 21 +/− 5 °C | 5 | 30 | 70 | 5.39 (4.65–6.13) | 52 | 37 | 1.32 (0.33–2.31) | |
| 14 | 12 | 42 | 7.09 (4.87–9.31) | 0 | 0 | n.a. | ||
| 24 +/− 5 °C | 5 | 30 | 77 | 6.86 (5.84–7.89) | 43 | 33 | 1.10 (0.33–1.87) | |
| 14 | 52 | 37 | 6.15 (5.39–6.91) | 47 | 17 | 1.28 (0.77–1.80) | ||
| 27 +/− 5 °C | 5 | 30 | 27 | 7.36 (4.71–10.01) | 63 | 17 | 1.70 (0.00–3.74) | |
| 14 | 36 | 50 | 5.38 (3.97–6.80) | 13 | 7 | 1.00 (0.00–7.35) | ||
| Aedes albopictus | 18 +/− 5 °C | 5 | 30 | 90 | 6.96 (5.94–7.98) | 30 | 27 | 1.13 (0.13–2.12) |
| 14 | 30 | 73 | 9.92 (9.53–10.31) | 91 | 67 | 1.55 (0.92–2.18) | ||
| 21 +/− 5 °C | 5 | 38 | 97 | 7.62 (6.93–8.30) | 30 | 29 | 1.95 (1.26–2.65) | |
| 14 | 29 | 86 | 8.96 (7.99–9.92) | 84 | 72 | 2.02 (1.32–2.72) | ||
| 24 +/− 5 °C | 5 | 30 | 97 | 9.15 (8.57–9.74) | 59 | 57 | 1.15 (0.84–1.46) | |
| 14 | 30 | 90 | 9.44 (8.74–10.14) | 89 | 80 | 2.33 (1.71–2.95) | ||
| 27 +/− 5 °C | 5 | 30 | 100 | 8.46 (7.78–9.14) | 47 | 47 | 1.57 (0.65–2.49) | |
| 14 | 31 | 94 | 8.72 (8.22–9.22) | 72 | 68 | 1.40 (1.00–1.81) | ||
| Aedes koreicus | 21 +/− 5 °C | 14 | 2 | 100 | 7.60 (0.00–52.79) | 100 | 100 | 1.50 (−11.21–14.21) |
| 27 +/− 5 °C | 14 | 15 | 60 | 5.38 (3.90–6.87) | 44 | 27 | 1.00 (0.00–2.59) | |
| Aedes sticticus | 24 +/− 5 °C | 14 | 16 | 69 | 7.90 (7.01–8.72) | 27 | 19 | 2.50 (0.00–7.47) |
| Aedes geniculatus | 21 +/− 5 °C | 14 | 9 | 33 | 4.00 (3.53–4.47) | 33 | 11 | 1.50 (n.a.) |
| Species | Temperature | Infection Status | SR 28 Days Post Feed (%) | IR (%) | Mean Body Titer log10 FFU/Mosquito (95% Confidence Interval) | TR (%) | TE (%) |
|---|---|---|---|---|---|---|---|
| Culex pipiens biotype molestus | 18 +/− 5 °C | SINV | 47.9 (57/119) | 100 | 8.29 (8.18–8.41) | 81 | 81 |
| None | 73.0 (92/126) | n.a. | n.a. | n.a. | n.a. | ||
| 21 +/− 5 °C | SINV | 9.6 (17/178) | 100 | 9.38 (9.21–9.54) | 71 | 71 | |
| none | 9.2 (12/130) | n.a. | n.a. | n.a. | n.a. | ||
| 24 +/− 5 °C | SINV | 5.5 (7/127) | 100 | 9.21 (8.93–9.49) | 43 | 43 | |
| none | 0.0 (0/158) | n.a. | n.a. | n.a. | n.a. | ||
| 27 +/− 5 °C | SINV | 0.0 (0/124) | n.a. | n.a. | n.a. | n.a. | |
| none | 0.0 (0/155) | n.a. | n.a. | n.a. | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, S.; Lühken, R.; Helms, M.; Pluskota, B.; Pfitzner, W.P.; Oerther, S.; Becker, N.; Schmidt-Chanasit, J.; Heitmann, A. Vector Competence of Mosquitoes from Germany for Sindbis Virus. Viruses 2022, 14, 2644. https://doi.org/10.3390/v14122644
Jansen S, Lühken R, Helms M, Pluskota B, Pfitzner WP, Oerther S, Becker N, Schmidt-Chanasit J, Heitmann A. Vector Competence of Mosquitoes from Germany for Sindbis Virus. Viruses. 2022; 14(12):2644. https://doi.org/10.3390/v14122644
Chicago/Turabian StyleJansen, Stephanie, Renke Lühken, Michelle Helms, Björn Pluskota, Wolf Peter Pfitzner, Sandra Oerther, Norbert Becker, Jonas Schmidt-Chanasit, and Anna Heitmann. 2022. "Vector Competence of Mosquitoes from Germany for Sindbis Virus" Viruses 14, no. 12: 2644. https://doi.org/10.3390/v14122644
APA StyleJansen, S., Lühken, R., Helms, M., Pluskota, B., Pfitzner, W. P., Oerther, S., Becker, N., Schmidt-Chanasit, J., & Heitmann, A. (2022). Vector Competence of Mosquitoes from Germany for Sindbis Virus. Viruses, 14(12), 2644. https://doi.org/10.3390/v14122644





