Overexpression Bombyx mori HEXIM1 Facilitates Immune Escape of Bombyx mori Nucleopolyhedrovirus by Suppressing BmRelish-Driven Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect, Cell Culture, Virus, and Chemical Treatment of BmN Cells
2.2. Phylogenetic Analysis
2.3. Tissue Sample Collection
2.4. The Construction of pIZ/V5-BmHEXIM1-Flag and Two Mutants Expression Vector
2.5. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.6. Immunofluorescence (IF) and Fluorescence Microscopy
2.7. RNA Interference Assay
2.8. Co-Immunoprecipitation (Co-IP) Assay
2.9. Western Blotting
2.10. Viral Titer Determination and Measurement of BmNPV Proliferation in BmN Cells
2.11. Statistical Analysis
3. Results
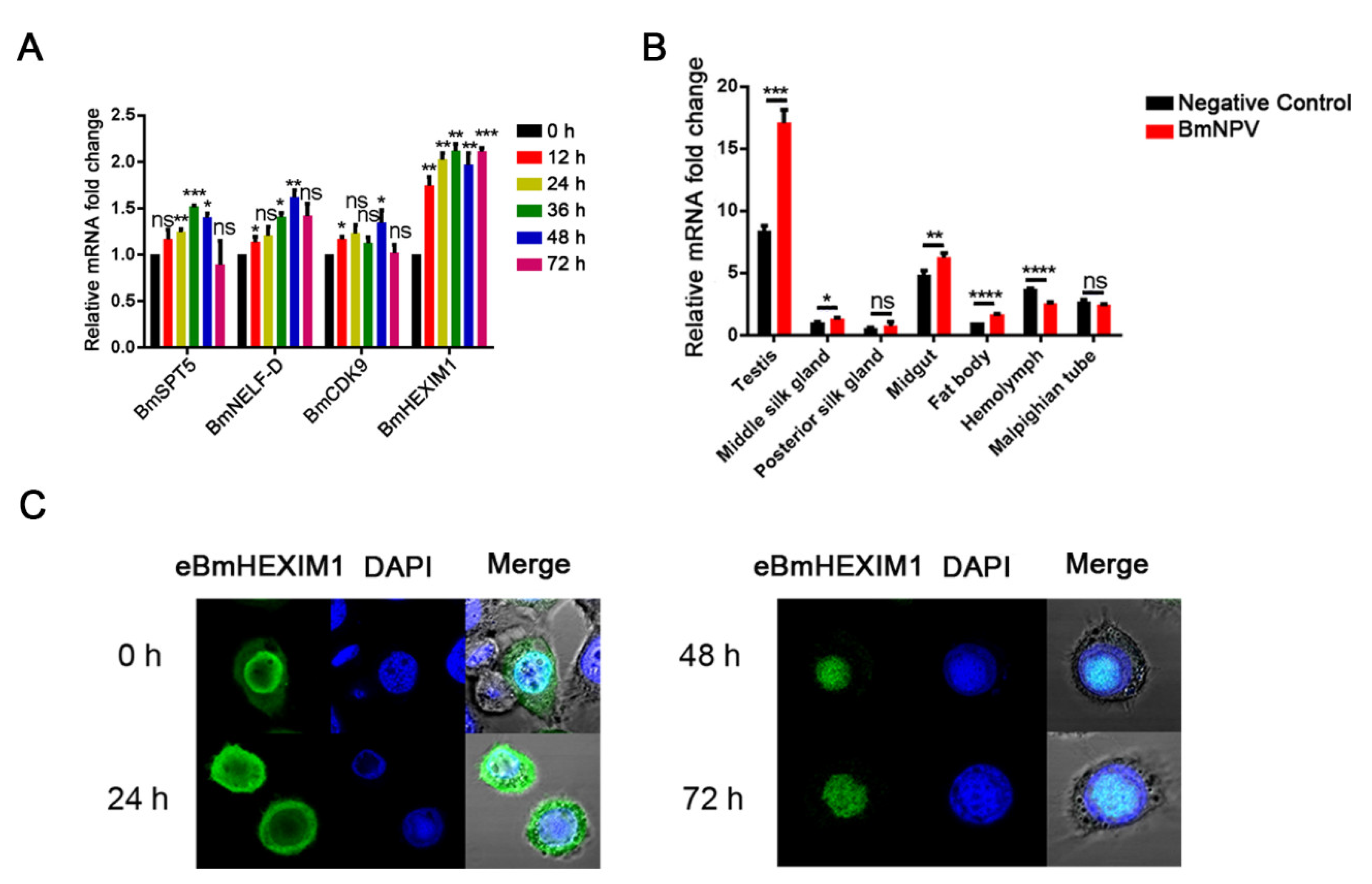
3.1. Identification and Characterization of BmHEXIM1
3.2. BmHEXIM1 Showed a Significant Response to BmNPV Infection
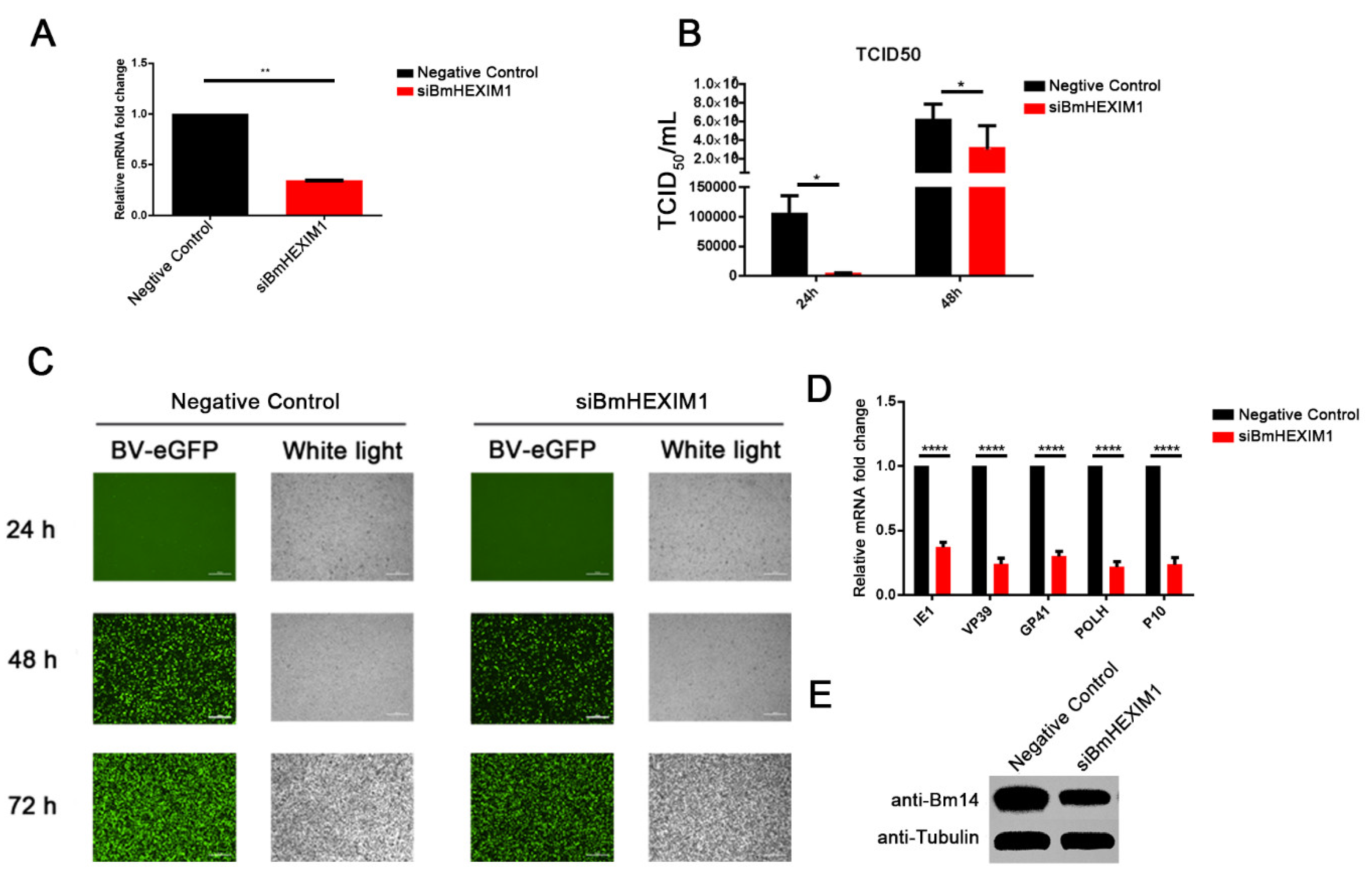
3.3. Knockdown of BmHEXIM1 Significantly Suppressed BmNPV Proliferation
3.4. Overexpression of BmHEXIM1 Significantly Promoted BmNPV Proliferation
3.5. The Full Length of BmHEXIM1 Was Essential for Promoting BmNPV Proliferation
3.6. BmHEXIM1 Is Not Involved in Controlling Viral Transcriptional Elongation by Regulating Viral Polymerase Phosphorylation
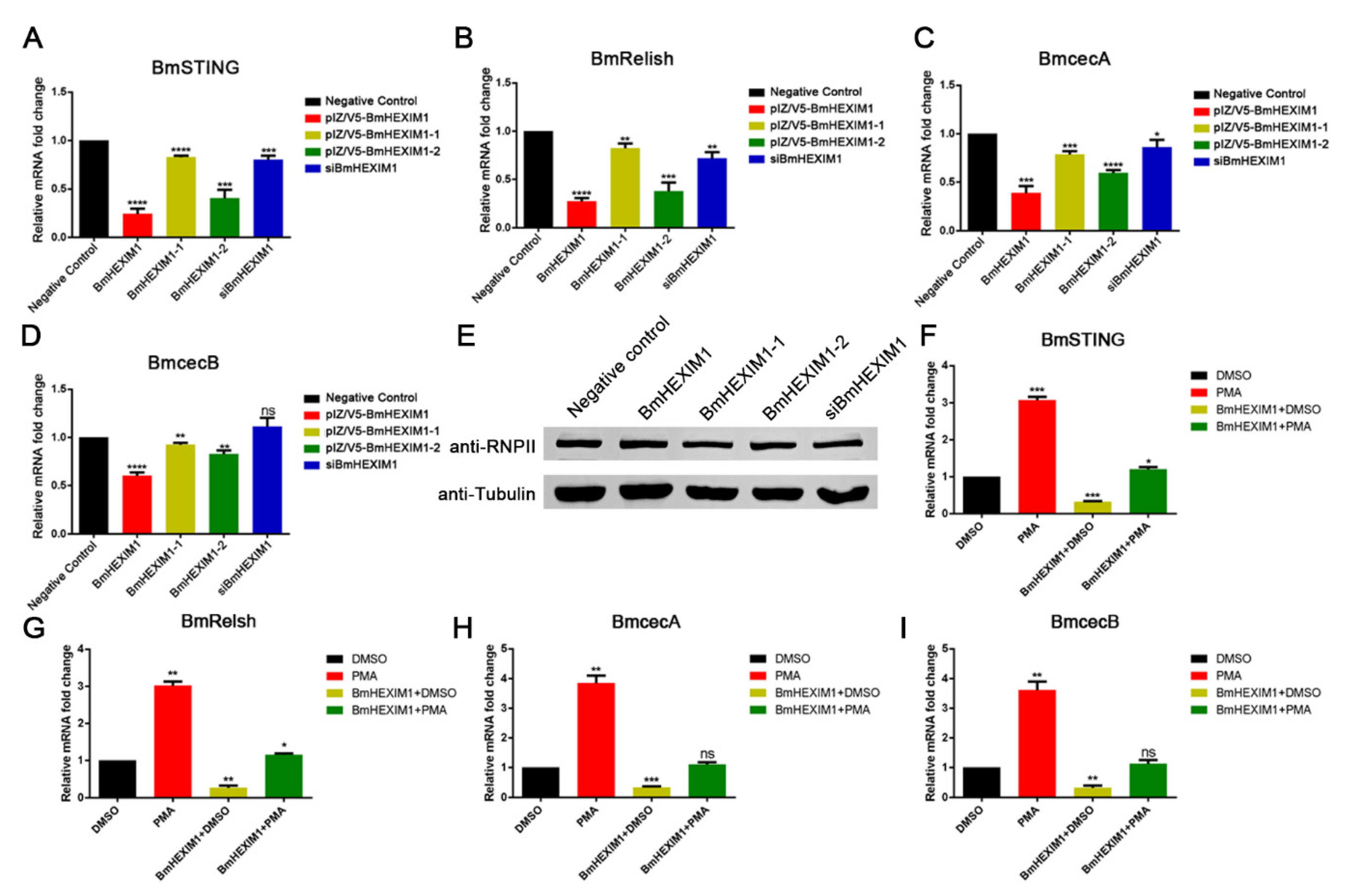
3.7. Overexpression of BmHEXIM1 Contributed to BmNPV Immune Escape by Suppressing BmRelish-Driven Immune Responses
3.8. HMBA Induced BmHEXIM1 Expression Leading to the Promotion of Viral Proliferation
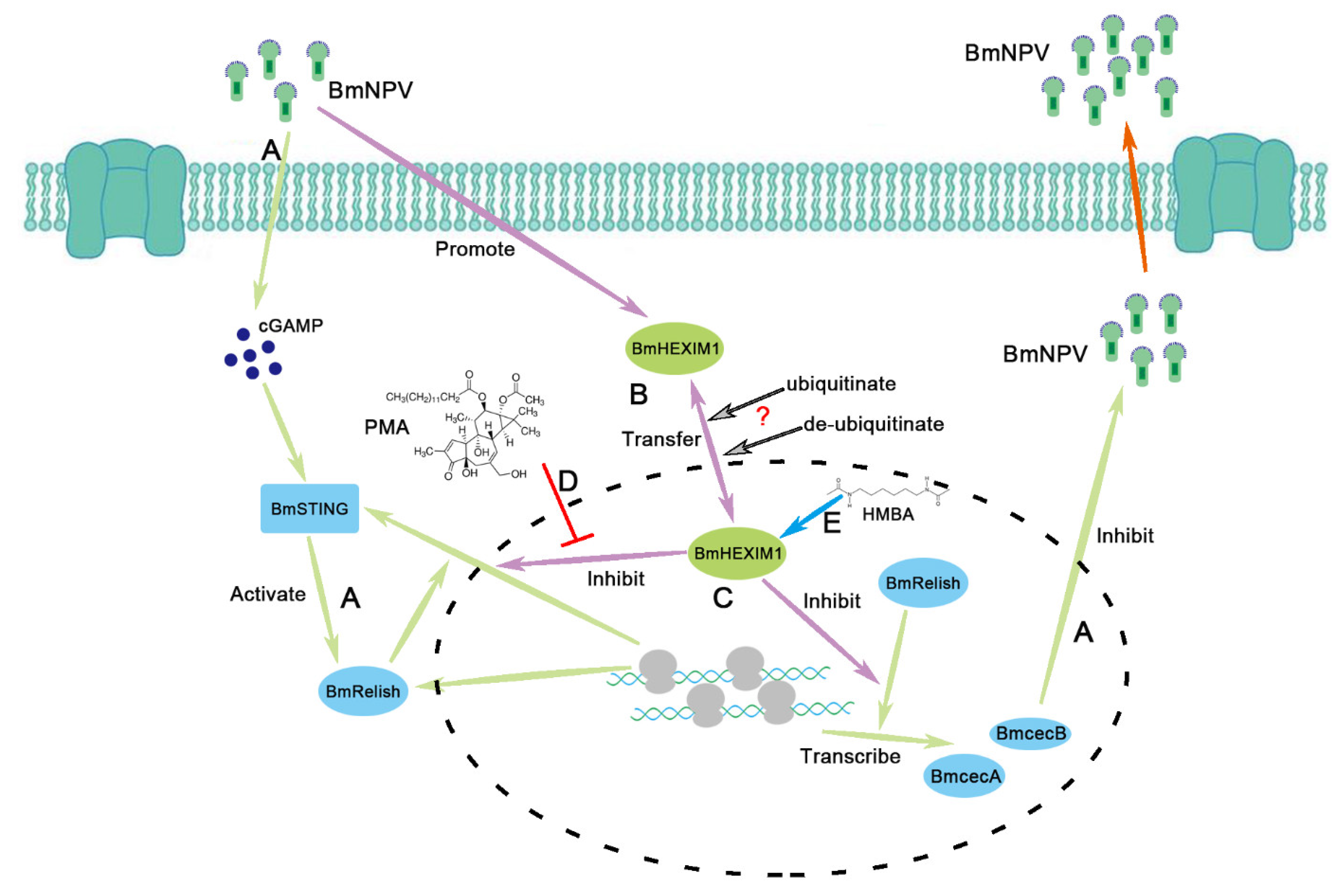
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BmcecA | Bombyx mori cecropin A |
| BmcecB | Bombyx mori cecropin B |
| BmNPV | Bombyx mori nucleopolyhedrovirus |
| BmNHR96 | Bombyx mori nuclear hormone receptor 96 |
| BmREEPa | Bombyx mori receptor expression-enhancing protein |
| BmSINAL10 | Bombyx mori E3 ubiquitin–protein ligase SINA-like 10 |
| BVs | Budded viruses |
| cGAMP | Cyclic GMP-AMP |
| Co-IP | Co-immunoprecipitation |
| CSLM | Confocal scanning laser microscopy |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GR | Glucocorticoid receptor |
| HEXIM1 | Hexamethylene bisacetamide-inducible protein 1 |
| HMBA | Hexamethylene bisacetamide |
| HSP | Heat shock protein |
| IF | Immunofluorescence |
| IMD | Immune deficiency |
| NCBI | National Center for Biotechnology Information |
| NJ | Neighbor joining |
| PAGE | Polyacrylamide gel electrophoresis |
| PKC | Protein kinase C |
| PMA | Phorbol 12-myristate acetate |
| P-TEFb | Positive transcriptional elongation factor b |
| RT-qPCR | Quantitative real-time polymerase chain reaction |
| snRNP | Small nuclear ribonucleoprotein particles |
| STING | Stimulator of interferon genes |
| VS | Virogenic stroma |
References
- Seth, R.B.; Sun, L.; Chen, Z.J. Antiviral innate immunity pathways. Cell Res. 2006, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.L.; Liu, T.H.; Wang, W.; Pan, C.X.; Du, G.Y.; Wu, Y.F.; Pan, M.H.; Lu, C. BmNHR96 participate BV entry of BmN-SWU1 cells via affecting the cellular cholesterol level. Biochem. Biophys. Res. Commun. 2017, 482, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-L.; Liu, T.-H.; Wang, W.; Pan, C.-X.; Du, G.-Y.; Wu, Y.-F.; Adur, M.; Zhang, M.-J.; Pan, M.-H.; Lu, C. Transgenic RNAi of BmREEPa in silkworms can enhance the resistance of silkworm to Bombyxmori Nucleopolyhedrovirus. Biochem. Biophys. Res. Commun. 2017, 483, 855–859. [Google Scholar] [CrossRef]
- Feng, M.; Kong, X.; Zhang, J.; Xu, W.; Wu, X. Identification of a novel host protein SINAL10 interacting with GP64 and its role in Bombyx mori nucleopolyhedrovirus infection. Virus Res. 2018, 247, 102–110. [Google Scholar] [CrossRef]
- Wu, P.; Shang, Q.; Huang, H.; Zhang, S.; Zhong, J.; Hou, Q.; Guo, X. Quantitative proteomics analysis provides insight into the biological role of Hsp90 in BmNPV infection in Bombyx mori. J. Proteom. 2019, 203, 103379. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Fei, S.; Awais, M.M.; Zheng, H.; Feng, M.; Sun, J. Heat Shock Protein 75 (TRAP1) facilitate the proliferation of the Bombyx mori nucleopolyhedrovirus. Int. J. Biol. Macromol. 2021, 175, 372–378. [Google Scholar] [CrossRef]
- Mao, F.; Zhu, Y.; Gao, X.; Chen, X.; Ngowo, J.; Miao, M.; Quan, Y.; Yu, W. HSP/HSC70 activity is required for Bombyx mori nucleopolyhedrovirus replication at the early infectious phase. Microb. Pathog. 2020, 153, 104647. [Google Scholar] [CrossRef]
- Ketchart, W.; Yeh, I.-J.; Zhou, H.; Thiagarajan, P.S.; Lathia, J.; Reizes, O.; Exner, A.; Su, B.; Montano, M.M. Induction of HEXIM1 activities by HMBA derivative 4a1: Functional consequences and mechanism. Cancer Lett. 2016, 379, 60–69. [Google Scholar] [CrossRef][Green Version]
- Liu, P.; Xiang, Y.; Fujinaga, K.; Bartholomeeusen, K.; Nilson, K.A.; Price, D.H.; Peterlin, B.M. Release of Positive Transcription Elongation Factor b (P-TEFb) from 7SK Small Nuclear Ribonucleoprotein (snRNP) Activates Hexamethylene Bisacetamide-inducible Protein (HEXIM1) Transcription. J. Biol. Chem. 2014, 289, 9918–9925. [Google Scholar] [CrossRef]
- Tan, J.L.; Fogley, R.D.; Flynn, R.A.; Ablain, J.; Yang, S.; Saint-André, V.; Fan, Z.P.; Do, B.T.; Laga, A.C.; Fujinaga, K.; et al. Stress from Nucleotide Depletion Activates the Transcriptional Regulator HEXIM1 to Suppress Melanoma. Mol. Cell 2016, 62, 34–46. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, T.; Price, D.H. RNA Polymerase II Elongation Control. Annu. Rev. Biochem. 2012, 81, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.A.; Nguyen, V.T.; Fraldi, A.; Labas, V.; Edwards, M.; Bonnet, F.; Lania, L.; Bensaude, O. MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol. Cell. Biol. 2003, 23, 4859–4869. [Google Scholar] [CrossRef] [PubMed]
- Yik, J.H.; Chen, R.; Nishimura, R.; Jennings, J.L.; Link, A.J.; Zhou, Q. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell 2003, 12, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Diribarne, G.; Bensaude, O. 7SK RNA, a non-coding RNA regulating P-TEFb, a general transcription factor. RNA Biol. 2009, 6, 122–128. [Google Scholar] [CrossRef]
- Morchikh, M.; Cribier, A.; Raffel, R.; Amraoui, S.; Cau, J.; Severac, D.; Dubois, E.; Schwartz, O.; Bennasser, Y.; Benkirane, M. HEXIM1 and NEAT1 Long Non-coding RNA Form a Multi-subunit Complex that Regulates DNA-Mediated Innate Immune Response. Mol. Cell 2017, 67, 387–399.e5. [Google Scholar] [CrossRef]
- Ouchida, R.; Kusuhara, M.; Shimizu, N.; Hisada, T.; Makino, Y.; Morimoto, C.; Handa, H.; Ohsuzu, F.; Tanaka, H. Suppression of NF-kappaB-dependent gene expression by a hexamethylene bisacetamide-inducible protein HEXIM1 in human vascular smooth muscle cells. Genes Cells Devoted Mol. Cell. Mech. 2003, 8, 95–107. [Google Scholar] [CrossRef]
- Shimizu, N.; Ouchida, R.; Yoshikawa, N.; Hisada, T.; Watanabe, H.; Okamoto, K.; Kusuhara, M.; Handa, H.; Morimoto, C.; Tanaka, H. HEXIM1 forms a transcriptionally abortive complex with glucocorticoid receptor without involving 7SK RNA and positive transcription elongation factor b. Proc. Natl. Acad. Sci. USA 2005, 102, 8555–8560. [Google Scholar] [CrossRef]
- Wittmann, B.M.; Fujinaga, K.; Deng, H.; Ogba, N.; Montano, M.M. The breast cell growth inhibitor, estrogen down regulated gene 1, modulates a novel functional interaction between estrogen receptor alpha and transcriptional elongation factor cyclin T1. Oncogene 2005, 24, 5576–5588. [Google Scholar] [CrossRef]
- Hellweg, C.E.; Arenz, A.; Bogner, S.; Schmitz, C.; Baumstark-Khan, C. Activation of nuclear factor kappa B by different agents: Influence of culture conditions in a cell-based assay. Ann. N. Y. Acad. Sci. 2006, 1091, 191–204. [Google Scholar] [CrossRef]
- Schroy, P.C.; Rustgi, A.K.; Ikonomu, E.; Liu, X.P.; Polito, J.; Andry, C.; O’Keane, J.C. Growth and intestinal differentiation are independently regulated in HT29 colon cancer cells. J. Cell. Physiol. 1994, 161, 111–123. [Google Scholar] [CrossRef]
- Palumbo, C.; Albonici, L.; Bei, R.; Bocci, C.; Scarpa, S.; DI Nardo, P.; Modesti, A. HMBA induces cell death and potentiates doxorubicin toxicity in malignant mesothelioma cells. Cancer Chemother. Pharmacol. 2004, 54, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Zhang, X.; Feinman, R.; Teitz, T.; Zelenetz, A.; Richon, V.M.; Rifkind, R.A.; Marks, P.A.; Michaeli, J. Hexamethylene bisacetamide induces programmed cell death (apoptosis) and down-regulates BCL-2 expression in human myeloma cells. Proc. Natl. Acad. Sci. USA 1998, 95, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kawai, T.; Obinata, M.; Fujiwara, H.; Horiuchi, T.; Saeki, Y.; Sato, Y.; Furusawa, M. Production of human α-interferon in silkworm using a baculovirus vector. Nature 1985, 315, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Western Blotting: An Introduction. West. Blotting 2015, 1312, 17–30. [Google Scholar]
- Summers, M.D.; Smith, G. A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures. 1987. [Google Scholar]
- Williams, G.V.; Faulkner, P. Cytological changes and viral morphogenesis during baculovirus infection. In The Baculoviruses; Miller, L.K., Ed.; Springer US: Boston, MA, USA, 1997; pp. 61–107. [Google Scholar]
- Okano, K.; Mikhailov, V.S.; Maeda, S. Colocalization of baculovirus IE-1 and two DNA-binding proteins, DBP and LEF-3, to viral replication factories. J Virol 1999, 73, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Matsumoto, S.; Nagamine, T. Analysis of baculovirus IE1 in living cells: Dynamics and spatial relationships to viral structural proteins. J. Gen. Virol. 2004, 85, 3575–3583. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, H.; Wu, X. Bombyx mori nucleopolyhedrovirus F-like protein Bm14 is a cofactor for GP64-Mediated efficient infection via forming a complex on the envelope of budded virus. Virology 2020, 539, 61–68. [Google Scholar] [CrossRef]
- Michels, A.A.; Bensaude, O. Hexim1, an RNA-controlled protein hub. Transcription 2018, 9, 262–271. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Cao, X.; Lu, Y.; Mi, Z.; He, C.; Liu, J.; Zheng, Z.; Li, M.J.; Li, T.; et al. Activation of P-TEFb by cAMP-PKA signaling in autosomal dominant polycystic kidney disease. Sci. Adv. 2019, 5, eaaw3593. [Google Scholar] [CrossRef]
- Liang, C.; Su, X.; Xu, G.; Dai, X.; Zhao, S. Autographa californica multiple nucleopolyhedrovirus PK1 is a factor that regulates high-level expression of very late genes in viral infection. Virology 2017, 512, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Chadha, P.; Das, R.H. Serine/threonine kinase (pk-1) is a component of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) very late gene transcription complex and it phosphorylates a 102kDa polypeptide of the complex. Virus Res. 2008, 137, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Dames, S.A.; Schönichen, A.; Schulte, A.; Barboric, M.; Peterlin, B.M.; Grzesiek, S.; Geyer, M. Structure of the Cyclin T binding domain of Hexim1 and molecular basis for its recognition of P-TEFb. Proc. Natl. Acad. Sci. USA 2007, 104, 14312–14317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L. Insights into the Antiviral Pathways of the Silkworm Bombyx mori. Front. Immunol. 2021, 12, 639092. [Google Scholar] [CrossRef]
- Hua, X.; Li, B.; Song, L.; Hu, C.; Li, X.; Wang, D.; Xiong, Y.; Zhao, P.; He, H.; Xia, Q.; et al. Stimulator of interferon genes (STING) provides insect antiviral immunity by promoting Dredd caspase-mediated NF-κB activation. J. Biol. Chem. 2018, 293, 11878–11890. [Google Scholar] [CrossRef]
- Andreeff, M.; Stone, R.; Michaeli, J.; Young, C.W.; Tong, W.P.; Sogoloff, H.; Ervin, T.; Kufe, D.; Rifkind, R.A.; Marks, P.A. Hexamethylene bisacetamide in myelodysplastic syndrome and acute myelogenous leukemia: A phase II clinical trial with a differentiation-inducing agent. Blood 1992, 80, 2604–2609. [Google Scholar] [CrossRef]
- Young, C.W.; Fanucchi, M.P.; Walsh, D.; Baltzer, L.; Yaldaei, S.; Stevens, Y.W.; Gordon, C.; Tong, W.; Rifkind, A.R.; Marks, A.P. Phase I trial and clinical pharmacological evaluation of hexamethylene bisacetamide administration by ten-day continuous intravenous infusion at twenty-eight-day intervals. Cancer Res. 1988, 48, 7304–7309. [Google Scholar]
- Faust, T.B.; Li, Y.; Bacon, C.W.; Jang, G.M.; Weiss, A.; Jayaraman, B.; Newton, B.W.; Krogan, N.J.; D’Orso, I.; Frankel, A.D. The HIV-1 Tat protein recruits a ubiquitin ligase to reorganize the 7SK snRNP for transcriptional activation. eLife 2018, 7, e31879. [Google Scholar] [CrossRef]
- Katsuma, S.; Tsuchida, A.; Matsuda-Imai, N.; Kang, W.; Shimada, T. Role of the ubiquitin-proteasome system in Bombyx mori nucleopolyhedrovirus infection. J. Gen. Virol. 2010, 92, 699–705. [Google Scholar] [CrossRef]
- Lü, P.; Pan, Y.; Yang, Y.; Zhu, F.; Li, C.; Guo, Z.; Yao, Q.; Chen, K. Discovery of anti-viral molecules and their vital functions in Bombyx mori. J. Invertebr. Pathol. 2018, 154, 12–18. [Google Scholar] [CrossRef]
- Kingsolver, M.B.; Huang, Z.; Hardy, R.W. Insect Antiviral Innate Immunity: Pathways, Effectors, and Connections. J. Mol. Biol. 2013, 425, 4921–4936. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, W.; Guo, H.; Dang, Y.; Cheng, T.; Yang, W.; Sun, Q.; Wang, B.; Wang, Y.; Xie, E.; et al. Distinct Functions of Bombyx mori Peptidoglycan Recognition Protein 2 in Immune Responses to Bacteria and Viruses. Front. Immunol. 2019, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Okado, K.; Martins, N.; Cai, H.; Barbier, V.; Lamiable, O.; Troxler, L.; Santiago, E.; Kuhn, L.; Paik, D.; et al. The Kinase IKKβ Regulates a STING- and NF-κB-Dependent Antiviral Response Pathway in Drosophila. Immunity 2018, 49, 225–234.e4. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, M.; Kleinschmidt, A.; Winder, D.; Von Pechmann, N.; Ludvigsen, A.; Neumann, M.; Holle, R.; Salmons, B.; Erfle, V.; Brack-Werner, R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998, 79, 731–740. [Google Scholar] [CrossRef]
- Crack, L.R.; Jones, L.; Malavige, G.N.; Patel, V.; Ogg, G.S. Human antimicrobial peptides LL-37 and human β-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin. Exp. Dermatol. 2012, 37, 534–543. [Google Scholar] [CrossRef]
- Yashwant, R.S.; Thomas, D.S.; Manoharan, C.; Roy, G.; Kunjupillai, V.; Mishra, R.K.; Nongthomba, U.; Gopalapillai, R. Transgenic Silkworms Overexpressing Relish and Expressing Drosomycin Confer Enhanced Immunity to Multiple Pathogens. Mol. Biotechnol. 2022, 64, 711–724. [Google Scholar] [CrossRef]
- Dey, A.; Wong, E.; Kua, N.; Teo, H.L.; Tergaonkar, V.; Lane, D. Hexamethylene bisacetamide (HMBA) simultaneously targets AKT and MAPK pathway and represses NF kappaB activity: Implications for cancer therapy. Cell Cycle 2008, 7, 3759–3767. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H.; Aweya, J.J.; Chen, Y.; Chen, M.; Wu, X.; Chen, X.; Lu, J.; Chen, R.; Liu, M. HMBA Enhances Prostratin-Induced Activation of Latent HIV-1 via Suppressing the Expression of Negative Feedback Regulator A20/TNFAIP3 in NF-κB Signaling. BioMed Res. Int. 2016, 2016, 5173205. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Li, Y.; Kong, X.; Zhao, S.; Li, J.; Wu, X. Overexpression Bombyx mori HEXIM1 Facilitates Immune Escape of Bombyx mori Nucleopolyhedrovirus by Suppressing BmRelish-Driven Immune Responses. Viruses 2022, 14, 2636. https://doi.org/10.3390/v14122636
Chen G, Li Y, Kong X, Zhao S, Li J, Wu X. Overexpression Bombyx mori HEXIM1 Facilitates Immune Escape of Bombyx mori Nucleopolyhedrovirus by Suppressing BmRelish-Driven Immune Responses. Viruses. 2022; 14(12):2636. https://doi.org/10.3390/v14122636
Chicago/Turabian StyleChen, Guanping, Yuedong Li, Xiangshuo Kong, Shudi Zhao, Jiale Li, and Xiaofeng Wu. 2022. "Overexpression Bombyx mori HEXIM1 Facilitates Immune Escape of Bombyx mori Nucleopolyhedrovirus by Suppressing BmRelish-Driven Immune Responses" Viruses 14, no. 12: 2636. https://doi.org/10.3390/v14122636
APA StyleChen, G., Li, Y., Kong, X., Zhao, S., Li, J., & Wu, X. (2022). Overexpression Bombyx mori HEXIM1 Facilitates Immune Escape of Bombyx mori Nucleopolyhedrovirus by Suppressing BmRelish-Driven Immune Responses. Viruses, 14(12), 2636. https://doi.org/10.3390/v14122636




