Evolution of Blood Safety in Switzerland over the Last 25 Years for HIV, HCV, HBV and Treponema pallidum
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Donors
2.2. Evolution of the Screening Strategy in Switzerland
2.3. The Current Mandatory Testing Strategy in Switzerland
2.4. Confirmation of Screening Reactive Samples
2.5. Theoretical Residual Risk Estimations
2.6. Lookback Procedures (LBP)
3. Results
3.1. Blood Donors
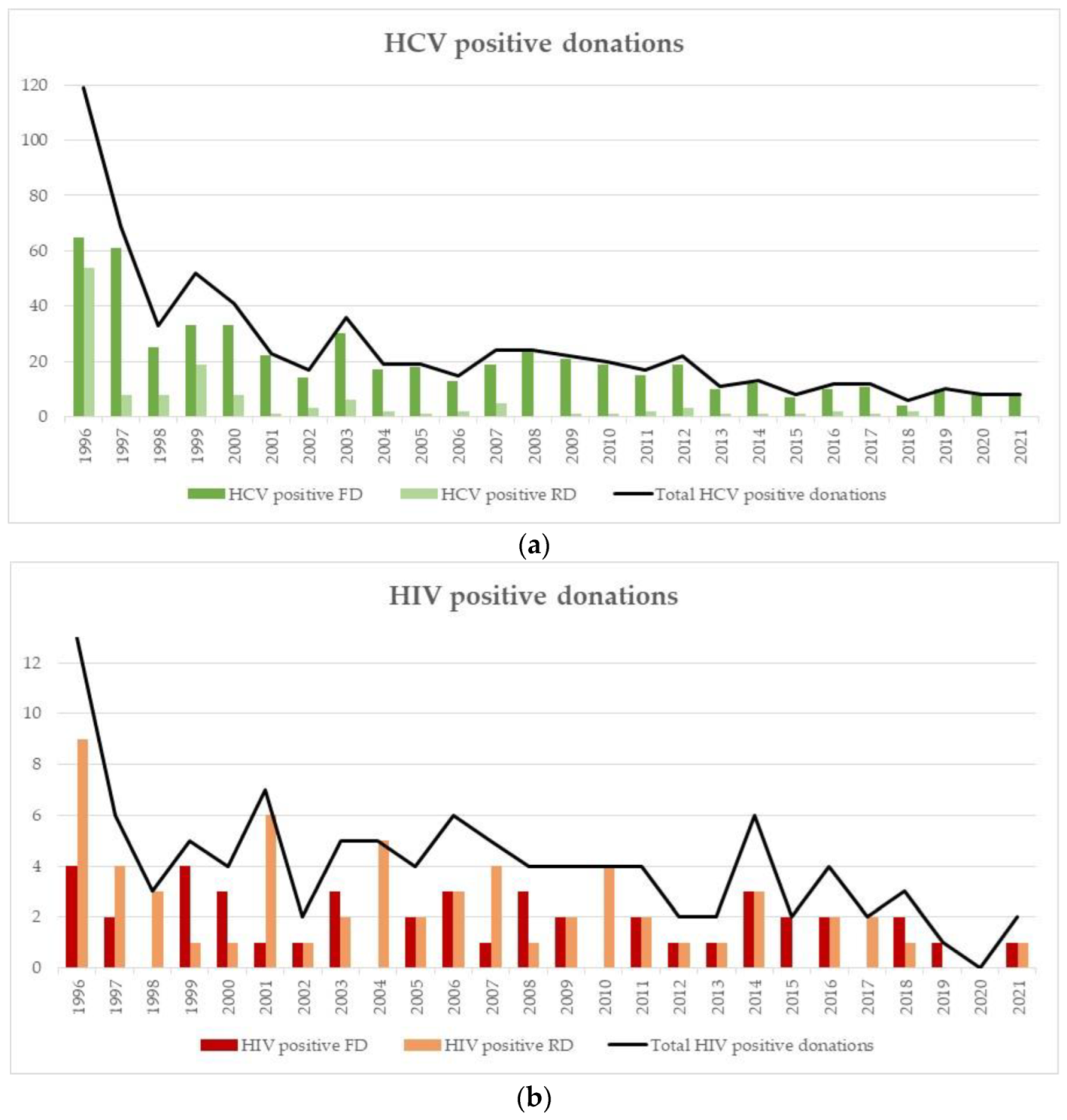
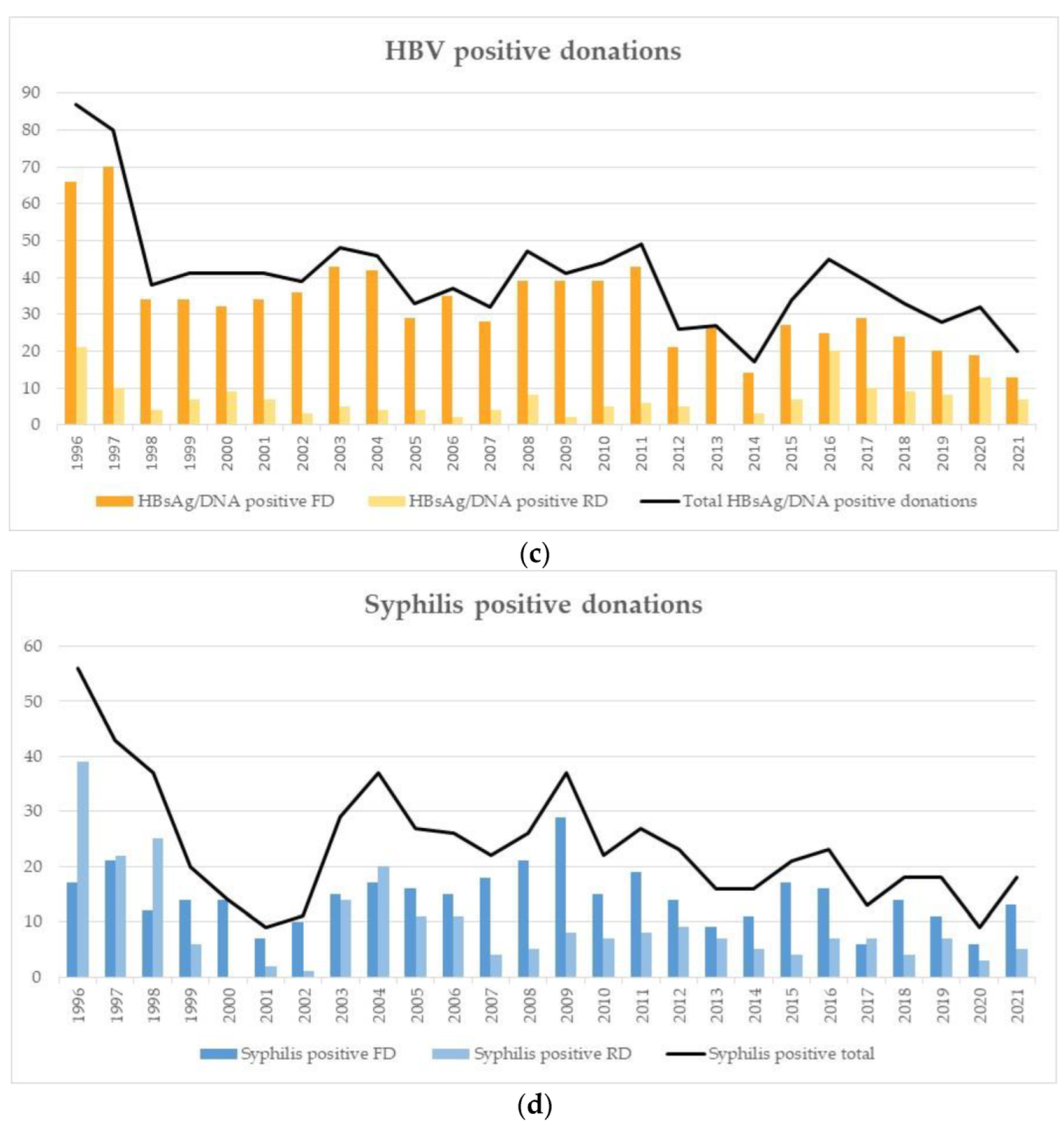
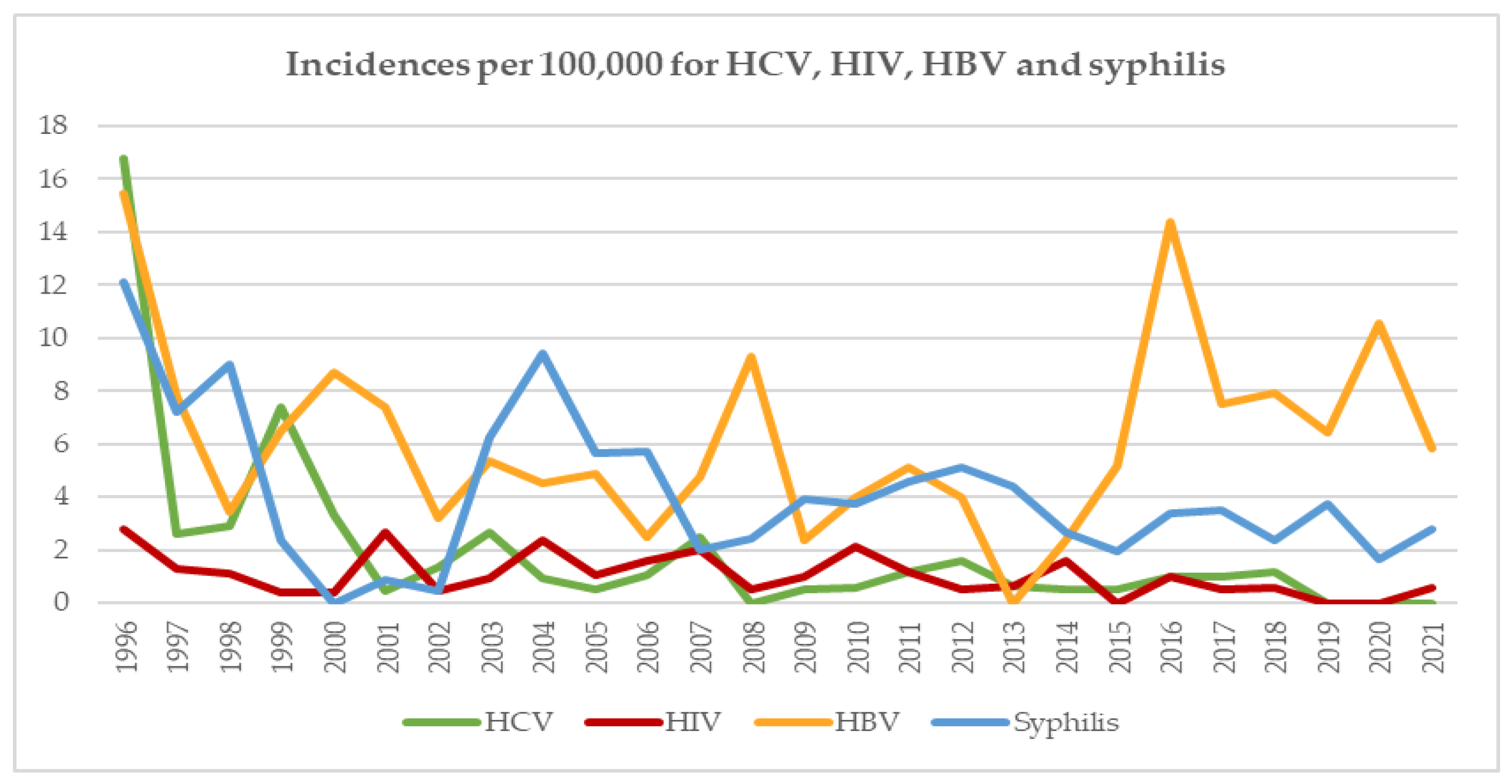
3.2. Incidences in RD
3.3. Prevalences in FD
3.4. Residual Risks (RR)
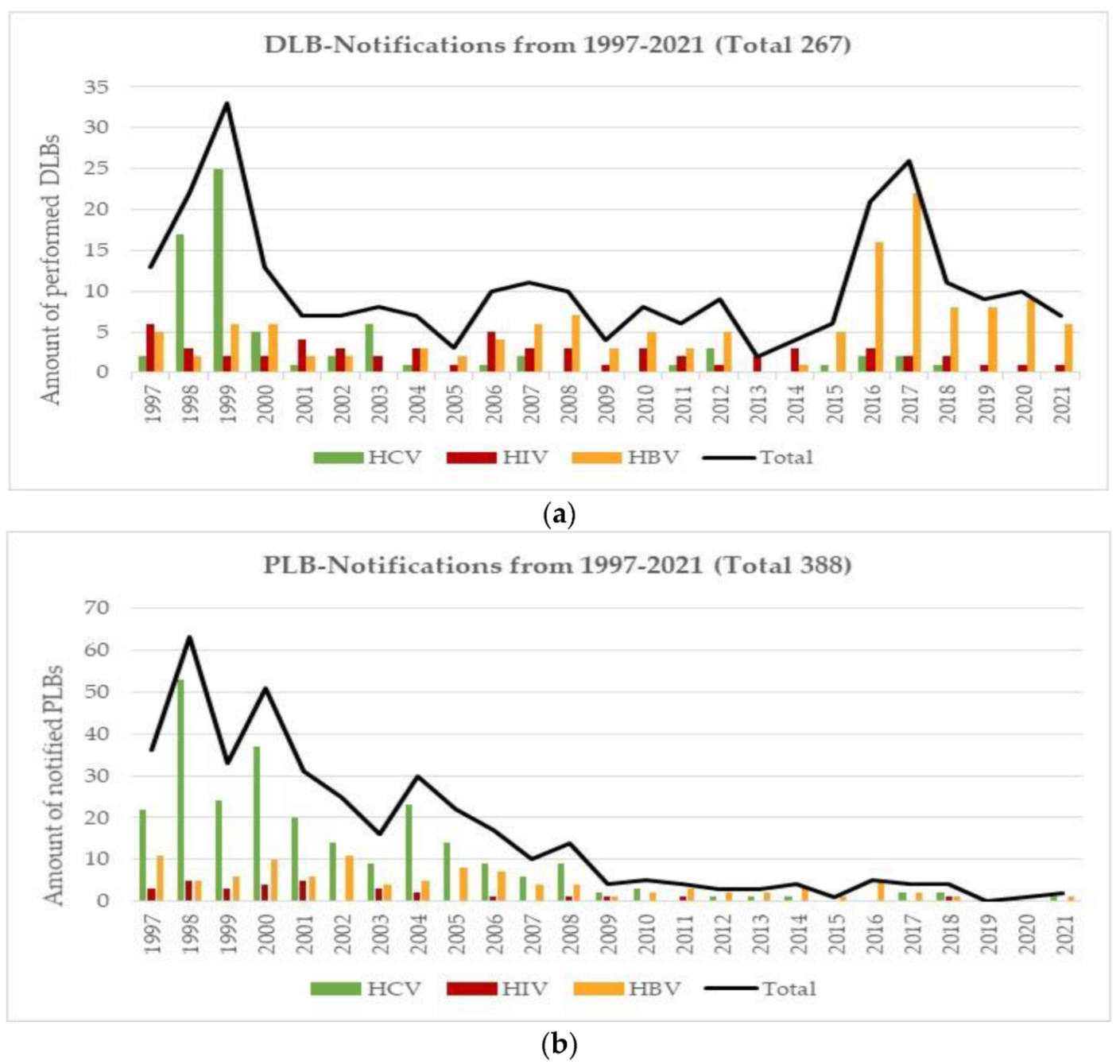
3.5. Lookback Procedures (LB)
3.6. NAT Yields
3.7. Haemovigilance
4. Discussion
4.1. General
4.2. Blood Donations
4.3. Evolution of the Testing Systems
4.4. Positive Cases
4.5. NAT Yields
4.6. Incidences/Prevalences
4.7. Residual Risk (RR)
5. Conclusions/Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| HCV | HIV | HBV (Variant 1, Including OBI) | HBV (Variant 2, Excluding OBI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1/RR | 95% Confidence Interval | 1/RR | 95% Confidence Interval | 1/RR | 95% Confidence Interval | 1/RR | 95% Confidence Interval | ||||
| 1996 | 3.3 × 104 | 2.5 × 104 | 4.4 × 104 | 5.9 × 105 | 3.1 × 105 | 1.3 × 106 | 4.0 × 104 | 2.6 × 104 | 6.4 × 104 | 4.0 × 104 | 2.6 × 104 | 6.4 × 104 |
| 1997 | 2.1 × 105 | 1.1 × 105 | 4.9 × 105 | 1.3 × 106 | 4.9 × 105 | 4.6 × 106 | 7.9 × 104 | 4.3 × 104 | 1.7 × 105 | 7.9 × 104 | 4.3 × 104 | 1.7 × 105 |
| 1998 | 1.9 × 105 | 9.7 × 104 | 4.4 × 105 | 1.5 × 106 | 5.3 × 105 | 7.4 × 106 | 1.8 × 105 | 7.0 × 104 | 6.6 × 105 | 1.8 × 105 | 7.0 × 104 | 6.6 × 105 |
| 1999 | 2.3 × 105 | 1.5 × 105 | 3.9 × 105 | 4.2 × 106 | 7.6 × 105 | 1.7 × 108 | 9.5 × 104 | 4.6 × 104 | 2.4 × 105 | 9.5 × 104 | 4.6 × 104 | 2.4 × 105 |
| 2000 | 5.3 × 105 | 2.7 × 105 | 1.2 × 106 | 4.1 × 106 | 7.3 × 105 | 1.6 × 108 | 7.1 × 104 | 3.7 × 104 | 1.6 × 105 | 7.1 × 104 | 3.7 × 104 | 1.6 × 105 |
| 2001 | 3.9 × 106 | 7.0 × 105 | 1.5 × 108 | 6.2 × 105 | 2.9 × 105 | 1.7 × 106 | 8.4 × 104 | 4.1 × 104 | 2.1 × 105 | 8.4 × 104 | 4.1 × 104 | 2.1 × 105 |
| 2002 | 1.3 × 106 | 4.4 × 105 | 6.2 × 106 | 7.3 × 106 | 1.3 × 106 | 2.9 × 108 | 1.9 × 105 | 6.5 × 104 | 9.3 × 105 | 1.9 × 105 | 6.5 × 104 | 9.3 × 105 |
| 2003 | 6.5 × 105 | 3.0 × 105 | 1.8 × 106 | 3.7 × 106 | 1.0 × 106 | 3.1 × 107 | 1.2 × 105 | 5.0 × 104 | 3.6 × 105 | 1.2 × 105 | 5.0 × 104 | 3.6 × 105 |
| 2004 | 1.8 × 106 | 5.1 × 105 | 1.5 × 107 | 1.4 × 106 | 6.0 × 105 | 4.3 × 106 | 1.4 × 105 | 5.4 × 104 | 5.1 × 105 | 1.4 × 105 | 5.4 × 104 | 5.1 × 105 |
| 2005 | 3.4 × 106 | 6.1 × 105 | 1.3 × 108 | 3.2 × 106 | 9.0 × 105 | 2.7 × 107 | 1.3 × 105 | 5.0 × 104 | 4.7 × 105 | 1.3 × 105 | 5.0 × 104 | 4.7 × 105 |
| 2006 | 1.7 × 106 | 4.6 × 105 | 1.4 × 107 | 2.1 × 106 | 7.3 × 105 | 1.0 × 107 | 2.5 × 105 | 6.9 × 104 | 2.1 × 106 | 2.5 × 105 | 6.9 × 104 | 2.1 × 106 |
| 2007 | 6.9 × 105 | 3.0 × 105 | 2.1 × 106 | 1.7 × 106 | 6.5 × 105 | 6.1 × 106 | 1.3 × 105 | 5.1 × 104 | 4.8 × 105 | 1.3 × 105 | 5.1 × 104 | 4.8 × 105 |
| 2008 | ∞ | 2.9 × 106 | ∞ | 9.3 × 106 | 1.7 × 106 | 3.7 × 108 | 1.3 × 105 | 6.6 × 104 | 3.0 × 105 | 1.3 × 105 | 6.6 × 104 | 3.0 × 105 |
| 2009 | 1.1 × 107 | 1.9 × 106 | 4.2 × 108 | 4.6 × 106 | 1.3 × 106 | 3.8 × 107 | 5.2 × 105 | 1.4 × 105 | 4.3 × 106 | 5.2 × 105 | 1.4 × 105 | 4.3 × 106 |
| 2010 | 1.4 × 107 | 2.4 × 106 | 5.4 × 108 | 2.4 × 106 | 9.5 × 105 | 8.9 × 106 | 4.3 × 105 | 1.8 × 105 | 1.3 × 106 | 4.3 × 105 | 1.8 × 105 | 1.3 × 106 |
| 2011 | 6.4 × 106 | 1.8 × 106 | 5.3 × 107 | 4.6 × 106 | 1.3 × 106 | 3.8 × 107 | 3.4 × 105 | 1.6 × 105 | 9.2 × 105 | 3.4 × 105 | 1.6 × 105 | 9.2 × 105 |
| 2012 | 4.5 × 106 | 1.5 × 106 | 2.2 × 107 | 9.7 × 106 | 1.7 × 106 | 3.8 × 108 | 4.3 × 105 | 1.8 × 105 | 1.3 × 106 | 4.3 × 105 | 1.8 × 105 | 1.3 × 106 |
| 2013 | 1.2 × 107 | 2.1 × 106 | 4.6 × 108 | 8.3 × 106 | 1.5 × 106 | 3.3 × 108 | ∞ | 5.0 × 105 | ∞ | ∞ | 5.0 × 105 | ∞ |
| 2014 | 1.4 × 107 | 2.5 × 106 | 5.4 × 108 | 3.3 × 106 | 1.1 × 106 | 1.6 × 107 | 7.3 × 105 | 2.5 × 105 | 3.5 × 106 | 7.3 × 105 | 2.5 × 105 | 3.5 × 106 |
| 2015 | 1.5 × 107 | 2.7 × 106 | 5.8 × 108 | ∞ | 2.9 × 106 | ∞ | 3.4 × 105 | 1.6 × 105 | 8.3 × 105 | 3.4 × 105 | 1.6 × 105 | 8.3 × 105 |
| 2016 | 9.4 × 106 | 2.6 × 106 | 7.8 × 107 | 6.3 × 106 | 1.7 × 106 | 5.2 × 107 | 1.7 × 105 | 1.1 × 105 | 2.7 × 105 | 8.4 × 105 | 3.3 × 105 | 3.1 × 106 |
| 2017 | 1.9 × 107 | 3.4 × 106 | 7.5 × 108 | 6.3 × 106 | 1.7 × 106 | 5.2 × 107 | 3.4 × 105 | 1.8 × 105 | 7.0 × 105 | ∞ | 9.1 × 105 | ∞ |
| 2018 | 1.6 × 107 | 2.9 × 106 | 6.3 × 108 | 1.1 × 107 | 1.9 × 106 | 4.2 × 108 | 3.2 × 105 | 1.7 × 105 | 6.9 × 105 | 2.8 × 106 | 5.1 × 105 | 1.1 × 108 |
| 2019 | ∞ | 4.7 × 106 | ∞ | ∞ | 3.1 × 106 | ∞ | 3.9 × 105 | 2.0 × 105 | 9.0 × 105 | ∞ | 8.4 × 105 | ∞ |
| 2020 | ∞ | 4.6 × 106 | ∞ | ∞ | 3.1 × 106 | ∞ | 2.3 × 105 | 1.4 × 105 | 4.3 × 105 | 3.0 × 106 | 5.4 × 105 | 1.2 × 108 |
| 2021 | ∞ | 4.5 × 106 | ∞ | 1.1 × 107 | 2.0 × 106 | 4.3 × 108 | 4.2 × 105 | 2.0 × 105 | 1.0 × 106 | ∞ | 7.9 × 105 | ∞ |
References
- Regulations and Guidelines BTS SRC. Blutspende SRK Schweiz. Guidelines and Regulations. Available online: https://dokuman.sbsc-bsd.ch/de-de/vorschriftenbsd/vorschriftenkapitelbeschl%C3%BCsse/kapitel.aspx (accessed on 25 October 2022).
- Lackritz, E.M.; Satten, G.A.; Aberle-Grasse, J.; Dodd, R.Y.; Raimondi, V.P.; Janssen, R.S.; Lewis, W.F.; Notari, E.P.; Petersen, L.R. Estimated risk of transmission of the human immunodeficiency virus by screened blood in the United States. N. Engl. J. Med. 1995, 333, 1721–1725. [Google Scholar] [CrossRef]
- Schreiber, G.B.; Busch, M.P.; Kleinman, S.H.; Korelitz, J.J. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N. Engl. J. Med. 1996, 334, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Korelitz, J.J.; Busch, M.P.; Kleinman, S.H.; Williams, A.E.; Gilcher, R.O.; Ownby, H.E.; Schreiber, G. A method for estimating hepatitis B virus incidence rates in volunteer blood donors. National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study. Transfusion 1997, 37, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Glynn, S.A.; Kleinman, S.H.; Wright, D.J.; Busch, M.P. International application of the incidence rate/window period model. Transfusion 2002, 42, 966–972. [Google Scholar] [CrossRef]
- Roth, W.K.; Busch, M.P.; Schuller, A.; Ismay, S.; Cheng, A.; Seed, C.R.; Jungbauer, C.; Minsk, P.M.; Sondag-Thull, D.; Wendel, S.; et al. International survey on NAT testing of blood donations: Expanding implementation and yield from 1999 to 2009. Vox Sang. 2012, 102, 82–90. [Google Scholar] [CrossRef]
- Bruhn, R.; Lelie, N.; Busch, M.; Kleinman, S.; the International NAT Study Group. Relative efficacy of nucleic acid amplification testing and serologic screening in preventing hepatitis C virus transmission risk in seven international regions. Transfusion 2015, 55, 1195–1205. [Google Scholar] [CrossRef]
- Lelie, N.; Bruhn, R.; Busch, M.; Vermeulen, M.; Tsoi, W.; Kleinman, S.; Coleman, C.; Reddy, R.; Bird, A.; Cable, R.; et al. Detection of different categories of hepatitis B virus (HBV) infection in a multi-regional study comparing the clinical sensitivity of hepatitis B surface antigen and HBV-DNA testing. Transfusion 2017, 57, 24–35. [Google Scholar] [CrossRef]
- Fiedler, S.A.; Oberle, D.; Chudy, M.; Scheiblauer, H.; Henseler, O.; Halbauer, J.; Heiden, M.; Funk, M. Effectiveness of blood donor screening by HIV, HCV, HBV-NAT assays, as well as HBsAg and anti-HBc immunoassays in Germany (2008–2015). Vox Sang. 2019, 114, 443–450. [Google Scholar] [CrossRef]
- 812.212.1 Medicinal Products Licensing Ordinance, MPLO. The Swiss Federal Council. 2018. Updated 1 July 2022. Available online: https://www.fedlex.admin.ch/eli/cc/2018/786/en (accessed on 25 October 2022).
- Weusten, J.; Vermeulen, M.; Van Drimmelen, H.; Lelie, N. Refinement of a viral transmission risk model for blood donations in seroconversion window phase screened by nucleic acid testing in different pool sizes and repeat test algorithms. Transfusion 2011, 51, 203–215. [Google Scholar] [CrossRef]
- Barrera, J.M.; Francis, B.; Ercilla, G.; Nelles, M.; Achord, D.; Darner, J.; Lee, S.R. Improved detection of anti-HCV in post-transfusion hepatitis by a third-generation ELISA. Vox Sang. 1995, 68, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Busch, M. Closing the Windows on Viral Transmission by Blood Transfusion; American Association of Blood Banks: Bethesda, MD, USA, 2001. [Google Scholar]
- Galel, S.A.; Simon, T.L.; Williamson, P.C.; AuBuchon, J.P.; Waxman, D.A.; Erickson, Y.; Bertuzis, R.; Duncan, J.R.; Malhotra, K.; Vaks, J.; et al. Sensitivity and specificity of a new automated system for the detection of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus nucleic acid in blood and plasma donations. Transfusion 2018, 58, 649–659. [Google Scholar] [CrossRef]
- Niederhauser, C.; Weingand, T.; Candotti, D.; Maier, A.; Tinguely, C.; Wuillemin, W.A.; Gowland, P.; Allain, J.P.; Stolz, M. Fatal outcome of a hepatitis B virus transfusion-transmitted infection. Vox Sang. 2010, 98, 504–507. [Google Scholar] [CrossRef]
- Niederhauser, C.; Candotti, D.; Weingand, T.; Maier, A.; Tinguely, C.; Stolz, M.; Allain, J.-P. Reverse vertical transmission of hepatitis B virus (HBV) infection from a transfusion-infected newborn to her mother. J. Hepatol. 2012, 56, 734–737. [Google Scholar] [CrossRef][Green Version]
- Gottschalk, J.; Achermann, Y.; Niederhauser, C.; Tinguely, C.; Glauser, A.; Lodemann, P.; Frey, B. First case of HIV window donation in Switzerland after 8 years of universal HIV NAT screening. Transfus. Apher. Sci. 2010, 43, S25. [Google Scholar] [CrossRef]
- Stolz, M.; Gilgen, M.; Niederhauser, C. Hepatitis C virus-polymerase chain reaction minipool testing: 3 years in the largest Swiss blood transfusion service. Vox Sang. 2003, 84, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, C.; Mansouri Taleghani, B.; Graziani, M.; Stolz, M.; Tinguely, C.; Schneider, P. Blood donor screening: How to decrease the risk of transfusion-transmitted hepatitis B virus? Swiss Med. Wkly. 2008, 138, 134–141. [Google Scholar] [CrossRef]
- Velati, C.; Romanò, L.; Piccinini, V.; Marano, G.; Catalano, L.; Pupella, S.; Facco, G.; Pati, I.; Tosti, M.E.; Vaglio, S.; et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis C virus and human immunodeficiency virus after the implementation of nucleic acid testing in Italy: A 7-year (2009–2015) survey. Blood Transfus. 2018, 16, 422–432. [Google Scholar] [PubMed]
- Velati, C.; Romanò, L.; Pati, I.; Marano, G.; Piccinini, V.; Catalano, L.; Pupella, S.; Vaglio, S.; Veropalumbo, E.; Masiello, F.; et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis B virus infection in Italy from 2009 to 2018. Blood Transfus. 2019, 17, 409–417. [Google Scholar] [PubMed]
- López-Menchero, C.; Alvarez, M.; Fernández, P.; Guzmán, M.; Ortiz-De-Salazar, M.I.; Arbona, C. Evolution of the residual risk of HBV, HCV and HIV transmission through blood transfusion in the Region of Valencia, Spain, during a 15-year period (2003–2017). Blood Transfus. 2019, 17, 418–427. [Google Scholar] [PubMed]
- Roth, W.K.; Buhr, S.; Drosten, C.; Seifried, E. NAT and viral safety in blood transfusion. Vox Sang. 2000, 78 (Suppl. 2), 257–259. [Google Scholar]
- Busch, M.P.; Kleinman, S.H.; Nemo, G.J. Current and emerging infectious risks of blood transfusions. JAMA 2003, 289, 959–962. [Google Scholar] [CrossRef]
- Busch, M.P. Transfusion-transmitted viral infections: Building bridges to transfusion medicine to reduce risks and understand epidemiology and pathogenesis. Transfusion 2006, 46, 1624–1640. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, R.; Lelie, N.; Custer, B.; Busch, M.; Kleinman, S.; International NAT Study Group. Prevalence of human immunodeficiency virus RNA and antibody in first-time, lapsed, and repeat blood donations across five international regions and relative efficacy of alternative screening scenarios. Transfusion 2013, 53, 2399–2412. [Google Scholar] [CrossRef]
- Vučetić, D.; Jovičić, M.; Maslovarić, I.; Bogdanović, S.; Antić, A.; Stanojković, Z.; Filimović, G.; Ilić, V. Transfusion-transmissible infections among Serbian blood donors: Declining trends over the period 2005–2017. Blood Transfus. 2019, 17, 336–346. [Google Scholar] [PubMed]
- Grubyte, S.; Urboniene, J.; Nedzinskiene, L.; Jelinskaite, A.; Zagminas, K.; Ambrozaitis, A.; Jancoriene, L. Prevalence, incidence and residual risk of transfusion transmitted viruses (HBV, HCV and HIV infections) in Lithuanian blood donors from 2004 to 2018: The incidence/window-period model study. PLoS ONE 2021, 16, e0246704. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis C in der Schweiz im Jahr 2020. 29 November 2021. Report No.: BAG-Bulletin 48/2021. Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/hiv-sti-statistiken-analysen-und-trends/hiv-sti-epizahlen-2020.pdf.download.pdf/bu-48-hiv-sti-hepbc-2020-de.pdf (accessed on 25 October 2022).
- HIV und Aids in der Schweiz im Jahr 2020. 29 November 2021. Report No.: BAG-Bulletin 48/2021. Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/hiv-sti-statistiken-analysen-und-trends/hiv-sti-epizahlen-2020.pdf.download.pdf/bu-48-hiv-sti-hepbc-2020-de.pdf (accessed on 25 October 2022).
- Hepatitis B in der Schweiz im Jahr 2020. 29 November 2021. Report No.: BAG-Bulletin 48/2021. Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/hiv-sti-statistiken-analysen-und-trends/hiv-sti-epizahlen-2020.pdf.download.pdf/bu-48-hiv-sti-hepbc-2020-de.pdf (accessed on 25 October 2022).
- Syphilis in der Schweiz im Jahr 2020. 29 November 2021. Report No.: BAG-Bulletin 48/2021. Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/hiv-sti-statistiken-analysen-und-trends/hiv-sti-epizahlen-2020.pdf.download.pdf/bu-48-hiv-sti-hepbc-2020-de.pdf (accessed on 25 October 2022).
- Coste, J.; Reesink, H.W.; Engelfriet, C.P.; Laperche, S.; Brown, S.; Busch, M.P.; Cuijpers, H.T.; Elgin, R.; Ekermo, B.; Epstein, J.S.; et al. Implementation of donor screening for infectious agents transmitted by blood by nucleic acid technology: Update to 2003. Vox Sang. 2005, 88, 289–303. [Google Scholar] [CrossRef]
- Reesink, H.W.; Engelfriet, C.P.; Henn, G.; Mayr, W.R.; Delage, G.; Bernier, F.; Krusius, T.; Assal, A.; Gallian, P.; Corbi, C.; et al. Occult hepatitis B infection in blood donors. Vox Sang. 2008, 94, 153–166.tabl. [Google Scholar] [CrossRef]
- Hourfar, M.K.; Jork, C.; Schottstedt, V.; Weber-Schehl, M.; Brixner, V.; Busch, M.P.; Geusendam, G.; Gubbe, K.; Mahnhardt, C.; Mayr-Wohlfart, U.; et al. Experience of German Red Cross blood donor services with nucleic acid testing: Results of screening more than 30 million blood donations for human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus. Transfusion 2008, 48, 1558–1566. [Google Scholar] [CrossRef]
- Zou, S.; Dorsey, K.A.; Notari, E.P.; Foster, G.A.; Krysztof, D.E.; Musavi, F.; Dodd, R.Y.; Stramer, S.L. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 2010, 50, 1495–1504. [Google Scholar] [CrossRef]
- Laperche, S.; Tiberghien, P.; Roche-Longin, C.; Pillonel, J. Fifteen years of Nucleic Acid Testing in France: Results and lessons. Transfus. Clin. Et Biol. J. De La Soc. Fr. De Transfus. Sang. 2017, 24, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.K. History and Future of Nucleic Acid Amplification Technology Blood Donor Testing. Transfus. Med. Hemotherapy Off. Organ Der Dtsch. Ges. Fur Transfus. Und Immunhamatol. 2019, 46, 67–75. [Google Scholar] [CrossRef]
- Alvarez, M.; Oyonarte, S.; Rodriguez, P.M.; Hernandez, J.M. Estimated risk of transfusion-transmitted viral infections in Spain. Transfusion 2002, 42, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Reynolds, C.; Gibney, Z.; Derrick, J.; Ijaz, S.; Davison, K.L.; Brailsford, S. Hepatitis B infections among blood donors in England between 2009 and 2018: Is an occult hepatitis B infection a risk for blood safety? Transfusion 2021, 61, 2402–2413. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.F.; Grégoire, Y.; Pillonel, J.; Steele, W.R.; Custer, B.; Davison, K.L.; Germain, M.; Lewin, A.; Seed, C.R. HIV residual risk in Canada under a three-month deferral for men who have sex with men. Vox Sang. 2020, 115, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nübling, C.M.; Heiden, M.; Chudy, M.; Kress, J.; Seitz, R.; Keller-Stanislawski, B.; Funk, M.B. Experience of mandatory nucleic acid test (NAT) screening across all blood organizations in Germany: NAT yield versus breakthrough transmissions. Transfusion 2009, 49, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Korn, K.; Nübling, C.M.; Chudy, M.; Kress, J.; Horst, H.A.; Geusendam, G.; Hennig, H.; Sireis, W.; Rabenau, H.F.; et al. First transmission of human immunodeficiency virus Type 1 by a cellular blood product after mandatory nucleic acid screening in Germany. Transfusion 2009, 49, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Servant-Delmas, A.; Chuteau, C.; Lefort, C.; Piquet, Y.; Chevaleyre, S.; Betbeze, V.; Delhoume, M.; Hantz, S.; Alain, S.; Laperche, S. Two cases of transfusion-transmitted hepatitis B virus (HBV) infection in a low-endemic country before implementation of HBV nucleic acid testing. Transfusion 2013, 53, 291–296. [Google Scholar] [CrossRef]
- Álvarez, M.; Luis-Hidalgo, M.; Bracho, M.A.; Blanquer, A.; Larrea, L.; Villalba, J.; Puig, N.; Planelles, D.; Montoro, J.; González-Candelas, F.; et al. Transmission of human immunodeficiency virus Type-1 by fresh-frozen plasma treated with methylene blue and light. Transfusion 2016, 56, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Cappy, P.; Barlet, V.; Lucas, Q.; Tinard, X.; Pillonel, J.; Gross, S.; Tiberghien, P.; Laperche, S. Transfusion of HIV-infected blood products despite highly sensitive nucleic acid testing. Transfusion 2019, 59, 2046–2053. [Google Scholar] [CrossRef] [PubMed]



| Virus | Year | Pool/ID | Sensitivity | Platform/Assay |
|---|---|---|---|---|
| HCV | Since 1999 | 48 | 1008 IU/ml | Cobas Amplicor (Roche Diagnostics) 1 |
| HCV | Since 2001 | 48 | 283 IU/mL | Cobas Ampliscreen (Roche Diagnostics) 2 |
| HCV | Since 2007 | ID | 3.7 IU/mL | Tigris/Procleix Ultrio (Chiron/Novartis) |
| HCV | Since 2015 | ID | 8.3 IU/mL | Cobas 8800/MPX (Roche Diagnostics) |
| HIV-1 | Since 2002 | 48 | 7680 IU/mL | Cobas Ampliscreen (Roche Diagnostics) 2 |
| HIV-1 | Since 2007 | ID | 24.9 IU/mL | Tigris/Procleix Ultrio (Chiron/Novartis) |
| HIV-1 M HIV-1 O HIV-2 | Since 2015 | ID | 25.7 IU/mL 8.2 IU/mL 4.0 IU/mL | Cobas 8800/MPX (Roche Diagnostics) |
| HBV | Since 2007 | ID | 7.6 IU/mL | Tigris/Procleix Ultrio (Chiron/Novartis) |
| HBV | Since 2015 | ID | 1.5 IU/mL | Cobas 8800/MPX (Roche Diagnostics) |
| Year | HCV [1/RR] | HIV [1/RR] | HBV (Variant 1, Including OBI) [1/RR] | HBV (Variant 2, Excluding OBI) [1/RR] |
|---|---|---|---|---|
| 1996 | 3.3 × 104 | 5.9 × 105 | 4.0 × 104 | 4.0 × 104 |
| 1997 | 2.1 × 105 | 1.3 × 106 | 7.9 × 104 | 7.9 × 104 |
| 1998 | 1.9 × 105 | 1.5 × 106 | 1.8 × 105 | 1.8 × 105 |
| 1999 | 2.3 × 105 | 4.2 × 106 | 9.5 × 104 | 9.5 × 104 |
| 2000 | 5.3 × 105 | 4.1 × 106 | 7.1 × 104 | 7.1 × 104 |
| 2001 | 3.9 × 106 | 6.2 × 105 | 8.4 × 104 | 8.4 × 104 |
| 2002 | 1.3 × 106 | 7.3 × 106 | 1.9 × 105 | 1.9 × 105 |
| 2003 | 6.5 × 105 | 3.7 × 106 | 1.2 × 105 | 1.2 × 105 |
| 2004 | 1.8 × 106 | 1.4 × 106 | 1.4 × 105 | 1.4 × 105 |
| 2005 | 3.4 × 106 | 3.2 × 106 | 1.3 × 105 | 1.3 × 105 |
| 2006 | 1.7 × 106 | 2.1 × 106 | 2.5 × 105 | 2.5 × 105 |
| 2007 | 6.9 × 105 | 1.7 × 106 | 1.3 × 105 | 1.3 × 105 |
| 2008 | ∞ | 9.3 × 106 | 1.3 × 105 | 1.3 × 105 |
| 2009 | 1.1 × 107 | 4.6 × 106 | 5.2 × 105 | 5.2 × 105 |
| 2010 | 1.4 × 107 | 2.4 × 106 | 4.3 × 105 | 4.3 × 105 |
| 2011 | 6.4 × 106 | 4.6 × 106 | 3.4 × 105 | 3.4 × 105 |
| 2012 | 4.5 × 106 | 9.7 × 106 | 4.3 × 105 | 4.3 × 105 |
| 2013 | 1.2 × 107 | 8.3 × 106 | ∞ | ∞ |
| 2014 | 1.4 × 107 | 3.3 × 106 | 7.3 × 105 | 7.3 × 105 |
| 2015 | 1.5 × 107 | ∞ | 3.4 × 105 | 3.4 × 105 |
| 2016 | 9.4 × 106 | 6.3 × 106 | 1.7 × 105 | 8.4 × 105 |
| 2017 | 1.9 × 107 | 6.3 × 106 | 3.4 × 105 | ∞ |
| 2018 | 1.6 × 107 | 1.1 × 107 | 3.2 × 105 | 2.8 × 106 |
| 2019 | ∞ | ∞ | 3.9 × 105 | ∞ |
| 2020 | ∞ | ∞ | 2.3 × 105 | 3.0 × 106 |
| 2021 | ∞ | 1.1 × 107 | 4.2 × 105 | ∞ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niederhauser, C.; Tinguely, C.; Stolz, M.; Vock, M.; El Dusouqui, S.A.; Gowland, P. Evolution of Blood Safety in Switzerland over the Last 25 Years for HIV, HCV, HBV and Treponema pallidum. Viruses 2022, 14, 2611. https://doi.org/10.3390/v14122611
Niederhauser C, Tinguely C, Stolz M, Vock M, El Dusouqui SA, Gowland P. Evolution of Blood Safety in Switzerland over the Last 25 Years for HIV, HCV, HBV and Treponema pallidum. Viruses. 2022; 14(12):2611. https://doi.org/10.3390/v14122611
Chicago/Turabian StyleNiederhauser, Christoph, Caroline Tinguely, Martin Stolz, Michael Vock, Soraya Amar El Dusouqui, and Peter Gowland. 2022. "Evolution of Blood Safety in Switzerland over the Last 25 Years for HIV, HCV, HBV and Treponema pallidum" Viruses 14, no. 12: 2611. https://doi.org/10.3390/v14122611
APA StyleNiederhauser, C., Tinguely, C., Stolz, M., Vock, M., El Dusouqui, S. A., & Gowland, P. (2022). Evolution of Blood Safety in Switzerland over the Last 25 Years for HIV, HCV, HBV and Treponema pallidum. Viruses, 14(12), 2611. https://doi.org/10.3390/v14122611





