Recent Information on Pan-Genotypic Direct-Acting Antiviral Agents for HCV in Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Statistical Methods
3. Results
3.1. Epidemiology
3.2. Natural History of HCV Infection
3.3. Antiviral Therapy of HCV and Its Purpose (Pan-Genotypic Regimens)
3.4. Natural History of HCV, HD Population, and Antivirals
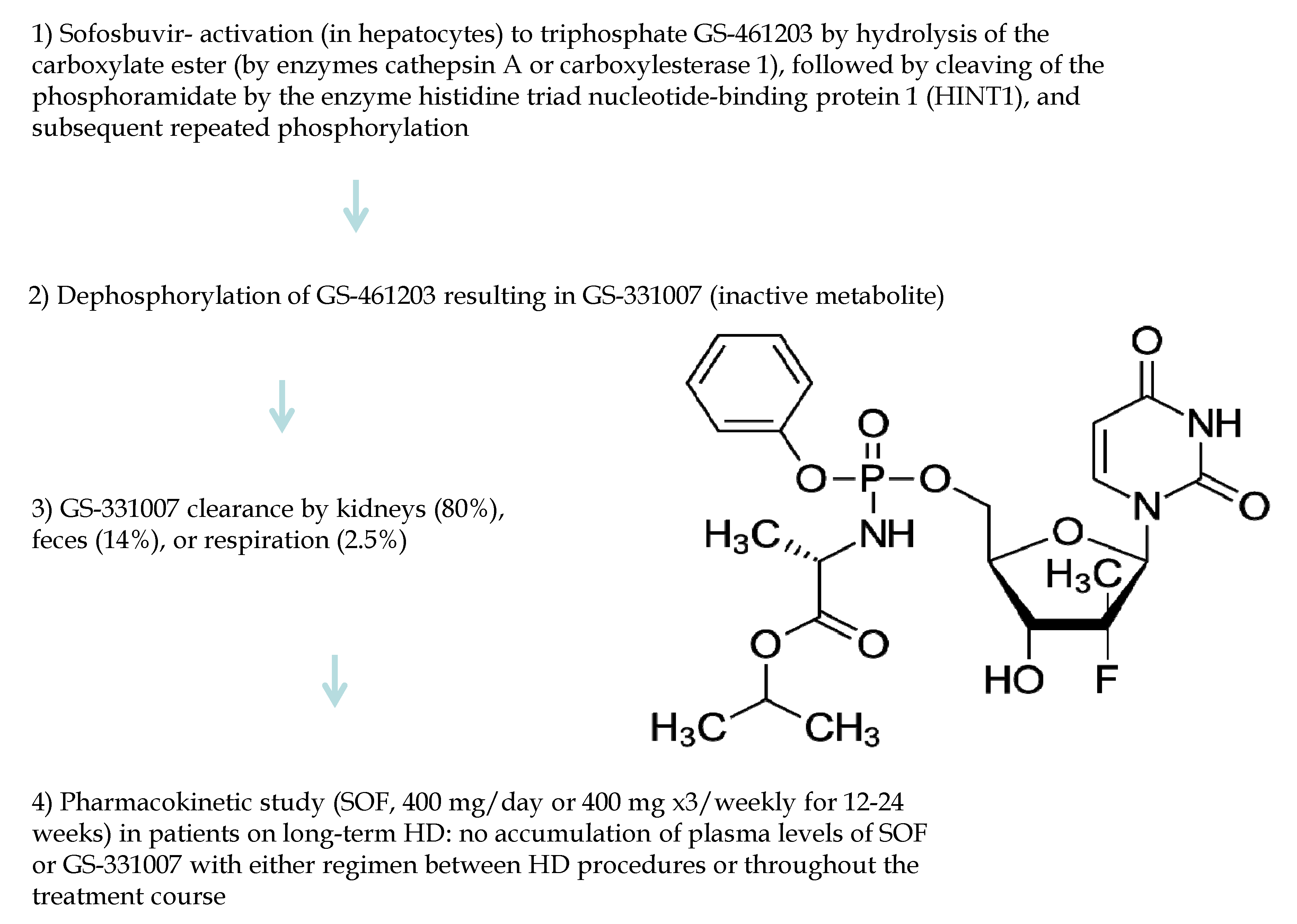
3.5. Pan-Genotypic DAAs (Sofosbuvir)
3.6. Pan-Genotypic DAAs (Glecaprevir/Pibrentasvir)
3.7. Pan-Genotypic DAAs (Sofosbuvir/Velpatasvir)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH | Arterial hypertension |
| AUC | Area under curve |
| CI | Confidence intervals |
| CKD | Chronic kidney disease |
| CLD | Chronic liver disease |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular disease |
| DAAs | Direct acting antiviral agents |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| ESRD | End-stage renal disease |
| GLE | Glecaprevir |
| HBsAg | Hepatitis B virus antigen |
| HCV | Hepatitis C virus |
| HCW | Health care worker |
| HD | Haemodialysis |
| IFN | Interferon |
| ITT | Intention-to-treat |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| NA | Not available |
| PD | Peritoneal dialysis |
| PIB | Pibrentasvir |
| RRT | Renal replacement therapy |
| SAE | Severe adverse event |
| SE | Standard error |
| SOF | Sofosbuvir |
| SVR | Sustained virological response |
| VEL | Velpatasvir |
| VOX | Voxilaprevir |
| WHO | World Health Organization |
References
- Fabrizi, F.; Cerutti, R.; Messa, P. An updated view on the antiviral therapy of hepatitis C in chronic kidney disease. Pathogens 2021, 1, 1381. [Google Scholar] [CrossRef]
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Fabrizi, F.; Donato, F.; Messa, P. Association between hepatitis C virus and chronic kidney disease: A systematic review and meta-analysis. Ann Hepatol. 2018, 17, 364–391. [Google Scholar] [CrossRef]
- Cox, A.; El-Sayed, M.; Kao, J.; Lazarus, J.; Lemoine, M.; Lok, A.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Der Simonian, R.; Laird, D. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Costa Bouzas, J.; Takkouche, B.; Cadarso-Suarez, C.; Spiegelman, D. HEpiMA: Software for the identification of heterogeneity in meta-analysis. Comput. Meth. Programs Biomed. 2001, 64, 101–107. [Google Scholar] [CrossRef]
- Jadoul, M.; Bieber, B.; Martin, P.; Akiba, T.; Nwanko, C.; Arduino, M.; Goodkin, D.; Pisoni, R. Prevalence, incidence, and risk factors for hepatitis C virus infection in haemodialysis patients. Kidney Int. 2019, 95, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Stepanova, M.; Golabi, P.; Brown, K.; Younossi, Z. Prevalence, mortality and healthcare utilization among Medicare beneficiaries with hepatitis C in haemodialysis units. J. Viral. Hepat. 2019, 26, 1293–1300. [Google Scholar] [CrossRef]
- Adane, T.; Getawa, S. The prevalence and associated factors of hepatitis B and C virus in haemodialysis patients in Africa: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251570. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, M.; Chemaitelly, H.; Mahmud, S.; Chabna, K.; Kouyoumjian, S.; Kanaani, Z.; Abu-Raddad, L. Epidemiology of hepatitis C virus among haemodialysis patients in the Middle East and North Africa: Systematic syntheses, meta-analyses, and meta-regressions. Epidemiol. Infect. 2017, 145, 3243–3263. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Nasir, J.; Usman, M.; Sarwar, A.; Majeed, R.; Billah, B. The prevalence of hepatitis C virus in haemodialysis in Pakistan: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232931. [Google Scholar] [CrossRef] [PubMed]
- Chuaypen, N.; Khlaiphuengsin, A.; Prasoppokakorn, T.; Susantitaphong, P.; Prasithsirikul, W.; Avihingsanon, A.; Tangkijvanich, P.; Praditpornsilpa, K. Prevalence and genotype distribution of hepatitis C virus within haemodialysis units in Thailand: Role of HCV core antigen in the assessment of viremia. BMC Infect. Dis. 2022, 22, 79. [Google Scholar] [CrossRef]
- Fabrizi, F.; Lunghi, G.; Andrulli, S.; Pagliari, B.; Mangano, S.; Faranna, P.; Pagano, A.; Locatelli, F. Influence of hepatitis C virus (HCV) viraemia upon serum aminotransferase activity in chronic dialysis patients. Nephrol. Dial. Transpl. 1997, 12, 1394–1398. [Google Scholar] [CrossRef][Green Version]
- Fabrizi, F.; Dixit, V.; Messa, P. Hepatitis C virus and mortality among patients on dialysis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Nagashima, S.; Yamamoto, C.; Takahashi, K.; Matsuo, J.; Ohisa, M.; Akita, T.; Matyakubov, J.; Mizeav, U.; Katayama, K.; et al. Eighteen-year follow-up cohort study on hepatitis B and C virus infections related long-term prognosis among haemodialysis patients in Hiroshima. J. Med. Virol. 2020, 92, 3436–3447. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kao, J. Pan-genotypic direct-acting antivirals for patients with hepatitis C virus infection and chronic kidney disease stage 4 or 5. Hepatol. Int. 2022, 16, 1001–1019. [Google Scholar] [CrossRef]
- AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C. Available online: https://www.hcvguidelines.org/uniquepopulations/renalimpairment (accessed on 24 October 2022).
- Fabrizi, F.; Dixit, V.; Messa, P.; Martin, P. Antiviral therapy (pegylated interferon and ribavirin) of hepatitis C in dialysis patients: Meta-analysis of clinical studies. J. Viral. Hepat. 2014, 21, 681–689. [Google Scholar] [CrossRef]
- Goodkin, D.; Bieber, B.; Gillespie, B.; Robinson, B.; Jadoul, M. Hepatitis C infection is very rarely treated among haemodialysis patients. Am. J. Nephrol. 2013, 38, 405–412. [Google Scholar] [CrossRef]
- Soderholm, J.; Millbourn, C.; Busch, K.; Kovamees, J.; Schvarcz, R.; Lindahl, K.; Bruchfeld, A. Higher risk of renal disease in chronic hepatitis C patients: Antiviral therapy survival benefit in patients on haemodialysis. J. Hepatol. 2018, 68, 904–911. [Google Scholar] [CrossRef]
- Hsu, Y.; Hung, P.; Muo, C.; Tsai, W.; Hsu, C.; Kao, C. Interferon-based treatment of hepatitis C virus infection reduces all-cause mortality in patients with end-stage renal disease. An 8-year nationwide cohort study in Taiwan. Medicine 2015, 94, e2113. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Tsai, S.; Chen, Y. Anti-hepatitis C virus therapy in chronic kidney disease patients improves long-term renal and patient survivals. World J. Clin. Cases 2019, 7, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Perez de Josè, A.; Carbayo, J.; Pocurull, A.; Bada-Bosch, T.; Corona, C.; Shabaka, A.; Terrada, N.; Valenzuela, L.; Huerta, A.; Lorente, F.; et al. Direct-acting antiviral therapy improves kidney survival in hepatitis C virus-associated cryoglobulinaemia: The RENALCRYOGLOBULINEMIC study. Clin. Kidney J. 2021, 14, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Marbury, T.; Kirby, B.; Au, N.; Mathias, A.; Stamm, L.; Sajwani, K.; Klein, G.; Gane, E. The effect of renal or hepatic impairment on the pharmacokinetics of GS-9857, a pangenotypic HCV NS3/4A protease inhibitor. J. Hepatol. 2016, 64, S613. [Google Scholar] [CrossRef]
- Desnoyer, A.; Pospai, D.; Lè, M.; Gervais, A.; Heurguè–Berlot, A.; Laradi, A.; Harent, S.; Pinto, A.; Salmon, D.; Hillaire, S.; et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in haemodialysis patients with chronic hepatitis C. J. Hepatol. 2016, 65, 40–47. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cerutti, R.; Dixit, V.; Ridruejo, E. Sofosbuvir-based regimens for HCV in stage 4–5 chronic kidney disease. A systematic review with meta-analysis. Nefrologia 2021, 41, 578–589. [Google Scholar] [CrossRef]
- Gane, E.; Lawitz, E.; Pugatch, D.; Papatheodoridis, G.; Brau, N.; Brown, A.; Pol, S.; Leroy, V.; Persico, M.; Moreno, C.; et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef]
- Lawitz, E.; Flisiak, R.; Abunimeh, M.; SIse, M.; Park, J.; Kaskas, M.; Bruchfeld, A.; Worns, M.; Aglitti, A.; Zamor, P.; et al. Efficacy and safety of glecaprevir/pibrentasvir in renally impaired patients with chronic HCV infection. Liver Int. 2020, 40, 1032–1041. [Google Scholar] [CrossRef]
- Suda, G.; Hasebe, C.; Abe, M.; Kurosaki, M.; Itakura, J.; Izumi, N.; Uchida, Y.; Mochida, S.; Haga, H.; Ueno, Y.; et al. Safety and efficacy of glecaprevir and pibrentasvir in Japanese haemodialysis patients with genotype 2 hepatitis C virus infection. J. Gastroenterol. 2019, 54, 641–649. [Google Scholar] [CrossRef]
- Atsukawa, M.; Tsubota, A.; Toyoda, H.; Takaguchi, K.; Nakamuta, M.; Watanabe, T.; Michitaka, K.; Ikegami, T.; Nozaki, A.; Uojima, H.; et al. The efficacy and safety of glecaprevir plus pibrentasvir in 141 patients with severe renal impairment; a prospective, multicenter study. Aliment. Pharmacol. Ther. 2019, 49, 1230–1241. [Google Scholar] [CrossRef]
- Yen, H.; Su, P.; Zeng, Y.; Liu, I.; Huang, S.; Hsu, Y.; Chen, Y.; Yang, C.; Wu, S.; Chou, K. Glecaprevir-pibrentasvir for chronic hepatitis C: Comparing treatment effect in patients with and without end-stage renal disease in a real-world setting. PLoS ONE 2020, 15, e02337582. [Google Scholar] [CrossRef]
- Yap, D.; Liu, K.; Hsu, Y.; Wong, G.; Tsai, M.; Chen, C.; Hsu, C.; Hui, Y.; Li, M.; Liu, C.; et al. Use of glecaprevir/pibrentasvir in patients with chronic hepatitis C virus infection and severe renal impairment. Clin. Mol. Hepatol. 2020, 26, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, S.; Peng, C.; Lin, W.; Liu, C.; Su, T.; Tseng, T.; Chen, P.; Chen, D.; Kao, J. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection and severe renal impairment. J. Viral. Hepat. 2020, 27, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Stoehr, A.; Klinker Hm Teuber, G.; Naumann, U.; Christine, J.; Heyne, R.; Serfert, Y.; Niederau, C.; Zeuzem, S.; Berg, T.; et al. Hepatitis C therapy with grazoprevir/elbasvir and glecaprevir/pibrentasvir in patients with advanced chronic kidney disease: Data from the Germany Hepatitis C-Registry (DHC-R). Eur. J. Gastroenterol. Hepatol. 2022, 34, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Borgia, S.; Dearden, J.; Yoshida, E.; Shafran, S.; Brown, A.; Ben-Ari, Z.; Cramp, M.; Cooper, C.; Foxton, M.; Rodriguez, F.; et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J. Hepatol. 2019, 71, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Begovac, J.; Krznaric, J.; Bogdanic, N.; Mocibob, L.; Zekan, S. Successful treatment of genotype 3 hepatitis C infection in a non-cirrhotic HIV infected patient on chronic dialysis with the combination of sofobuvir and velpatasvir. Medicine 2018, 97, e13671. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Malhotra, V.; Agrawal, D.; Singh, S.; Beniwal, P.; Sharma, S.; Jhorawat, R.; Rathore, V.; Joshi, H. Sofosbuvir-velpatasvir fixed drug combination for the treatment of chronic hepatitis C infection in patients with end-stage renal disease and kidney transplantation. J. Clin. Exp. Hepatol. 2020, 10, 189–193. [Google Scholar] [CrossRef]
- Taneja, S.; Duseja, A.; Mehta, M.; De, A.; Verma, N.; Premkumar, M.; Dhiman, R.; Singh, V.; Singh, M.; Ratho, R.; et al. Sofosbuvir and velpatasvir combination is safe and effective in treating chronic hepatitis C in end-stage renal disease on maintenance haemodialysis. Liver. Int. 2021, 41, 705–709. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Su, W.; Tseng, K.; Lo, C.; Liu, C.; Chen, J.; Peng, C.; Shih, Y.; Yang, S.; et al. Sofosbuvir/velpatasvir with or without low-dose ribavirin for patients with chronic hepatitis C virus infection and severe renal impairment. Gut 2022, 71, 176–184. [Google Scholar] [CrossRef]
- Yu, M.; Huang, C.; Wei, Y.; Lin, W.; Lin, Y.; Hsu, P.; Hsu, C.; Liu, T.; Lee, J.; Niu, S.; et al. Establishment of an outreach, grouping healthcare system to achieve micro-elimination of HCV for uremic patients in haemodialysis centres (ERASE-C). Gut 2020, 70, 2349–2358. [Google Scholar] [CrossRef]
- Gohel, K.; Borasadia, P. Sosfobuvir-based HCV treatment in maintenance haemodialysis patients: A single-center study. Transplant. Proc. 2020, 52, 1684–1686. [Google Scholar] [CrossRef]
- Mostafi, M.; Jabin, M.; Chowdhury, Z.; Khondoker, M.; Ali, S.; Tamanna, R.; Rezwan, R.; Alomgir, S. The outcome of daclatasvir and low dose sofosbuvir therapy in end-stage renal disease patients with hepatitis C virus infection. Ukrainian K Nephrol Dial 2020, 2, 3–8. [Google Scholar] [CrossRef]
- De, A.; Roy, A.; Verma, N.; Mishra, S.; Premkumar, M.; Taneja, S.; Singh, V.; Duseja, A. Sofosbuvir plus velpatasvir combination for the treatment of chronic hepatitis C in patients with end-stage renal disease on renal replacement therapy: A systematic review and meta-analysis. Nephrology 2022, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
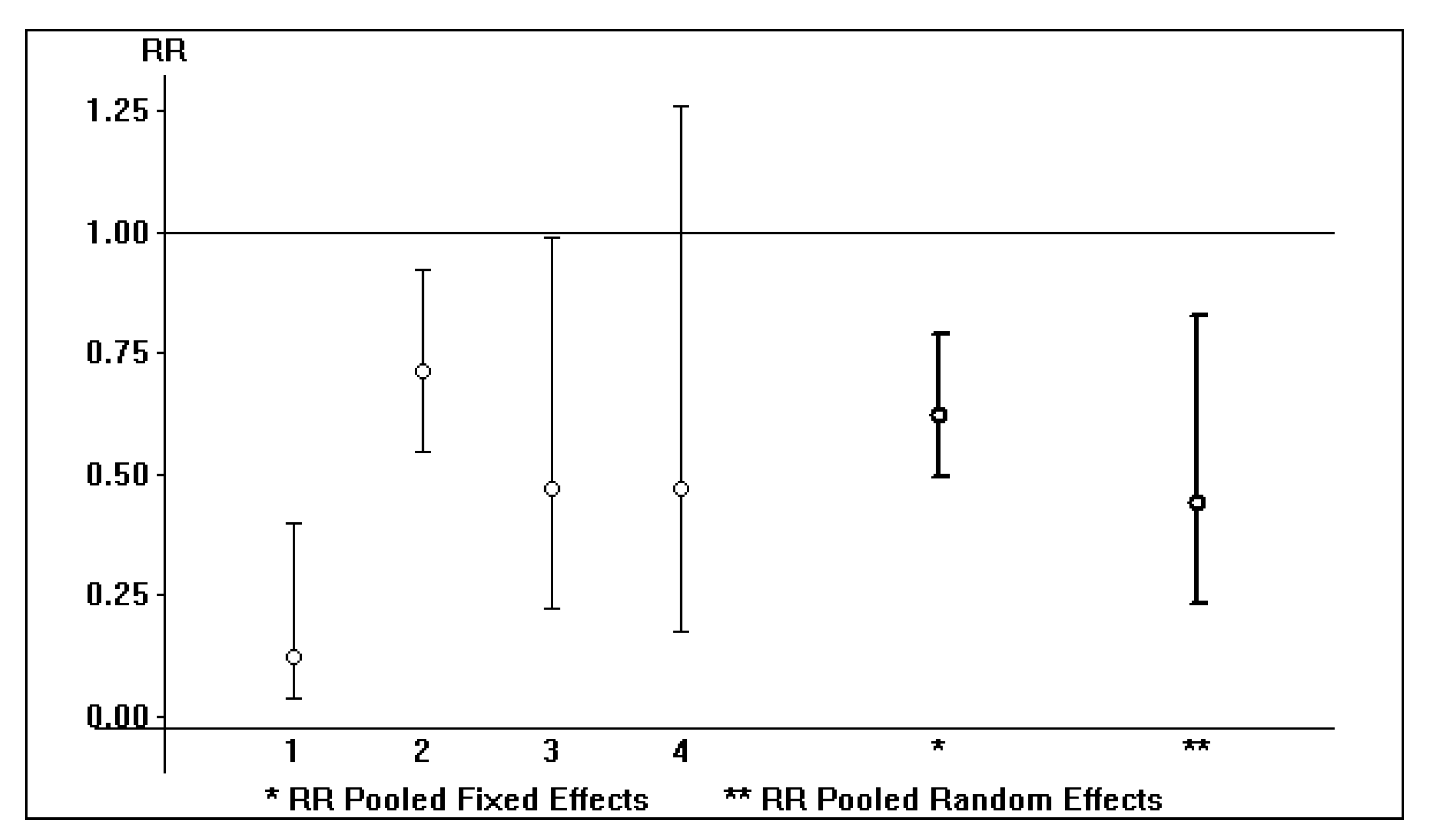
| Study | Reference Year | Death Rate (Treated) | Death Rate (Untreated) | p | Country |
|---|---|---|---|---|---|
| Goodkin D, et al. | 2013 | 4/42 (9.5%) | 638/3,307 (21%) | NS | USA |
| Hsu Y, et al. | 2015 | 7/134 (5.2%) | 581/2,097 (27.7%) | 0.0001 | Taiwan |
| Soderholm J, et al. | 2018 | 11/45 (24%) | 124/223 (56%) | 0.0001 | Sweden |
| Chen Y, et al. | 2019 | 61/482 (12.7%) | 648/1,928 (33.6%) | 0.0001 | Taiwan |
| Perez de Josè A, et al. | 2021 | 13/124 (10.5%) | 10/15 (67%) | 0.0001 | Spain |
| Study | Reference Year | SVR Rate | AEs Resulting in Drug Discontinuation | Country | Study Design |
|---|---|---|---|---|---|
| Suda G, et al. | 2019 | 26/27 (96.3%) | 2 (7.4%) | Japan | Prospective |
| Atsukawa M, et al. | 2019 | 140/141 (99.3%) | 3 (7.2%) | Japan | Prospective |
| Yen H, et al. | 2020 | 42/44 (95.5%) | 1 (2.3%) | Taiwan | Retrospective |
| Yap D, et al. | 2020 | 18/21 (85.7%) | 1 (4%) | Hong Kong/Taiwan | Prospective |
| Liu C, et al. | 2020 | 107/108 (99%) | 2 (3%) | Taiwan | Retrospective |
| Stein K, et al. | 2022 | 29/33 (87.9%) | 0% | Germany | Prospective |
| Study | Reference Year | Study Size | SVR Rate | Age, years | Males, n | Country |
|---|---|---|---|---|---|---|
| Borgia S, et al. | 2019 | 59 | 56/59 (94.9%) | 60 (33; 91) | 35 (59%) | Canada |
| Gohel K, et al. | 2020 | 3 | 3/3 (100%) | 46.5 | NA | India |
| Mostafi M, et al. | 2020 | 44 | 44/44 (100%) | 43.7 ± 12 | NA | Bangladesh |
| Gaur N, et al. | 2020 | 31 | 30/31 (96.8%) | 39.8 ± 10.8 | 24 (77.5%) | India |
| Yu M, et al. | 2021 | 105 | 94/105 (89.5%) | 66.2 ± 10 | 54 (51.4%) | Taiwan |
| Taneja S, et al. | 2021 | 51 | 49/51 (96%) | 42.8 ± 14.6 | 41 (80.4%) | India |
| Liu C, et al. | 2022 | 191 | 181/191 (94.8%) | 65 (23; 95) | 104 (54.5%) | Taiwan |
| Study | HBsAg, n | HCV Genotype 1, n | Cirrhosis, n | Treatment-Naïve, n | Diabetics, n | Drop-Out Rate (Due to AEs), n |
|---|---|---|---|---|---|---|
| Borgia S, et al. | NA | 27 (45.8%) | 17 (28.8%) | 46 (77.9%) | 19 (32%) | 0 |
| Gohel K, et al. | NA | NA | 0 | 3 (100%) | NA | 0 |
| Mostafi M, et al. | 0 | NA | 10 (23%) | 44 (100%) | 28 (63.6%) | 0 |
| Gaur N, et al. | 6 (19.3%) | 21 (67.7%) | 3 (9.6%) | 31 (100%) | 6 (19%) | 0 |
| Yu M, et al. | 8 (7.6%) | 46 (43.8%) | 37 (35.2%) | NA | 65 (61.9%) | 5/105 (4.8%) |
| Taneja S, et al. | NA | 15 (79%) | 10 (19.6%) | 43 (84.3%) | NA | 0 |
| Liu C, et al. | 5 (2.6%) | 112 (58.6%) | 27 (14.1%) | 175 (91.6%) | NA | 2/191 (1%) |
| Weight (%) | SVR Rate (SE) | SVR Rate (Random-Effects Model) 95% CI | Year | |
|---|---|---|---|---|
| Borgia A, et al. | 0.02 | 0.94 (0.0286) | 0.95 (0.89; 1.01) | 2019 |
| Gohel K, et al. | 49.95 | 1 (0.0001) | 1.0 (1.00; 1.00) | 2020 |
| Mostafi M, et al. | 49.95 | 1 (0.0001) | 1.0 (1.00; 1.00) | 2020 |
| Gaur N, et al. | 0.01 | 0.96 (0.0352) | 0.96 (0.89; 1.03) | 2020 |
| Yu M, et al. | 0.01 | 0.89 (0.0305) | 0.89 (0.83; 0.95) | 2021 |
| Taneja S, et al. | 0.02 | 0.96 (0.0270) | 0.96 (0.91; 0.97) | 2021 |
| Liu C, et al. | 0.05 | 0.94 (0.0170) | 0.94 (0.91; 0.97) | 2022 |
| Total (95% CI) | 100.00 | 1.0 (1.00; 1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabrizi, F.; Tripodi, F.; Cerutti, R.; Nardelli, L.; Alfieri, C.M.; Donato, M.F.; Castellano, G. Recent Information on Pan-Genotypic Direct-Acting Antiviral Agents for HCV in Chronic Kidney Disease. Viruses 2022, 14, 2570. https://doi.org/10.3390/v14112570
Fabrizi F, Tripodi F, Cerutti R, Nardelli L, Alfieri CM, Donato MF, Castellano G. Recent Information on Pan-Genotypic Direct-Acting Antiviral Agents for HCV in Chronic Kidney Disease. Viruses. 2022; 14(11):2570. https://doi.org/10.3390/v14112570
Chicago/Turabian StyleFabrizi, Fabrizio, Federica Tripodi, Roberta Cerutti, Luca Nardelli, Carlo M. Alfieri, Maria F. Donato, and Giuseppe Castellano. 2022. "Recent Information on Pan-Genotypic Direct-Acting Antiviral Agents for HCV in Chronic Kidney Disease" Viruses 14, no. 11: 2570. https://doi.org/10.3390/v14112570
APA StyleFabrizi, F., Tripodi, F., Cerutti, R., Nardelli, L., Alfieri, C. M., Donato, M. F., & Castellano, G. (2022). Recent Information on Pan-Genotypic Direct-Acting Antiviral Agents for HCV in Chronic Kidney Disease. Viruses, 14(11), 2570. https://doi.org/10.3390/v14112570







