Longitudinal Dynamics of HPV16 Antibodies in Saliva and Serum among Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collections
2.3. Anti-HPV16 Antibody Measurements
2.4. Statistical Analysis
3. Results
3.1. Study Population
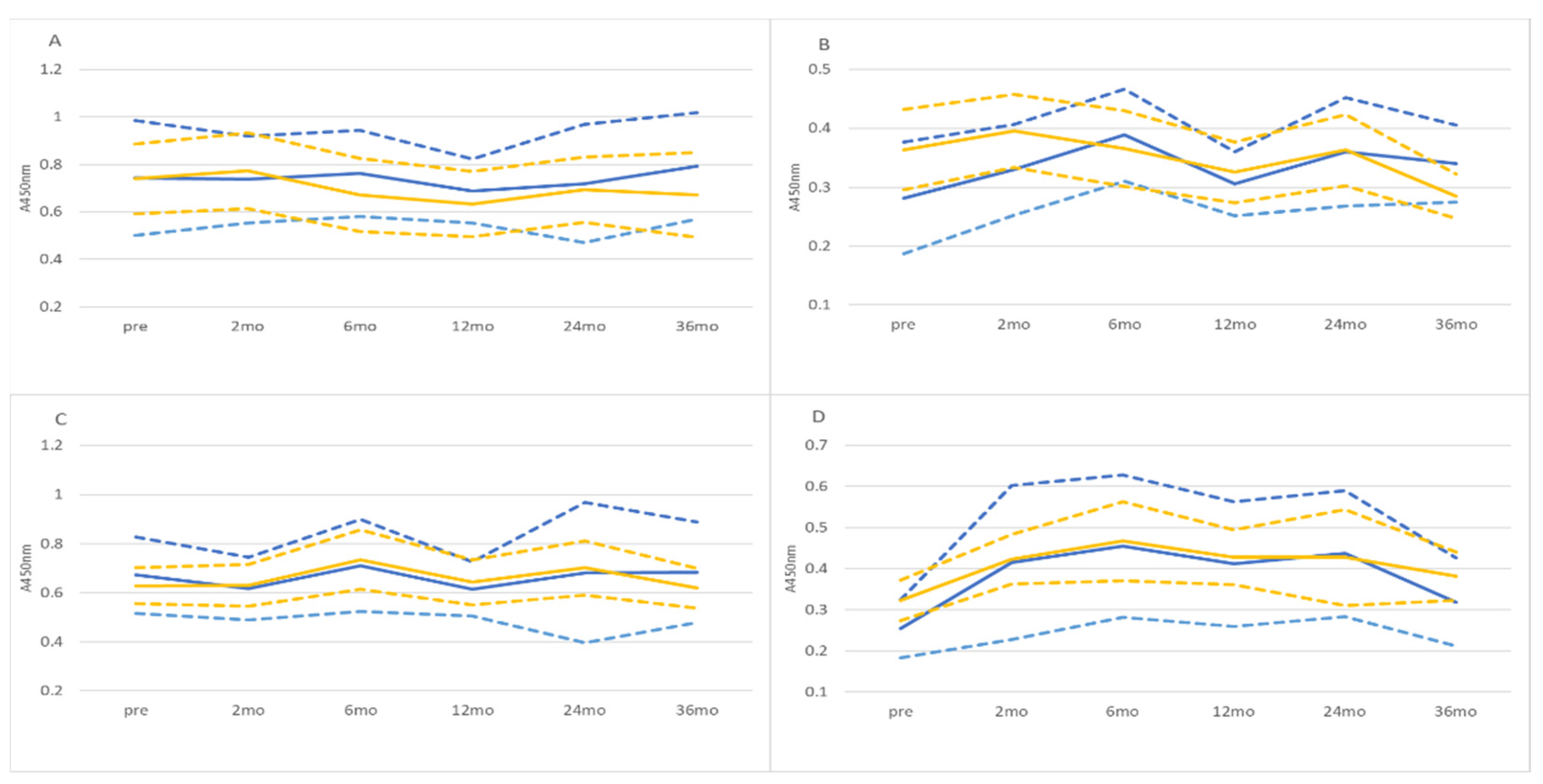
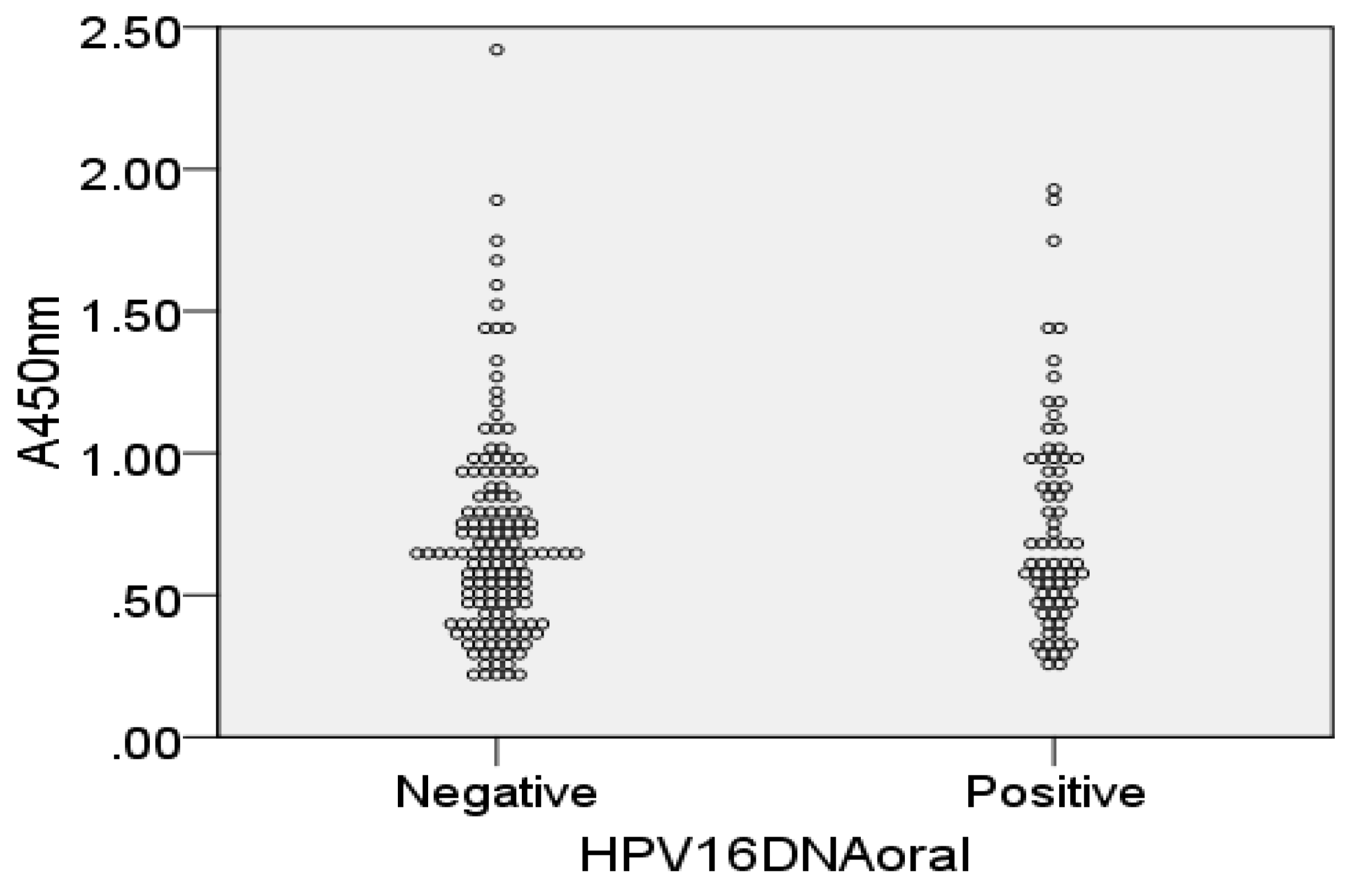
3.2. HPV16L1 and HPV16E7 IgG and IgA Antibodies in Saliva and Serum
3.3. Dynamics of Salivary Antibodies
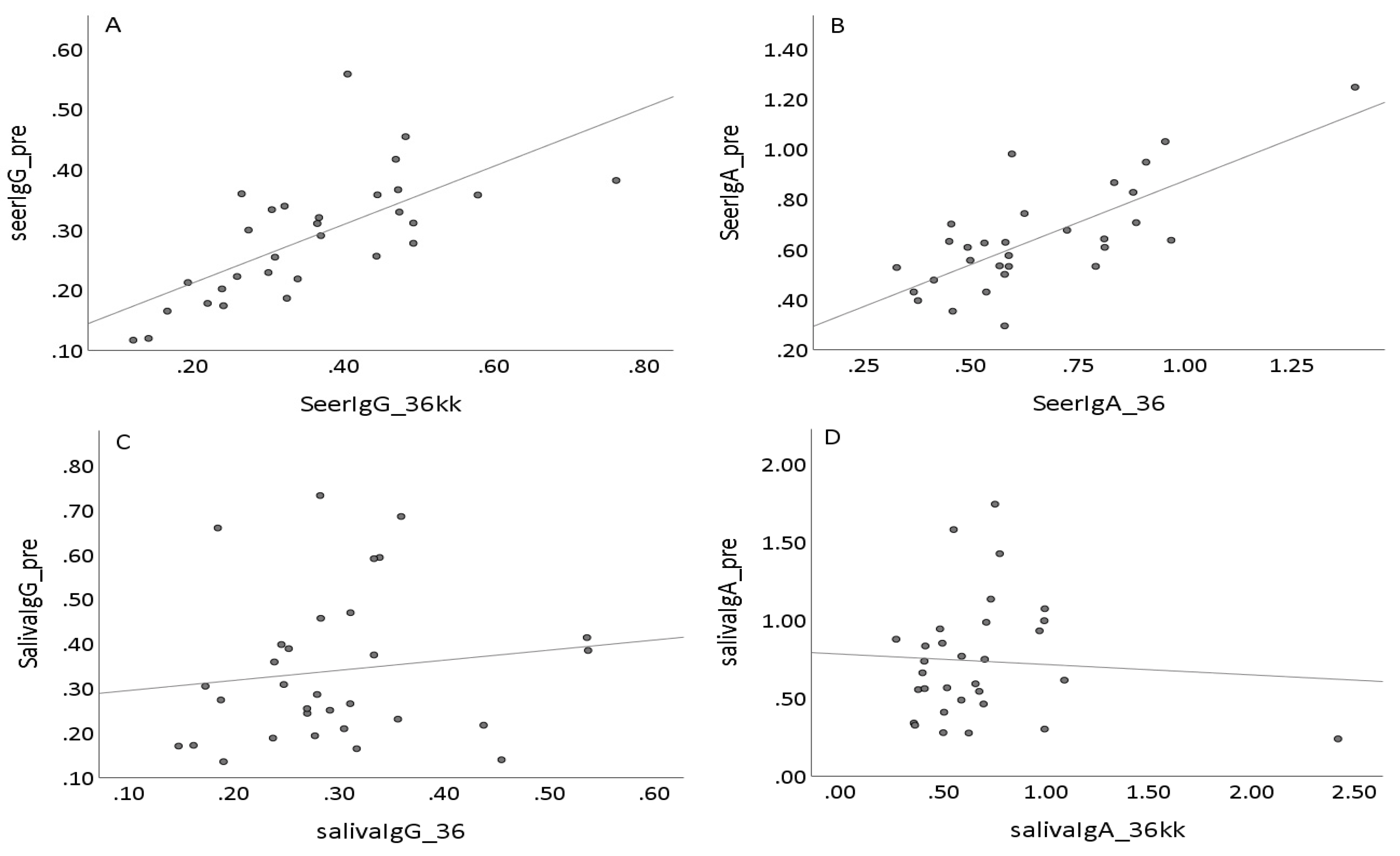
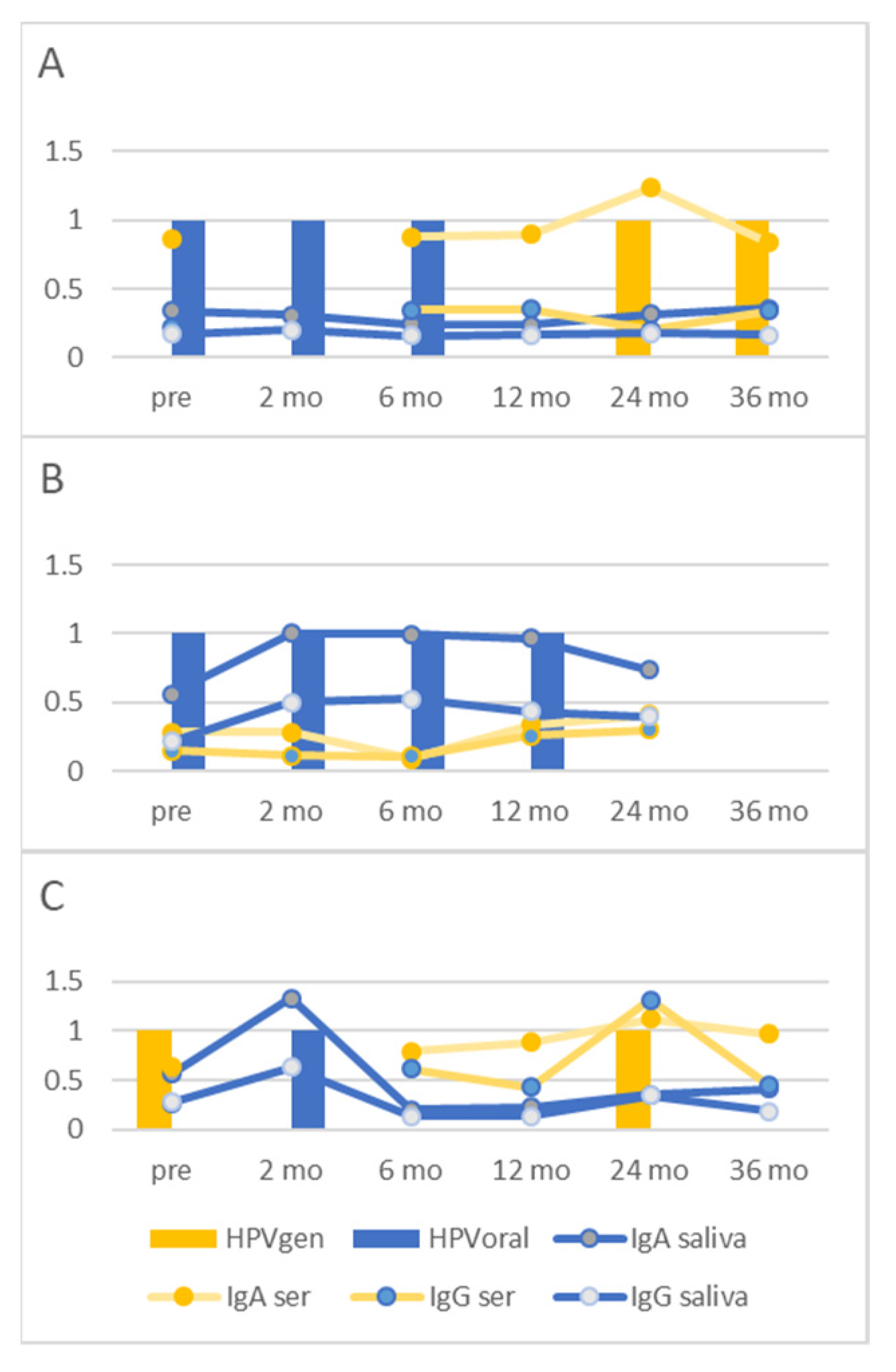
3.4. Individual HPV16 Responses in Saliva and Sera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de la Cour, C.D.; Sperling, C.D.; Belmonte, F.; Syrjänen, S.; Kjaer, S.K. Human Papillomavirus Prevalence in Oral Potentially Malignant Disorders: Systematic Review and Meta-Analysis. Oral. Dis. 2021, 27, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S. Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral. Sci. 2018, 126 (Suppl. S1), 49–66. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, V.; Chaturvedi, A.K.; Vignat, J.; Laversanne, M.; Bray, F.; Vaccarella, S. The Current Burden of Oropharyngeal Cancer: A Global Assessment Based on GLOBOCAN 2020. Cancer Epidemiol. Biomark. Prev. 2022, 31, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Rintala, M.; Grenman, S.; Puranen, M.; Syrjanen, S. Natural History of Oral Papillomavirus Infections in Spouses: A Prospective Finnish HPV Family Study. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2006, 35, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Rintala, M.; Sarkola, M.; Willberg, J.; Rautava, J.; Koskimaa, H.; Paaso, A.; Syrjänen, K.; Grénman, S.; Louvanto, K. Oral Human Papillomavirus Infection in Children during the First 6 Years of Life, Finland. Emerg Infect. Dis. 2021, 27, 759–766. [Google Scholar] [CrossRef]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Dillner, J. The Serological Response to Papillomaviruses. Semin. Cancer Biol. 1999, 9, 423–430. [Google Scholar] [CrossRef]
- Kirnbauer, R. Papillomavirus-like Particles for Serology and Vaccine Development. Intervirology 1996, 39, 54–61. [Google Scholar] [CrossRef]
- Onda, T.; Carter, J.J.; Koutsky, L.A.; Hughes, J.P.; Lee, S.-K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. Characterization of IgA Response among Women with Incident HPV 16 Infection. Virology 2003, 312, 213–221. [Google Scholar] [CrossRef][Green Version]
- Pattyn, J.; Van Keer, S.; Tjalma, W.; Matheeussen, V.; Van Damme, P.; Vorsters, A. Infection and Vaccine-Induced HPV-Specific Antibodies in Cervicovaginal Secretions. A Review of the Literature. Papillomavirus Res. 2019, 8, 100185. [Google Scholar] [CrossRef]
- Cameron, J.E.; Snowhite, I.V.; Chaturvedi, A.K.; Hagensee, M.E. Human Papillomavirus-Specific Antibody Status in Oral Fluids Modestly Reflects Serum Status in Human Immunodeficiency Virus-Positive Individuals. Clin. Diagn. Lab. Immunol. 2003, 10, 431–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loimaranta, V.; Sievi, K.; Werner, J.; Pawlita, M.; Waterboer, T.; Butt, J.; Syrjanen, S. Comparison of Multiplex-Serology and ELISA Based Methods in Detecting HPV16 L1 Antibody Responses in Paired Saliva and Serum Samples of Healthy Men. J. Virol. Methods 2019, 270, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Marais, D.J.; Sampson, C.; Jeftha, A.; Dhaya, D.; Passmore, J.A.; Denny, L.; Rybicki, E.P.; Walt, E.V.D.; Stephen, L.X.; Williamson, A.L. More Men than Women Make Mucosal IgA Antibodies to Human Papillomavirus Type 16 (HPV-16) and HPV-18: A Study of Oral HPV and Oral HPV Antibodies in a Normal Healthy Population. BMC Infect. Dis. 2006, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Marais, D.J.; Best, J.M.; Rose, R.C.; Keating, P.; Soeters, R.; Denny, L.; Dehaeck, C.M.; Nevin, J.; Kay, P.; Passmore, J.A.; et al. Oral Antibodies to Human Papillomavirus Type 16 in Women with Cervical Neoplasia. J. Med. Virol. 2001, 65, 149–154. [Google Scholar] [CrossRef]
- Passmore, J.A.; Marais, D.J.; Sampson, C.; Allan, B.; Parker, N.; Milner, M.; Denny, L.; Williamson, A.L. Cervicovaginal, Oral, and Serum IgG and IgA Responses to Human Papillomavirus Type 16 in Women with Cervical Intraepithelial Neoplasia. J. Med. Virol. 2007, 79, 1375–1380. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory Immunity with Special Reference to the Oral Cavity. J. Oral Microbiol. 2013, 5, 20401. [Google Scholar] [CrossRef]
- Rautava, J.; Willberg, J.; Louvanto, K.; Wideman, L.; Syrjanen, K.; Grenman, S.; Syrjanen, S. Prevalence, Genotype Distribution and Persistence of Human Papillomavirus in Oral Mucosa of Women: A Six-Year Follow-up Study. PLoS ONE 2012, 7, e42171. [Google Scholar] [CrossRef]
- Louvanto, K.; Rautava, J.; Willberg, J.; Wideman, L.; Syrjanen, K.; Grenman, S.; Syrjanen, S. Genotype-Specific Incidence and Clearance of Human Papillomavirus in Oral Mucosa of Women: A Six-Year Follow-up Study. PLoS ONE 2013, 8, e53413. [Google Scholar] [CrossRef]
- Rintala, M.A.; Grenman, S.E.; Jarvenkyla, M.E.; Syrjanen, K.J.; Syrjanen, S.M. High-Risk Types of Human Papillomavirus (HPV) DNA in Oral and Genital Mucosa of Infants during Their First 3 Years of Life: Experience from the Finnish HPV Family Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 1728–1733. [Google Scholar] [CrossRef]
- Laine, M.A. Effect of Pregnancy on Periodontal and Dental Health. Acta Odontol. Scand. 2002, 60, 257–264. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.-W.; Jiang, S.-Y. Relationship between Gingival Inflammation and Pregnancy. Mediat. Inflamm. 2015, 2015, 623427. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and Laboratory Studies: A Reference Table for Clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 2627. [Google Scholar] [CrossRef]
- Lima, J.; Cambridge, G.; Vilas-Boas, A.; Martins, C.; Borrego, L.-M.; Leandro, M. Serum Markers of B-Cell Activation in Pregnancy during Late Gestation, Delivery, and the Postpartum Period. Am. J. Reprod. Immunol. 2019, 81, e13090. [Google Scholar] [CrossRef]
- Tenovuo, J.; Laine, M.; Lehtonen, J. Salivary IgA antibodies reacting with Streptococcus mutans in relation to changes in salivary S. mutans counts: A longitudinal study in humans. Proceeding Finn. Dent. Soc. 1987, 83, 55–59. [Google Scholar]
- Wang, Z.H.; Kjellberg, L.; Abdalla, H.; Wiklund, F.; Eklund, C.; Knekt, P.; Lehtinen, M.; Kallings, I.; Lenner, P.; Hallmans, G.; et al. Type Specificity and Significance of Different Isotypes of Serum Antibodies to Human Papillomavirus Capsids. J. Infect. Dis. 2000, 181, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, A.; Rautava, J.; Kero, K.; Syrjänen, K.; Longatto-Filho, A.; Grenman, S.; Syrjänen, S. Physical state and copy numbers of HPV16 in oral asymptomatic infections that persisted or cleared during the 6-year follow-up. J. Gen. Virol. 2017, 98, 681–689. [Google Scholar] [CrossRef]
- Goncalves, A.K.; Giraldo, P.; Barros-Mazon, S.; Gondo, M.L.; Amaral, R.L.; Jacyntho, C. Secretory Immunoglobulin A in Saliva of Women with Oral and Genital HPV Infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Haukioja, A.; Asunta, M.; Soderling, E.; Syrjanen, S. Persistent Oral Human Papillomavirus Infection Is Associated with Smoking and Elevated Salivary Immunoglobulin G Concentration. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2014, 61, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Paaso, A.E.; Louvanto, K.; Syrjänen, K.J.; Waterboer, T.; Grénman, S.E.; Pawlita, M.; Syrjänen, S.M. Lack of Type-Specific Concordance between Human Papillomavirus (HPV) Serology and HPV DNA Detection in the Uterine Cervix and Oral Mucosa. J. Gen. Virol. 2011, 92, 2034–2046. [Google Scholar] [CrossRef]
- Beachler, D.C.; Viscidi, R.; Sugar, E.A.; Minkoff, H.; Strickler, H.D.; Cranston, R.D.; Wiley, D.J.; Jacobson, L.P.; Weber, K.M.; Margolick, J.B.; et al. A Longitudinal Study of Human Papillomavirus 16 L1, E6, and E7 Seropositivity and Oral Human Papillomavirus 16 Infection. Sex Transm Dis. 2015, 42, 93–97. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Johansson, M.; Waterboer, T.; Kaaks, R.; Chang-Claude, J.; Drogen, D.; Tjønneland, A.; Overvad, K.; Quirós, J.R.; González, C.A.; et al. Evaluation of Human Papillomavirus Antibodies and Risk of Subsequent Head and Neck Cancer. J. Clin. Oncol. 2013, 31, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, J.; Halec, G.; Baaken, D.; Waterboer, T.; Brenner, N. Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 3010. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, D.; Gheit, T.; Waterboer, T.; Abedi-Ardekani, B.; Carreira, C.; McKay-Chopin, S.; Gaborieau, V.; Marron, M.; Lagiou, P.; Ahrens, W.; et al. Human Papillomavirus Infections and Upper Aero-Digestive Tract Cancers: The ARCAGE Study. J. Natl Cancer Inst. 2013, 105, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Chatzistamatiou, K.; Sotiriadis, A.; Agorastos, T. Effect of Mode of Delivery on Vertical Human Papillomavirus Transmission—A Meta-Analysis. J. Obstet. Gynaecol. 2016, 36, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S. Current Concepts on Human Papillomavirus Infections in Children. APMIS 2010, 118, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Zouridis, A.; Kalampokas, T.; Panoulis, K.; Salakos, N.; Deligeoroglou, E. Intrauterine HPV Transmission: A Systematic Review of the Literature. Arch. Gynecol. Obstet. 2018, 298, 35–44. [Google Scholar] [CrossRef]
| HPV16 DNA Positive & | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serum Samples | Saliva Samples | Genital | Oral | |||||
| persist | trans | persist | trans | persist | trans | persist | trans | |
| pre | 11 | 26 | 11 | 26 | 1 | 2 | 4 | 7 |
| 2 mo | 10 | 23 | 10 | 25 | 0 | 1 | 7 | 12 |
| 6 mo | 13 | 26 | 12 | 26 | 0 | 0 | 7 | 7 |
| 12 mo | 12 | 23 | 13 | 23 | 5 | 6 | 9 | 4 |
| 24 mo | 9 | 21 | 12 | 26 | 7 | 12 | 9 | 3 |
| 36 mo | 10 | 22 | 10 | 23 | 8 | 12 | 6 | 2 |
| Saliva sIgA * | Saliva IgG # | Serum IgA & | Serum IgG ^ | |
|---|---|---|---|---|
| HPV16L1 | ||||
| persistent | 0.24–1.74 (0.66) | 0.13–0.69 (0.31) | 0.09–1.55 (0.65) | 0.11–1.10 (0.31) |
| transient | 0.20–2.42 (0.63) | 0.13–0.93 (0.32) | 0.30–1.89 (0.59) | 0.16–1.42 (0.37) |
| HPV16E7 | ||||
| persistent | NA | NA | 0.08–0.26 (0.19) | 0.19–0.56 (0.33) |
| transient | NA | NA | 0.11–0.51 (0.17) | 0.18–0.61 (0.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirttilä, T.; Syrjänen, S.; Louvanto, K.; Loimaranta, V. Longitudinal Dynamics of HPV16 Antibodies in Saliva and Serum among Pregnant Women. Viruses 2022, 14, 2567. https://doi.org/10.3390/v14112567
Pirttilä T, Syrjänen S, Louvanto K, Loimaranta V. Longitudinal Dynamics of HPV16 Antibodies in Saliva and Serum among Pregnant Women. Viruses. 2022; 14(11):2567. https://doi.org/10.3390/v14112567
Chicago/Turabian StylePirttilä, Tiina, Stina Syrjänen, Karolina Louvanto, and Vuokko Loimaranta. 2022. "Longitudinal Dynamics of HPV16 Antibodies in Saliva and Serum among Pregnant Women" Viruses 14, no. 11: 2567. https://doi.org/10.3390/v14112567
APA StylePirttilä, T., Syrjänen, S., Louvanto, K., & Loimaranta, V. (2022). Longitudinal Dynamics of HPV16 Antibodies in Saliva and Serum among Pregnant Women. Viruses, 14(11), 2567. https://doi.org/10.3390/v14112567






