Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea
Abstract
1. Introduction
2. Materials and Methods
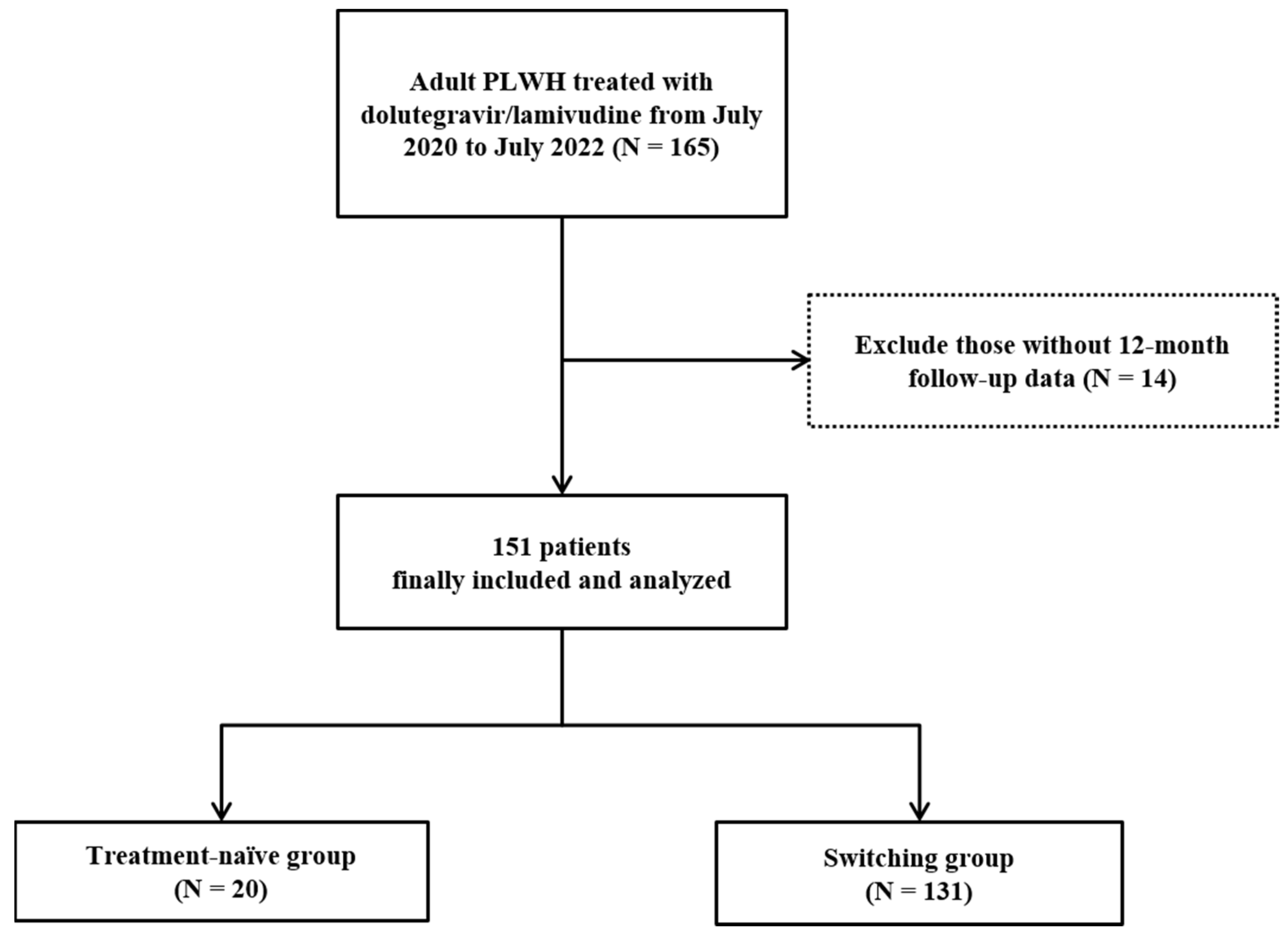
2.1. Study Design and Population
2.2. Study Outcomes and Covariates
2.3. Virological Analyses
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Antiretroviral Resistance Test
3.3. Treatment Outcomes
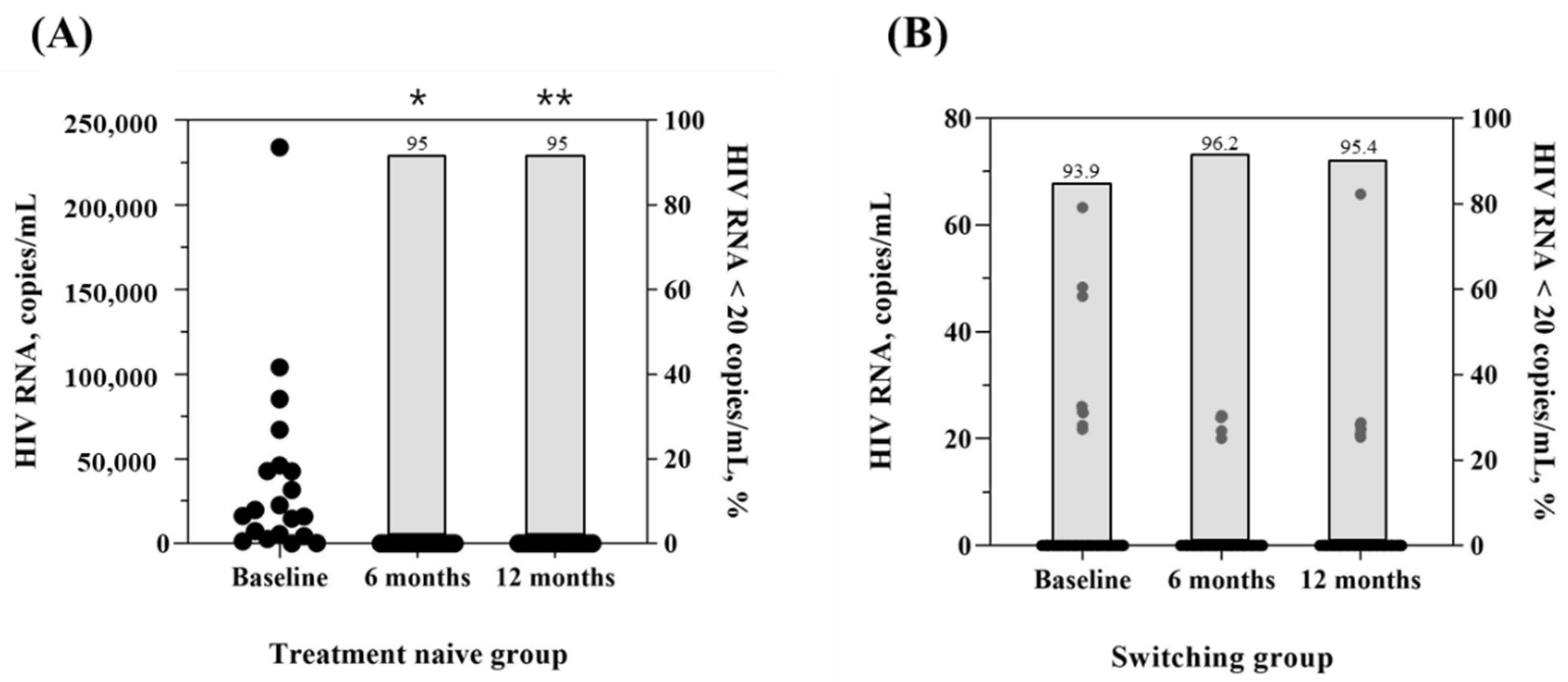
3.3.1. Effectiveness
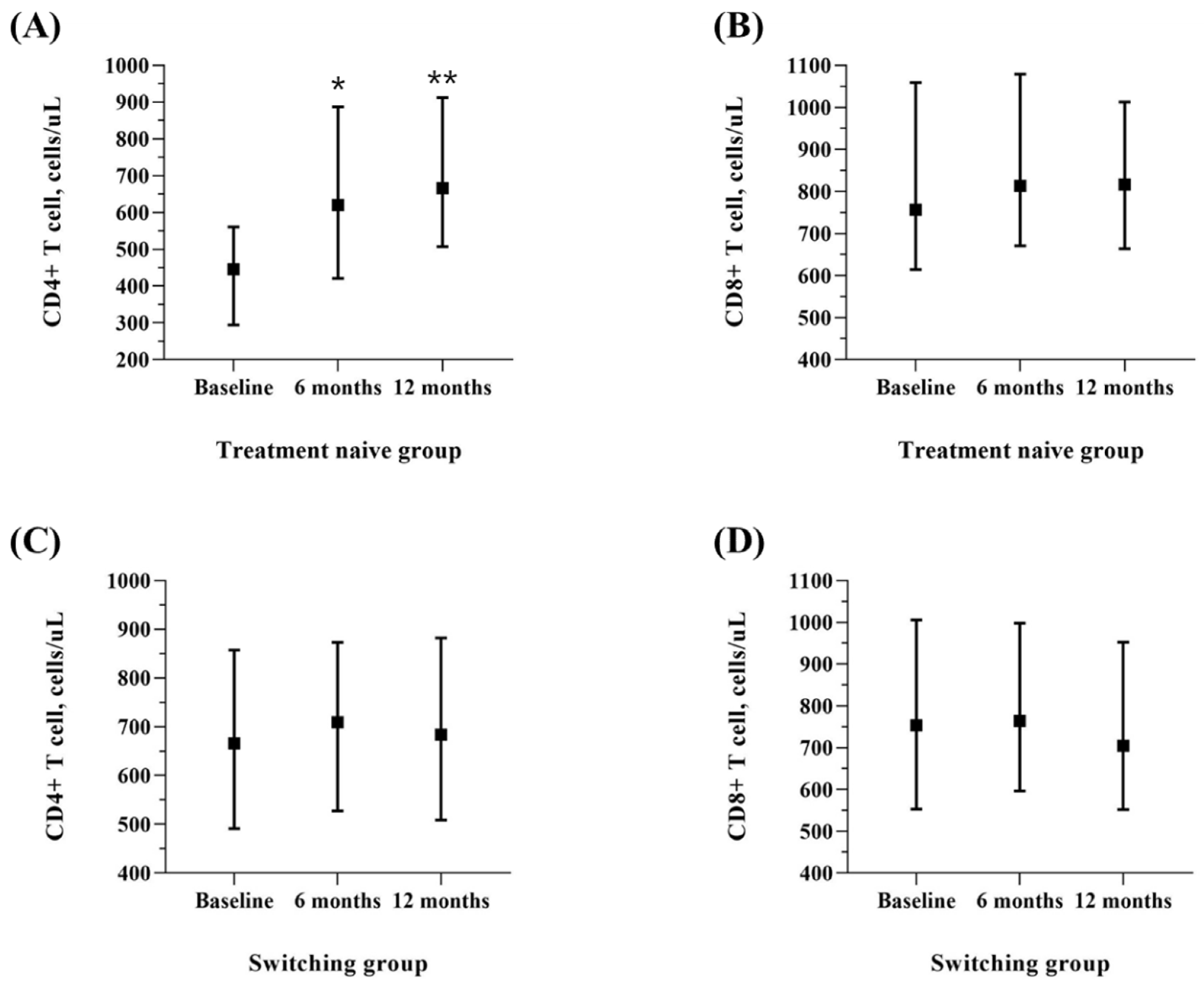
3.3.2. Tolerability and Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flepp, M.; Schiffer, V. Modern anti-hiv therapy. Swiss Med. Wkly. 2001, 131, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, C.S.; Beck, E.J.; Stelzle, D.; Mallouris, C.; Mahy, M.; Ghys, P. Global and regional trends of people living with hiv aged 50 and over: Estimates and projections for 2000–2020. PLoS ONE 2018, 13, e0207005. [Google Scholar] [CrossRef] [PubMed]
- Arancón Pardo, A.; Moreno Palomino, M.; Jiménez-Nácher, I.; Moreno, F.; González Fernández, M.Á.; González-García, J.; Herrero Ambrosio, A. Real-world experience with two-drug regimens in hiv-1-infected patients beyond the indication of clinical trials: 48 weeks’ results. AIDS Res. Hum. Retrovir. 2021, 37, 761–767. [Google Scholar] [CrossRef]
- Galizzi, N.; Poli, A.; Galli, L.; Muccini, C.; Mastrangelo, A.; Dell’Acqua, R.; Maillard, M.; Bossolasco, S.; Cinque, P.; Lazzarin, A. Retrospective study on the outcome of two-drug regimens based on dolutegravir plus one reverse transcriptase inhibitor in virologically-suppressed hiv-infected patients. Int. J. Antimicrob. Agents 2020, 55, 105893. [Google Scholar] [CrossRef]
- Maggiolo, F.; Gulminetti, R.; Pagnucco, L.; Digaetano, M.; Benatti, S.; Valenti, D.; Callegaro, A.; Ripamonti, D.; Mussini, C. Lamivudine/dolutegravir dual therapy in hiv-infected, virologically suppressed patients. BMC Infect. Dis. 2017, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, J.; Ajana, F.; Bisshop, F.; De Wit, S.; Osiyemi, O.; Portilla Sogorb, J.; Routy, J.-P.; Wyen, C.; Ait-Khaled, M.; Nascimento, M.C. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3-or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: Phase 3, randomized, noninferiority tango study. Clin. Infect. Dis. 2020, 71, 1920–1929. [Google Scholar] [PubMed]
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.-C.; Rockstroh, J.K.; Girard, P.-M.; Sievers, J. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with hiv-1 infection (gemini-1 and gemini-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019, 393, 143–155. [Google Scholar]
- AIDS, K.S.f. Clinical guidelines for the diagnosis and treatment of hiv/aids in hiv-infected koreans. Infect. Chemother. 2011, 43, 89–128. [Google Scholar]
- Du, X.; Peng, W.; Fu, Q.; Ma, Q.; Zhu, Z.; Li, T. A review of clinical pharmacokinetic and pharmacodynamic profiles of select antiretrovirals: Focus on differences among chinese patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 1179–1189. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines: Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Osterholzer, D.A.; Goldman, M. Dolutegravir: A next-generation integrase inhibitor for treatment of hiv infection. Clin. Infect. Dis. 2014, 59, 265–271. [Google Scholar] [CrossRef]
- Wainberg, M.A.; Han, Y.-S. Will drug resistance against dolutegravir in initial therapy ever occur? Front. Pharmacol. 2015, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.G.; van den Berk, G.E.; van Holten, N.; Oryszcyn, J.E.; Dorama, W.; Brinkman, K. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. Aids 2016, 30, 2831–2834. [Google Scholar] [CrossRef] [PubMed]
- Cahn, P.; Rolón, M.J.; Figueroa, M.I.; Gun, A.; Patterson, P.; Sued, O. Dolutegravir–lamivudine as initial therapy in hiv-1 infected, arv-naive patients, 48-week results of the paddle (pilot antiretroviral design with dolutegravir lamivudine) study. J. Int. AIDS Soc. 2017, 20, 21678. [Google Scholar] [CrossRef] [PubMed]
- Canducci, F.; Sampaolo, M.; Marinozzi, M.C.; Boeri, E.; Spagnuolo, V.; Galli, A.; Castagna, A.; Lazzarin, A.; Clementi, M.; Gianotti, N. Dynamic patterns of human immunodeficiency virus type 1 integrase gene evolution in patients failing raltegravir-based salvage therapies. Aids 2009, 23, 455–460. [Google Scholar] [CrossRef] [PubMed]
- (UNAIDS), JUNPoHaA. Unaids ‘aids data 2020’. 2020. Available online: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf (accessed on 1 March 2021).
- Bourgi, K.; Jenkins, C.A.; Rebeiro, P.F.; Shepherd, B.E.; Palella, F.; Moore, R.D.; Althoff, K.N.; Gill, J.; Rabkin, C.S.; Gange, S.J. Weight gain among treatment-naïve persons with hiv starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the united states and canada. J. Int. AIDS Soc. 2020, 23, e25484. [Google Scholar] [CrossRef]
- Bourgi, K.; Rebeiro, P.F.; Turner, M.; Castilho, J.L.; Hulgan, T.; Raffanti, S.P.; Koethe, J.R.; Sterling, T.R. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin. Infect. Dis. 2020, 70, 1267–1274. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S. Kidney disease: Improving global outcomes (kdigo) acute kidney injury work group. Kdigo clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Koteff, J.; Borland, J.; Chen, S.; Song, I.; Peppercorn, A.; Koshiba, T.; Cannon, C.; Muster, H.; Piscitelli, S.C. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br. J. Clin. Pharmacol. 2013, 75, 990–996. [Google Scholar] [CrossRef]
- Reese, M.J.; Savina, P.M.; Generaux, G.T.; Tracey, H.; Humphreys, J.E.; Kanaoka, E.; Webster, L.O.; Harmon, K.A.; Clarke, J.D.; Polli, J.W. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a hiv integrase inhibitor. Drug Metab. Dispos. 2013, 41, 353–361. [Google Scholar] [CrossRef]
- Raffi, F.; Jaeger, H.; Quiros-Roldan, E.; Albrecht, H.; Belonosova, E.; Gatell, J.M.; Baril, J.-G.; Domingo, P.; Brennan, C.; Almond, S. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with hiv-1 infection (spring-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2013, 13, 927–935. [Google Scholar] [CrossRef]
- Clotet, B.; Feinberg, J.; Van Lunzen, J.; Khuong-Josses, M.-A.; Antinori, A.; Dumitru, I.; Pokrovskiy, V.; Fehr, J.; Ortiz, R.; Saag, M. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with hiv-1 infection (flamingo): 48 week results from the randomised open-label phase 3b study. Lancet 2014, 383, 2222–2231. [Google Scholar] [CrossRef]
- Hua, W.; Wang, S.; Su, B.; Wang, W.; Liu, A.; Li, Z.; Zhang, T.; Shao, Y.; Ye, J.; Li, J. Evaluation of the efficacy and tolerability of dolutegravir plus lamivudine as a switch strategy in treatment-experienced hiv-infected patients. 2020. Available online: https://www.researchsquare.com/article/rs-80151/v1 (accessed on 1 March 2021).
- Spagnuolo, V.; Galli, L.; Poli, A.; Bigoloni, A.; Fumagalli, L.; Gianotti, N.; Nozza, S.; Ferrari, D.; Locatelli, M.; Lazzarin, A. A nucleoside-sparing regimen of dolutegravir plus ritonavir-boosted atazanavir in hiv-1-infected patients with virological failure: The dolatav study. Drug Des. Dev. Ther. 2019, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Amusan, P.; Power, C.; Gill, M.J.; Gomez, D.; Johnson, E.; Rubin, L.H.; Fujiwara, E. Lifetime antiretroviral exposure and neurocognitive impairment in hiv. J. Neurovirol. 2020, 26, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cerrone, M.; Ferretti, F.; Castrillo, N.; Maartens, G.; McClure, M.; Boffito, M. Pharmacokinetics of dolutegravir 100 mg once daily with rifampicin. Int. J. Antimicrob. Agents 2019, 54, 202–206. [Google Scholar] [CrossRef]
- Song, I.H.; Zong, J.; Borland, J.; Jerva, F.; Wynne, B.; Zamek-Gliszczynski, M.J.; Humphreys, J.E.; Bowers, G.D.; Choukour, M. The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J. Acquir. Immune Defic. Syndr. 1999 2016, 72, 400. [Google Scholar] [CrossRef]
| Treatment Naive (N = 20) | Switching Group (N = 131) | p-Value | |
|---|---|---|---|
| Gender, male | 20 (100.0) | 122 (93.1) | 0.608 |
| Age, years | 28.0 (24.3, 41.3) | 45.0 (35.0, 55.0) | <0.001 |
| BMI, kg/m2 | 23.4 (22.3, 24.3) | 23.7 (21.7, 25.9) | 0.001 |
| Sexual orientation * | |||
| Heterosexual | 4/13 (30.8) | 30/82 (36.6) | 0.765 |
| MSM | 6/13 (46.2) | 44/82 (53.7) | 0.615 |
| Bisexual | 2/13 (15.4) | 5/82 (6.1) | 0.244 |
| Alcohol | 13 (81.3) | 59 (59.6) | 0.097 |
| Smoking | 8 (47.1) | 46 (44.2) | 0.828 |
| Initial CD4+ T cell, cell/uL | 445.5 (294.3, 560.8) | 665.0 (490.0, 858.0) | <0.001 |
| Initial CD8+ T cell, cell/uL | 756.5 (614.5, 1059.0) | 746.0 (553.0, 1006.0) | 0.308 |
| Treatment duration of Dolutegravir/lamivudine, days | 507.5 (421.5, 628.3) | 525.0 (455.0, 550.0) | 0.001 |
| Resistance test | 18 (90.0) | 51 (38.9) | <0.001 |
| Underlying disease | |||
| Hypertension | 2 (10.0) | 30 (22.9) | 0.189 |
| Diabetes mellitus | 2 (10.0) | 25 (19.1) | 0.531 |
| Dyslipidemia | 2 (10.0) | 52 (40.0) | 0.009 |
| Cardiovascular disease | 2 (10.0) | 3 (2.3) | 0.131 |
| Osteoporosis | 0 (0.0) | 9 (6.9) | 0.608 |
| Osteopenia | 3 (15.0) | 20 (15.3) | 0.999 |
| Chronic kidney disease | 1 (5.0) | 9 (6.9) | 0.999 |
| Chronic lung disease | 0 (0.0) | 1 (0.8) | 0.999 |
| Tuberculosis | 1 (5.0) | 19 (14.5) | 0.476 |
| Syphilis | 3 (15.0) | 45 (34.6) | 0.080 |
| Solid tumor | 1 (5.0) | 3 (2.3) | 0.437 |
| Hematologic malignancy | 0 (0.0) | 4 (3.1) | 0.999 |
| Psychiatric illness | 2 (10.0) | 10 (7.6) | 0.661 |
| Hepatitis profile | |||
| Hepatitis C virus antibody positivity | 0 (0.0) | 1 (0.8) | 0.999 |
| Recent diagnosed sexually transmitted disease | 1 (5.0) | 5 (3.8) | 0.580 |
| Latest Regimen | Latest ART | Number |
|---|---|---|
| 2NRTIs + PIs | ABC/3TC/ATV/COBI | 2 |
| 3TC/ABC + ATV | 1 | |
| 3TC/ABC + LPV/r | 1 | |
| 2NRTIs + INSTIs | DTG/ABC/3TC | 101 |
| BIC/FTC/TAF | 8 | |
| ABC/3TC + RAL | 5 | |
| TDF/FTC + RAL | 3 | |
| TAF/FTC + RAL | 1 | |
| TAF/FTC + DTG | 1 | |
| 2NRTIs + NNRTIs | ETR + ABC + 3TC | 1 |
| ZDV/3TC + EFV | 2 | |
| 3TC/ABC + EFV | 2 | |
| TAF/FTC + EFV | 1 | |
| NRTIs + PIs + INSTIs | EVG/COBI/FTC/TAF | 2 |
| Treatment Naive (N = 20) | Switching Group (N = 131) | |
|---|---|---|
| Antiretroviral resistance test | 18 (90.0) | 51 (38.9) |
| Resistant to antiviral drugs | 5 (25.0) | 14 (10.7) |
| Major resistance type | ||
| NRTIs | 0 (0.0) | 1 (0.7) |
| NNRTIs | 4 (20.0) | 11 (8.4) |
| INSTIs | 2 (10.0) | 3 (2.3) |
| PIs | 0 (0.0) | 0 (0.0) |
| Parameter | Treatment Naïve | Switching Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | 12 Months | p Value + | BASELINE | 6 months | 12 Months | p Value + | |
| HIV RNA, copies/mL | 18050.0 (4682.5, 45350.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.005 * | 2.13 ± 9.14 | 0.88 ± 4.43 | 1.33 ± 7.05 | 0.252 * |
| CD4, cells/uL | 445.5 (294.3, 560.8) | 620.0 (420.8, 887.5) | 666.5 (507.8, 912.3) | <0.001 | 692.50 ± 279.70 | 728.36 ± 293.71 | 703.66 ± 278.99 | 0.080 |
| CD8, cells/uL | 756.5 (614.5, 1059.0) | 813.5 (671.3, 1079.3) | 817.0 (664.0, 1013.0) | 0.583 | 805.98 ± 348.62 | 841.16 ± 356.35 | 784.05 ± 347.54 | 0.007 |
| Lipid profile | ||||||||
| Triglyceride, mg/dL | 89.0 (71.5, 139.5) | 126.5 (78.8, 159.3) | 92.0 (78.0, 196.5) | 0.135 | 188.91 ± 137.66 | 180.19 ± 115.32 | 161.52 ± 102.54 | 0.026 * |
| LDL, mg/dL | 106.4 (81.1, 122.9) | 104.1 (83.6, 129.6) | 107.3 (89.2, 120.0) | 0.993 | 109.70 ± 79.87 | 105.03 ± 31.75 | 103.96 ± 33.17 | 0.510 * |
| cholesterol, mg/dL | 172.5 (158.8, 202.3) | 178.0 (158.0, 212.8) | 181.0 (160.1, 202.8) | 0.719 | 184.62 ± 38.64 | 185.00 ± 36.56 | 177.70 ± 37.47 | 0.004 |
| Renal profile | ||||||||
| blood urea nitrogen, mg/dL | 12.0 (10.8, 15.2) | 15.0 (11.8, 16.4) | 14.7 (11.5, 15.8) | 0.088 | 14.64 ± 3.75 | 15.23 ± 3.94 | 15.12 ± 3.82 | 0.177 |
| creatinine, mg/dL | 0.9 (0.8, 1.0) | 1.0 (1.0, 1.1) | 1.1 (1.0, 1.2) | <0.001 | 0.99 ± 0.17 | 1.04 ± 0.16 | 1.02 ± 0.16 | <0.001 * |
| Liver function | ||||||||
| AST, IU/L | 23.0 (17.5, 33.5) | 20.0 (18.0, 30.0) | 23.0 (17.0, 29.3) | 0.342 * | 25.89 ± 22.65 | 24.53 ± 13.26 | 26.14 ± 10.77 | 0.511 * |
| ALT, IU/L | 26.5 (13.5, 32.3) | 21.0 (16.0, 24.0) | 21.0 (14.5, 35.0) | 0.283 * | 30.86 ± 30.23 | 29.28 ± 22.51 | 30.05 ± 20.32 | 0.726 * |
| Total bilirubin, mg/dL | 0.8 (0.5, 1.0) | 0.9 (0.7, 1.2) | 0.8 (0.6, 1.0) | 0.182 | 0.79 ± 0.40 | 0.89 ± 0.41 | 0.88 ± 0.35 | 0.003 |
| Glucose, mg/dL | 94.5 (90.5, 102.8) | 93.0 (87.3, 94.8) | 96.5 (89.0, 100.8) | 0.361 * | 104.85 ± 41.61 | 102.30 ± 19.11 | 105.05 ± 34.74 | 0.915 |
| Body weight, kg | 66.5 (62.3, 75.3) | 70.0 (64.0, 78.0) | 71.0 (64.0, 78.0) | 0.070 | 70.0 (63.3, 78.8) | 70.0 (63.0, 79.0) | 70.0 (63.2, 78.8) | 0.268 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.H.; Kim, J.; Lee, J.A.; Kim, C.H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.-S.; Song, Y.G.; et al. Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea. Viruses 2022, 14, 2558. https://doi.org/10.3390/v14112558
Lee KH, Kim J, Lee JA, Kim CH, Ahn JY, Jeong SJ, Ku NS, Choi JY, Yeom J-S, Song YG, et al. Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea. Viruses. 2022; 14(11):2558. https://doi.org/10.3390/v14112558
Chicago/Turabian StyleLee, Ki Hyun, Jinnam Kim, Jung Ah Lee, Chang Hyup Kim, Jin Young Ahn, Su Jin Jeong, Nam Su Ku, Jun Yong Choi, Joon-Sup Yeom, Young Goo Song, and et al. 2022. "Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea" Viruses 14, no. 11: 2558. https://doi.org/10.3390/v14112558
APA StyleLee, K. H., Kim, J., Lee, J. A., Kim, C. H., Ahn, J. Y., Jeong, S. J., Ku, N. S., Choi, J. Y., Yeom, J.-S., Song, Y. G., & Kim, J. H. (2022). Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea. Viruses, 14(11), 2558. https://doi.org/10.3390/v14112558







