Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants
Abstract
1. Introduction
2. Induction of Host Immunity against SARS-CoV-2 Entry and Infection Stages
2.1. Viral Entry and the Initiation of SARS-CoV-2 Pathogenesis
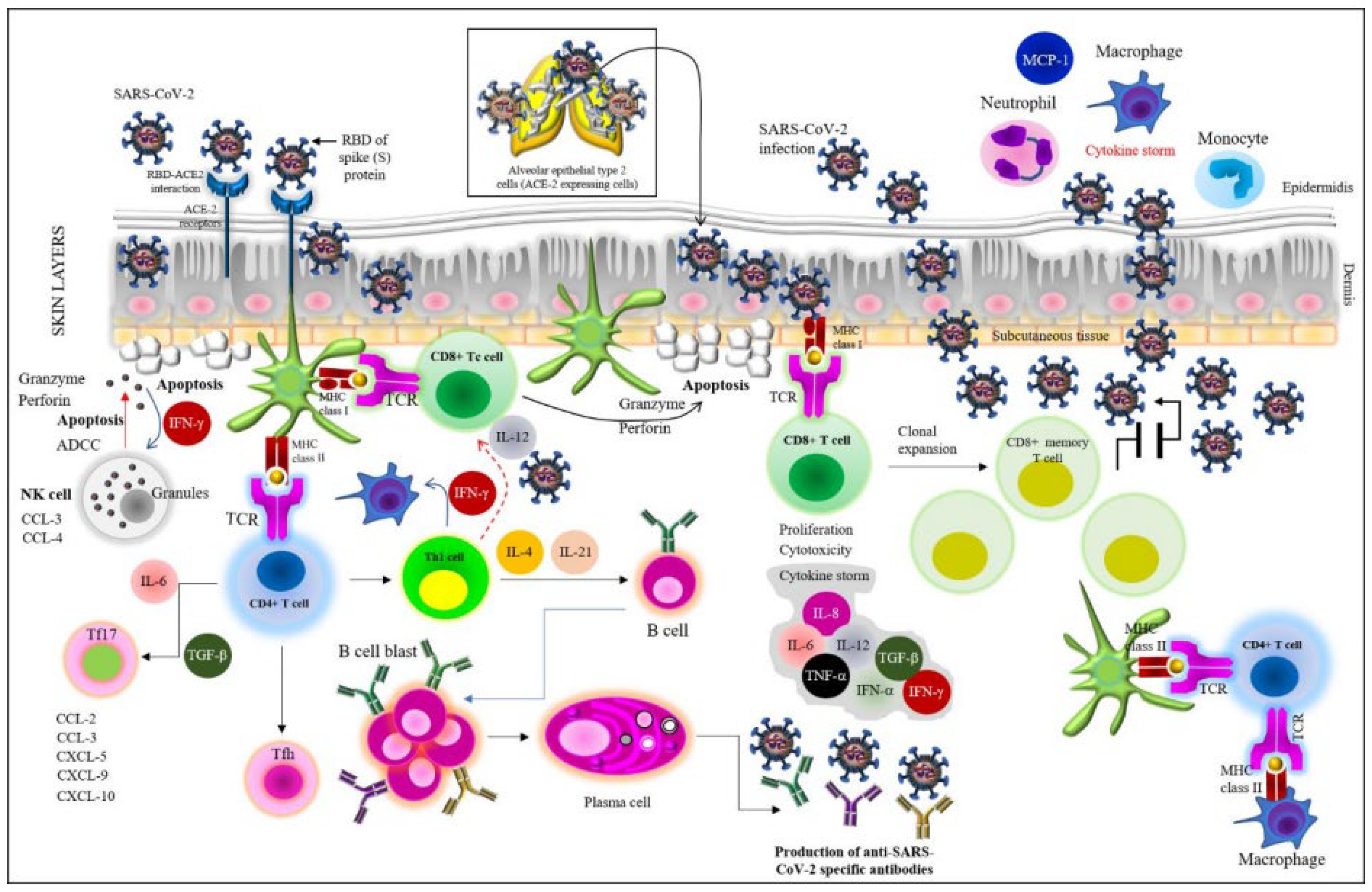
2.1.1. Immunological Consequences during Viral Entry and Hyperinflammation Effects
2.2. Innate Immune Response Is Largely Mediated by the Interferons (IFNs)
2.2.1. Molecular Mechanism of IFN Production in the Antiviral Innate Immunity
2.2.2. Production of IFN-Stimulated Genes (ISGs) for Regulating Innate and Adaptive Immunity
2.2.3. Evasion of IFN-Mediated Innate Immunity by SARS-CoV-2
3. Induction of Both Cell-Mediated Immunity and Humoral (Adaptive) Immunity
3.1. Cell-Mediated Immunity
3.2. Humoral Immunity
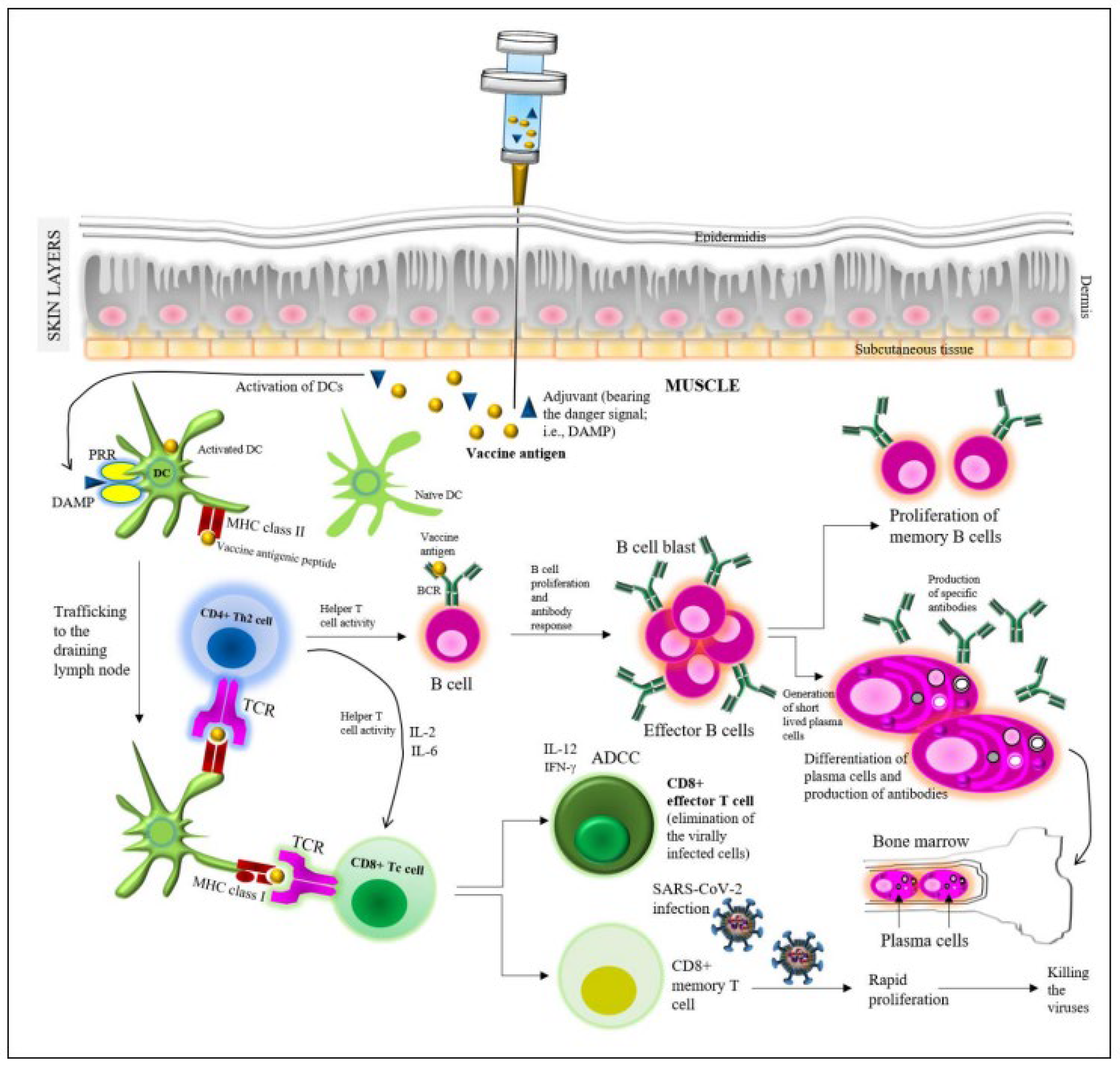
4. Vaccine-Induced Protective Immunity
4.1. Immunity Induced by the Major COVID-19 Vaccines
4.1.1. mRNA Vaccines
4.1.2. Viral Vector Vaccines
4.1.3. Protein Subunit Vaccines
4.1.4. Whole-Cell Inactivated Virus Vaccines
4.2. Effectiveness of COVID-19 Vaccines
4.2.1. Perspectives on the COVID-19 Vaccine-Induced Durability of NAb Responses
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO (World Health Organization). Coronavirus Diseases (COVID-19) Dashboard. Updated on 5:41 pm CET. Available online: https://covid19.who.int/ (accessed on 10 November 2022).
- Pulliam, J.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef] [PubMed]
- Zamorano Cuervo, N.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. eLife 2020, 9, e61390. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Sinai Immunology Review Project Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Aliyari, S.R.; Quanquin, N.; Pernet, O.; Zhang, S.; Wang, L.; Cheng, G. The Evolutionary Dance between Innate Host Antiviral Pathways and SARS-CoV-2. Pathogens 2022, 11, 538. [Google Scholar] [CrossRef]
- Belleudi, V.; Rosa, A.C.; Poggi, F.R.; Armuzzi, A.; Nicastri, E.; Goletti, D.; Diamanti, A.P.; Davoli, M.; Agabiti, N.; Addis, A. Direct and Indirect Impact of COVID-19 for Patients with Immune-Mediated Inflammatory Diseases: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Noor, R. A comparative review of pathogenesis and host innate immunity evasion strategies among the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). Arch. Microbiol. 2021, 203, 1943–1951. [Google Scholar] [CrossRef]
- Brant, A.C.; Tian, W.; Majerciak, V.; Yang, W.; Zheng, Z.M. SARS-CoV-2: From its discovery to genome structure, transcription, and replication. Cell Biosci. 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, J.; Gao, K.; Hozumi, Y.; Yin, C.; Wei, G.W. Analysis of SARS-CoV-2 mutations in the United States suggests presence of four substrains and novel variants. Commun. Biol. 2021, 4, 228. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.K.; Forero, A. Mechanisms of Antiviral Immune Evasion of SARS-CoV-2. J. Mol. Biol. 2022, 434, 167265. [Google Scholar] [CrossRef] [PubMed]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021, 147, 545–557.e9. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, U.; Barouch, D.H.; Rizzi, R.; Lagkadinou, E.; Türeci, Ö.; Pather, S.; Neels, P. Clinical development and approval of COVID-19 vaccines. Expert Rev. Vaccines 2022, 21, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Petrone, L.; Picchianti-Diamanti, A.; Sebastiani, G.D.; Aiello, A.; Laganà, B.; Cuzzi, G.; Vanini, V.; Gualano, G.; Grifoni, A.; Ferraioli, M.; et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int. J. Infect. Dis. Off. Publ. Int. Soc. Infect. Dis. 2022, 121, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. SARS-CoV-2: A Master of Immune Evasion. Biomedicines 2022, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Chia, T.; Young, B.E. SARS-CoV-2 variants of concern and vaccine escape, from Alpha to Omicron and beyond. Expert Rev. Respir. Med. 2022, 16, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Busà, R.; Sorrentino, M.C.; Russelli, G.; Amico, G.; Miceli, V.; Miele, M.; Di Bella, M.; Timoneri, F.; Gallo, A.; Zito, G.; et al. Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components. Front. Immunol. 2022, 13, 856657. [Google Scholar] [CrossRef]
- Jamshidi, E.; Asgary, A.; Shafiekhani, P.; Khajeamiri, Y.; Mohamed, K.; Esmaily, H.; Jamal Rahi, S.; Mansouri, N. Longevity of immunity following COVID-19 vaccination: A comprehensive review of the currently approved vaccines. Hum. Vaccines Immunother. 2022, 18, 2037384. [Google Scholar] [CrossRef] [PubMed]
- Sa Ribero, M.; Jouvenet, N.; Dreux, M.; Nisole, S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef]
- Huang, W.T.; Weng, S.W.; Tzeng, H.T.; Yen, F.C.; Chiang, Y.S.; You, H.L. Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination. Vaccines 2022, 10, 1550. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Teixeira, T.A.; Bernardes, F.S.; Oliveira, Y.C.; Hsieh, M.K.; Esteves, S.C.; Duarte-Neto, A.N.; Kallas, E.G.; Hallak, J. SARS-CoV-2 and Multi-Organ damage—What men’s health specialists should know about the COVID-19 pathophysiology. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2021, 47, 637–646. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wu, J.; Yu, Y.; Liu, S.; Li, T.; Li, Q.; Ding, R.; Wang, H.; Nie, J.; et al. A second functional furin site in the SARS-CoV-2 spike protein. Emerg. Microbes Infect. 2022, 11, 182–194. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Ji, T.T.; Dong, J.R.; Feng, H.; Chen, F.Q.; Chen, X.; Zhao, H.Y.; Chen, D.K.; Ma, W.T. Pathogenesis and Treatment of Cytokine Storm Induced by Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 13009. [Google Scholar] [CrossRef]
- Kaushal, A.; Noor, R. Association of Gut Microbiota with Inflammatory Bowel Disease and COVID-19 Severity: A Possible Outcome of the Altered Immune Response. Curr. Microbiol. 2022, 79, 184. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.Q.; Huang, M.; Sun, X.; Deng, F.; Wang, H.; Ning, Y.J. Immune evasion of SARS-CoV-2 from interferon antiviral system. Comput. Struct. Biotechnol. J. 2021, 19, 4217–4225. [Google Scholar] [CrossRef] [PubMed]
- Lowery, S.A.; Sariol, A.; Perlman, S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe 2021, 29, 1052–1062. [Google Scholar] [CrossRef]
- Gu, W.; Gan, H.; Ma, Y.; Xu, L.; Cheng, Z.J.; Li, B.; Zhang, X.; Jiang, W.; Sun, J.; Sun, B.; et al. The molecular mechanism of SARS-CoV-2 evading host antiviral innate immunity. Virol. J. 2022, 19, 49. [Google Scholar] [CrossRef]
- Li, H.; Zhou, F.; Zhang, L. STING, a critical contributor to SARS-CoV-2 immunopathology. Signal Transduct. Target. Ther. 2022, 7, 106. [Google Scholar] [CrossRef]
- Znaidia, M.; Demeret, C.; van der Werf, S.; Komarova, A.V. Characterization of SARS-CoV-2 Evasion: Interferon Pathway and Therapeutic Options. Viruses 2022, 14, 1247. [Google Scholar] [CrossRef]
- Kimura, I.; Konno, Y.; Uriu, K.; Hopfensperger, K.; Sauter, D.; Nakagawa, S.; Sato, K. Sarbecovirus ORF6 proteins hamper induction of interferon signaling. Cell Rep. 2021, 34, 108916. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, X.; Zeng, X.; Pan, Y.; Xu, H. Analysis of Lymphocyte Subpopulations and Cytokines in COVID-19-Associated Pneumonia and Community-Acquired Pneumonia. J. Immunol. Res. 2021, 2021, 6657894. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Thevarajan, I.; Nguyen, T.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef]
- Gupta, S.L.; Jaiswal, R.K. Neutralizing antibody: A savior in the Covid-19 disease. Mol. Biol. Rep. 2022, 49, 2465–2474. [Google Scholar] [CrossRef]
- Stankov, M.V.; Cossmann, A.; Bonifacius, A.; Dopfer-Jablonka, A.; Ramos, G.M.; Gödecke, N.; Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Noor, R.; Tasnim, N.; Saha, C. COVID-19 Pandemic and the Convalescent Plasma Therapy: Possible Benefits and Risks. Curr. Clin. Microbiol. Rep. 2021, 8, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Schietzel, S.; Anderegg, M.; Limacher, A.; Born, A.; Horn, M.P.; Maurer, B.; Hirzel, C.; Sidler, D.; Moor, M.B. Humoral and cellular immune responses on SARS-CoV-2 vaccines in patients with anti-CD20 therapies: A systematic review and meta-analysis of 1342 patients. RMD Open 2022, 8, e002036. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wang, M.; Islam, M.S.; Liao, P.; Hu, Y.; Chen, X. Humoral and Cellular Immune Responses of COVID-19 vaccines against SARS-Cov-2 Omicron variant: A systemic review. Int. J. Biol. Sci. 2022, 18, 4629–4641. [Google Scholar] [CrossRef]
- Noor, R.; Shareen, S.; Billah, M. COVID-19 vaccines: Their effectiveness against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its emerging variants. Bull. Natl. Res. Cent. 2022, 46, 96. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Aiello, A.; Laganà, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Farroni, C.; Lapa, D.; Najafi Fard, S.; Cuzzi, G.; et al. ImmunosuppressiveTherapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 mRNA Vaccine in Rheumatoid Arthritis Patients. Front. Immunol. 2021, 12, 740249. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Zhu, F.; Jin, P.; Zhu, T.; Wang, W.; Ye, H.; Pan, H.; Hou, L.; Li, J.; Wang, X.; Wu, S.; et al. Safety and Immunogenicity of a Recombinant Adenovirus Type-5-Vectored Coronavirus Disease 2019 (COVID-19) Vaccine With a Homologous Prime-Boost Regimen in Healthy Participants Aged ≥6 Years: A Randomized, Double-Blind, Placebo-Controlled, Phase 2b Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, e783–e791. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Janssen Biotech. COVID-19 Vaccine Ad26.COV2.S. Sponsor Briefing Document (Vaccines and Related Biological Products Advisory Committee, 2021). Available online: https://www.fda.gov/media/146217/download (accessed on 8 October 2022).
- Kurup, D.; Schnell, M.J. SARS-CoV-2 vaccines—The biggest medical research project of the 21st century. Curr. Opin. Virol. 2021, 49, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: First Approval of the Protein-Based Adjuvanted Nuvaxovid (NVX-CoV2373) Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune Protection from Viral Variants. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e936523. [Google Scholar] [CrossRef]
- Schultz, B.M.; Melo-González, F.; Duarte, L.F.; Gálvez, N.; Pacheco, G.A.; Soto, J.A.; Berríos-Rojas, R.V.; González, L.A.; Moreno-Tapia, D.; Rivera-Pérez, D.; et al. A Booster Dose of CoronaVac Increases Neutralizing Antibodies and T Cells that Recognize Delta and Omicron Variants of Concern. mBio 2022, 13, e0142322. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- de la Torre, J.C.G.; Cáceres-DelAguila, J.A.; Muro-Rojo, C.; De La Cruz-Escurra, N.; Copaja-Corzo, C.; Hueda-Zavaleta, M.; Arenas Siles, D.; Benites-Zapata, V.A. Humoral Immune Response Induced by the BBIBP-CorV Vaccine (Sinopharm) in Healthcare Workers: A Cohort Study. Trop. Med. Infect. Dis. 2022, 7, 66. [Google Scholar] [CrossRef]
- Noor, R. A review on the induction of host immunity by the current COVID-19 vaccines and a brief non-pharmaceutical intervention to mitigate the pandemic. Bull. Natl. Res. Cent. 2022, 46, 31. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021, 398, 2173–2184. [Google Scholar] [CrossRef]
- Noor, R. Developmental Status of the Potential Vaccines for the Mitigation of the COVID-19 Pandemic and a Focus on the Effectiveness of the Pfizer-BioNTech and Moderna mRNA Vaccines. Curr. Clin. Microbiol. Rep. 2021, 8, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Noor, R. How do the severe acute respiratory coronavirus 2 (SARS-CoV-2) and its variants escape the host protective immunity and mediate pathogenesis? Bull. Natl. Res. Cent. 2022, 46, 255. [Google Scholar] [CrossRef] [PubMed]
- Muena, N.A.; García-Salum, T.; Pardo-Roa, C.; Serrano, E.F.; Levican, J.; Avendaño, M.J.; Almonacid, L.I.; Valenzuela, G.; Poblete, E.; Strohmeier, S.; et al. Long-lasting neutralizing antibody responses in SARS-CoV-2 seropositive individuals are robustly boosted by immunization with the CoronaVac and BNT162b2 vaccines. medRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, S.; Liang, D.; Hu, W.; Ke, C.; He, J.; Yuan, R.; Huang, Y.; Li, Y.; Liu, D.; et al. Differential Antibody Response to Inactivated COVID-19 Vaccines in Healthy Subjects. Front. Cell. Infect. Microbiol. 2021, 11, 791660. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.F.; Yen, C.L.; Fu, Y.C.; Cheng, C.M.; Shen, T.C.; Chang, P.D.; Cheng, K.H.; Liu, C.C.; Chang, Y.T.; Chen, P.L.; et al. Innate Immune Responses of Vaccinees Determine Early Neutralizing Antibody Production After ChAdOx1nCoV-19 Vaccination. Front. Immunol. 2022, 13, 807454. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Athalye, M.; Parikh, P.K.; Chhipa, A.S.; Patel, S.; Apostolopoulos, V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses 2022, 14, 759. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, R. Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants. Viruses 2022, 14, 2541. https://doi.org/10.3390/v14112541
Noor R. Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants. Viruses. 2022; 14(11):2541. https://doi.org/10.3390/v14112541
Chicago/Turabian StyleNoor, Rashed. 2022. "Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants" Viruses 14, no. 11: 2541. https://doi.org/10.3390/v14112541
APA StyleNoor, R. (2022). Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants. Viruses, 14(11), 2541. https://doi.org/10.3390/v14112541






