Proteomic Signatures of the Serological Response to Influenza Vaccination in a Large Human Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Sample Collection
2.2. Estimates of Seroconversion
2.3. Sample Preparation
2.4. Protein Identification and Quantification
2.5. Proteomics Data Preprocessing
2.6. Statistical Testing
3. Results
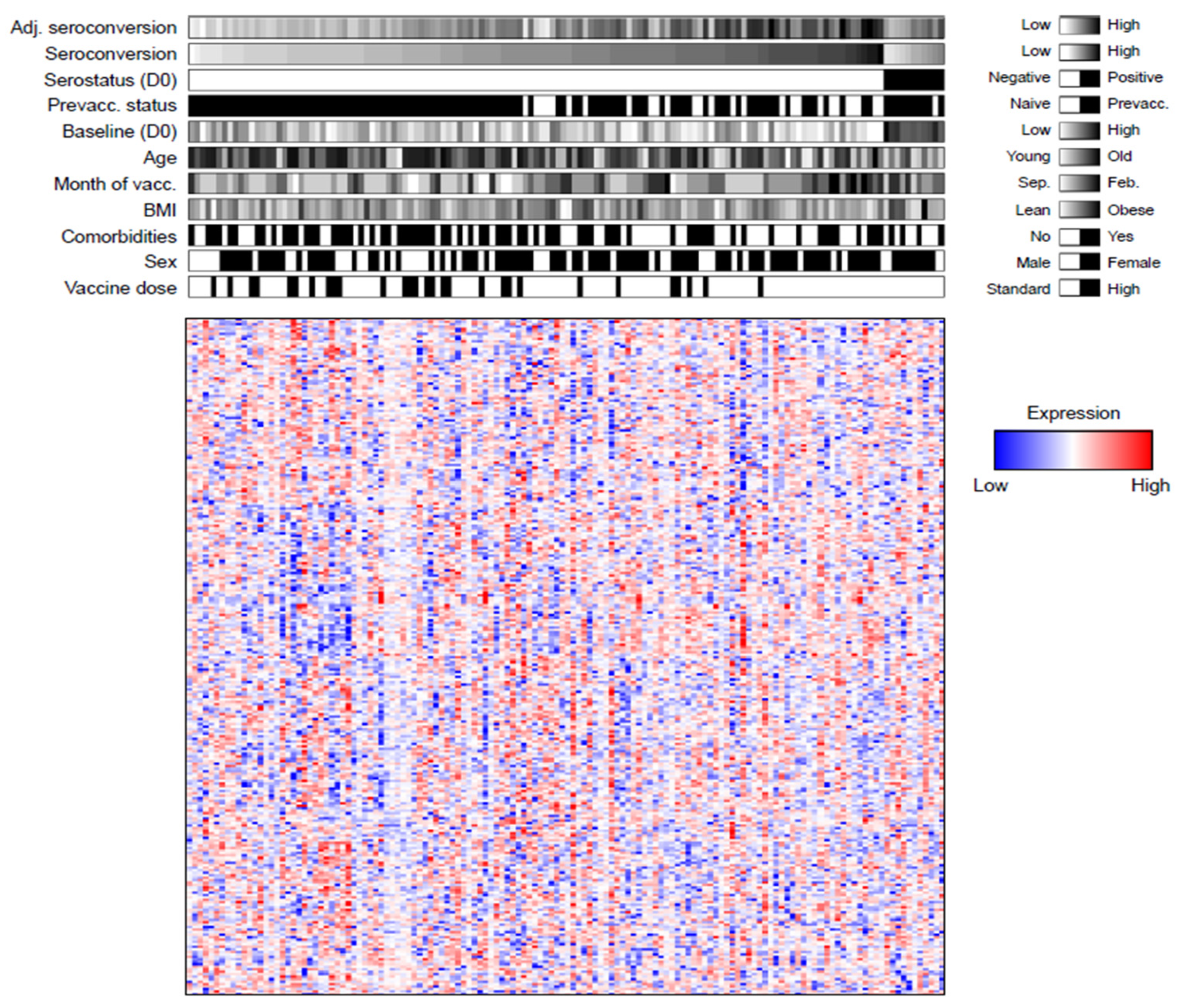
3.1. Quantification of ~300 Proteins in Influenza Vaccine Recipients
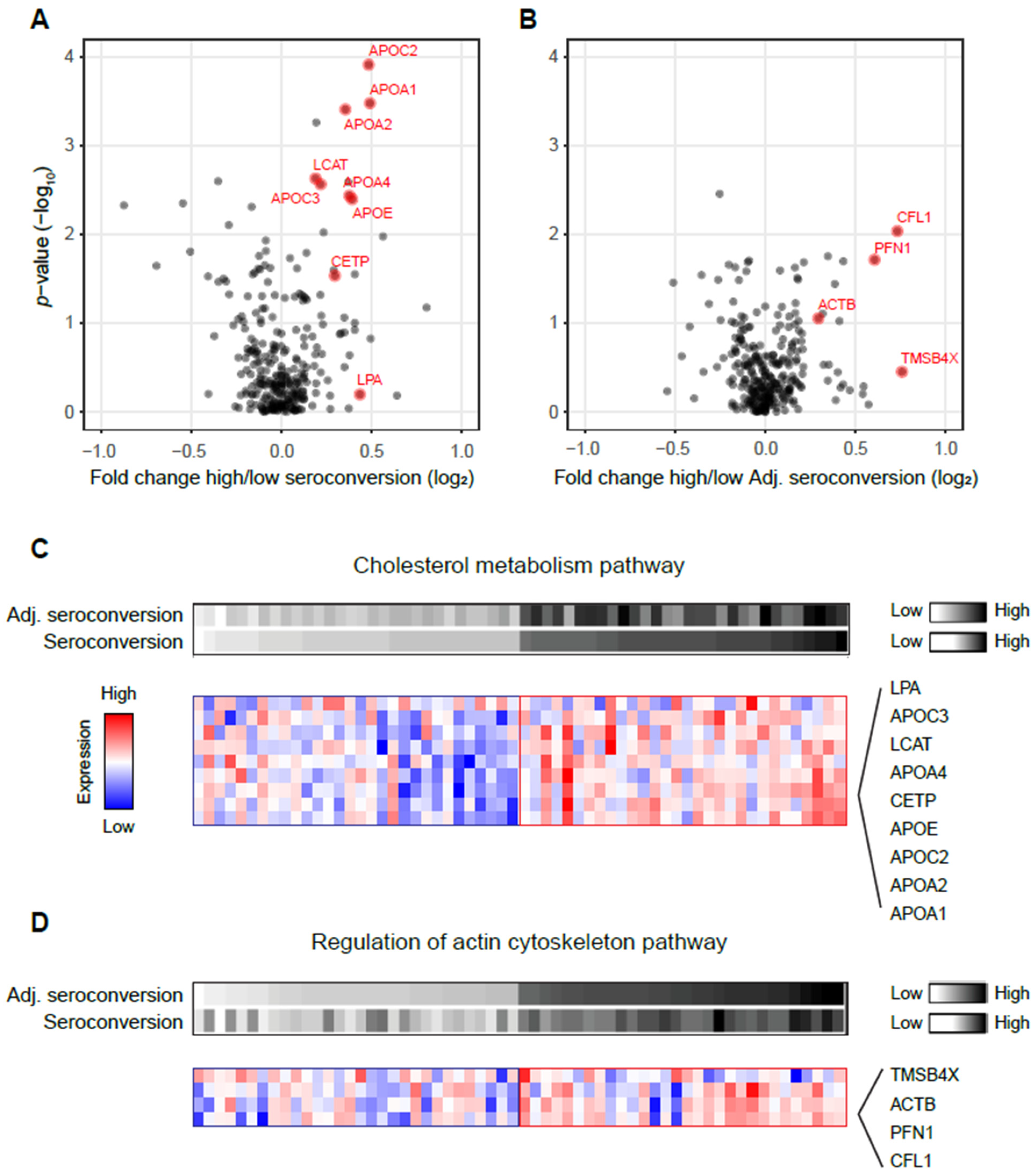
3.2. High Seroconversion Associated with Elevated Cholesterol Metabolism and Actin Cytoskeleton Pathways
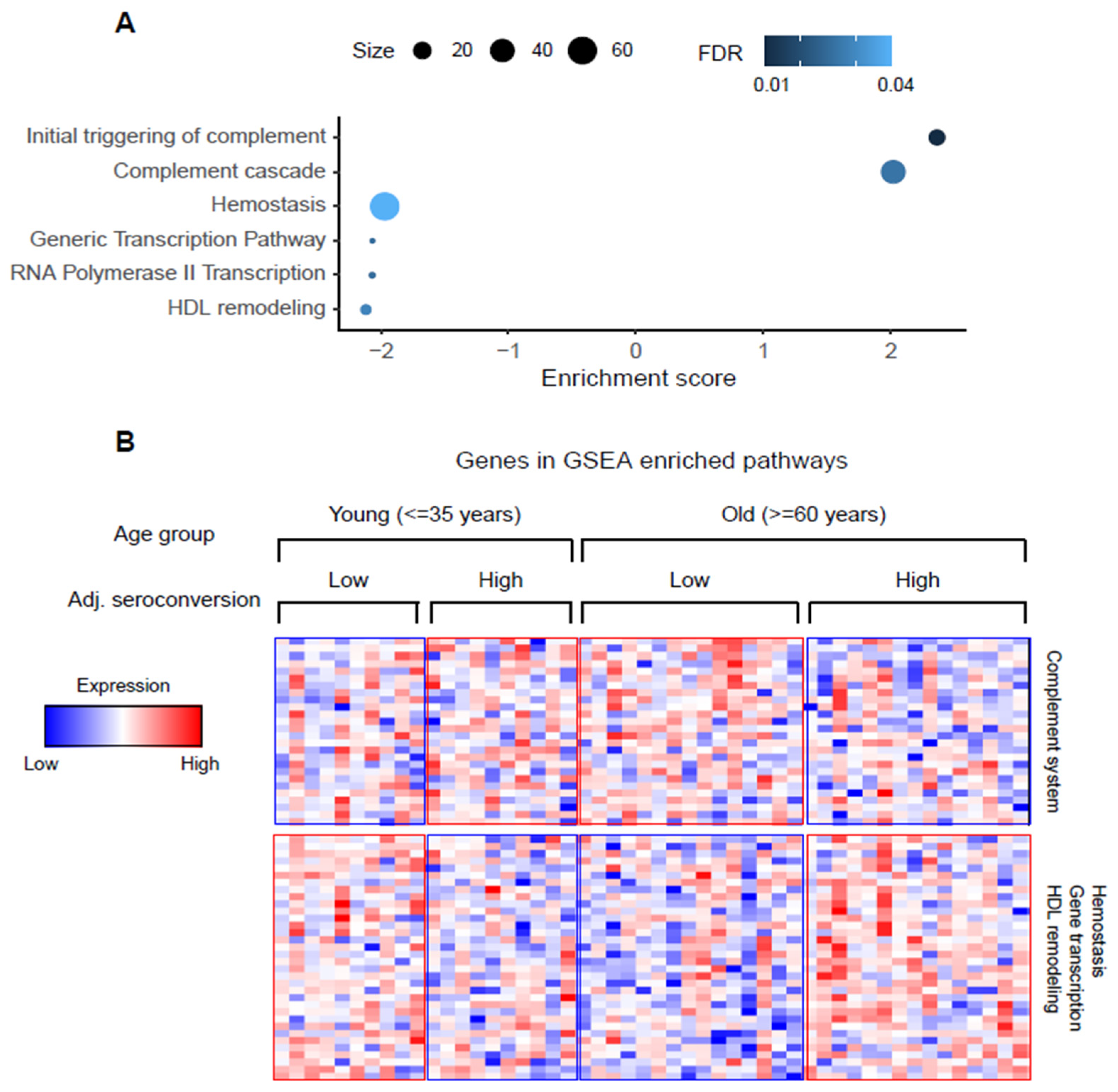
3.3. Association of Complement System, HDL Remodeling, Gene Transcription, and Hemostasis Pathways with Vaccine Response Changes with Age
3.4. Strain-Specific Differences in Vaccine-Response-Associated Protein Levels Changes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco, L.M.; Bucasas, K.L.; Wells, J.M.; Niño, D.; Wang, X.; Zapata, G.E.; Arden, N.; Renwick, A.; Yu, P.; Quarles, J.M.; et al. Integrative Genomic Analysis of the Human Immune Response to Influenza Vaccination. Elife 2013, 2, e00299. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.T.; Oberg, A.L.; Grill, D.E.; Ovsyannikova, I.G.; Haralambieva, I.H.; Kennedy, R.B.; Poland, G.A. System-Wide Associations between DNA-Methylation, Gene Expression, and Humoral Immune Response to Influenza Vaccination. PLoS ONE 2016, 11, e0152034. [Google Scholar] [CrossRef] [PubMed]
- Voth, D.W.; Feldman, H.A.; Steinschneider, A. Comparative Responses of Elderly Persons to Aqueous and Depot Influenza Vaccines. Arch. Environ. Health 1966, 13, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 Influenza Viruses Have a Glycosylation Site That Alters Binding of Antibodies Elicited by Egg-Adapted Vaccine Strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody Response to Influenza Vaccination in the Elderly: A Quantitative Review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Zheng, N.-Y.; Huang, M.; Cabanov, A.; Rojas, K.T.; Kaur, K.; Andrews, S.F.; Palm, A.-K.E.; Chen, Y.-Q.; Li, Y.; et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe 2019, 25, 357–366.e6. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Wu, S.; Ross, T.M.; Carlock, M.A.; Ghedin, E.; Choi, H.; Vogel, C. Evaluation of Determinants of the Serological Response to the Quadrivalent Split-inactivated Influenza Vaccine. Mol. Syst. Biol. 2022, 18, e10724. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of Immunological Markers in Predicting Responsiveness to Influenza Vaccination in Elderly Individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef]
- Van Duin, D.; Allore, H.G.; Mohanty, S.; Ginter, S.; Newman, F.K.; Belshe, R.B.; Medzhitov, R.; Shaw, A.C. Prevaccine Determination of the Expression of Costimulatory B7 Molecules in Activated Monocytes Predicts Influenza Vaccine Responses in Young and Older Adults. J. Infect. Dis. 2007, 195, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-Associated Decrease in TLR Function in Primary Human Dendritic Cells Predicts Influenza Vaccine Response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Poland, G.A. Vaccinomics, Predictive Vaccinology and the Future of Vaccine Development. Future Microbiol. 2010, 5, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- HIPC-CHI Signatures Project Team; HIPC-I Consortium. Multicohort Analysis Reveals Baseline Transcriptional Predictors of Influenza Vaccination Responses. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Qin, R.; Meng, G.; Pushalkar, S.; Carlock, M.A.; Ross, T.M.; Vogel, C.; Mahal, L.K. Prevaccination Glycan Markers of Response to an Influenza Vaccine Implicate the Complement Pathway. J. Proteome Res. 2022, 21, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.B.; Kirchenbaum, G.A.; Clutter, E.F.; Sautto, G.A.; Ross, T.M. Preexisting Subtype Immunodominance Shapes Memory B Cell Recall Response to Influenza Vaccination. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and Considerations for the Use of System Suitability and Quality Control Samples in Mass Spectrometry Assays Applied in Untargeted Clinical Metabolomic Studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Teo, G.; Chew, W.S.; Burla, B.J.; Herr, D.; Shyong Tai, E.; Wenk, M.R.; Torta, F.; Choi, H. MRMkit: Automated Data Processing for Large-Scale Targeted Metabolomics Analysis. Anal. Chem. 2020, 92, 13677–13682. [Google Scholar] [CrossRef]
- Eilers, P.H.C. A Perfect Smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef]
- Van der Kloet, F.M.; Bobeldijk, I.; Verheij, E.R.; Jellema, R.H. Analytical Error Reduction Using Single Point Calibration for Accurate and Precise Metabolomic Phenotyping. J. Proteome Res. 2009, 8, 5132–5141. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Teo, G.; Kim, S.; Tsou, C.-C.; Collins, B.; Gingras, A.-C.; Nesvizhskii, A.I.; Choi, H. mapDIA: Preprocessing and Statistical Analysis of Quantitative Proteomics Data from Data Independent Acquisition Mass Spectrometry. J. Proteom. 2015, 129, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Student. Student The Probable Error of a Mean. Biometrika 1908, 6, 1. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Alhammad, R.; Abu-Farha, M.; Hammad, M.M.; Thanaraj, T.A.; Channanath, A.; Alam-Eldin, N.; Al-Sabah, R.; Shaban, L.; Alduraywish, A.; Al-Mulla, F.; et al. Increased LRG1 Levels in Overweight and Obese Adolescents and Its Association with Obesity Markers, Including Leptin, Chemerin, and High Sensitivity C-Reactive Protein. Int. J. Mol. Sci. 2022, 23, 8564. [Google Scholar] [CrossRef]
- Tosheska Trajkovska, K.; Topuzovska, S. High-Density Lipoprotein Metabolism and Reverse Cholesterol Transport: Strategies for Raising HDL Cholesterol. Anatol. J. Cardiol. 2017, 18, 149–154. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Cholesterol Metabolism: From Lipidomics to Immunology. J. Lipid Res. 2022, 63, 100165. [Google Scholar] [CrossRef]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol Metabolism: A Review of How Ageing Disrupts the Biological Mechanisms Responsible for Its Regulation. Ageing Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Bedford, T.; Riley, S.; Barr, I.G.; Broor, S.; Chadha, M.; Cox, N.J.; Daniels, R.S.; Gunasekaran, C.P.; Hurt, A.C.; Kelso, A.; et al. Global Circulation Patterns of Seasonal Influenza Viruses Vary with Antigenic Drift. Nature 2015, 523, 217–220. [Google Scholar] [CrossRef]
- Satoh, M.; Takano, S.; Sogawa, K.; Noda, K.; Yoshitomi, H.; Ishibashi, M.; Mogushi, K.; Takizawa, H.; Otsuka, M.; Shimizu, H.; et al. Immune-Complex Level of Cofilin-1 in Sera Is Associated with Cancer Progression and Poor Prognosis in Pancreatic Cancer. Cancer Sci. 2017, 108, 795–803. [Google Scholar] [CrossRef]
- Paszek, E.; Zajdel, W.; Plens, K.; Żmudka, K.; Legutko, J.; Kleczyński, P. Low Profilin 1 Serum Levels Are Associated with Diabetes, Family History and Multivessel Lesions in Patients with Coronary Artery Disease. Postep. Kardiol. Interwencyjnej 2021, 17, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Penninger, J.M.; Crabtree, G.R. The Actin Cytoskeleton and Lymphocyte Activation. Cell 1999, 96, 9–12. [Google Scholar] [CrossRef]
- Papa, R.; Penco, F.; Volpi, S.; Gattorno, M. Actin Remodeling Defects Leading to Autoinflammation and Immune Dysregulation. Front. Immunol. 2020, 11, 604206. [Google Scholar] [CrossRef] [PubMed]
- Sprenkeler, E.G.G.; Webbers, S.D.S.; Kuijpers, T.W. When Actin Is Not Actin’ Like It Should: A New Category of Distinct Primary Immunodeficiency Disorders. J. Innate Immun. 2021, 13, 3–25. [Google Scholar] [CrossRef]
- Dustin, M.L.; Cooper, J.A. The Immunological Synapse and the Actin Cytoskeleton: Molecular Hardware for T Cell Signaling. Nat. Immunol. 2000, 1, 23–29. [Google Scholar] [CrossRef]
- Assinger, A. Platelets and Infection—An Emerging Role of Platelets in Viral Infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Pushalkar, S.; Maity, S.; Pressler, M.; Rendleman, J.; Vitrinel, B.; Carlock, M.; Ross, T.; Choi, H.; Vogel, C. Proteomic Signatures of the Serological Response to Influenza Vaccination in a Large Human Cohort Study. Viruses 2022, 14, 2479. https://doi.org/10.3390/v14112479
Wu S, Pushalkar S, Maity S, Pressler M, Rendleman J, Vitrinel B, Carlock M, Ross T, Choi H, Vogel C. Proteomic Signatures of the Serological Response to Influenza Vaccination in a Large Human Cohort Study. Viruses. 2022; 14(11):2479. https://doi.org/10.3390/v14112479
Chicago/Turabian StyleWu, Shaohuan, Smruti Pushalkar, Shuvadeep Maity, Matthew Pressler, Justin Rendleman, Burcu Vitrinel, Michael Carlock, Ted Ross, Hyungwon Choi, and Christine Vogel. 2022. "Proteomic Signatures of the Serological Response to Influenza Vaccination in a Large Human Cohort Study" Viruses 14, no. 11: 2479. https://doi.org/10.3390/v14112479
APA StyleWu, S., Pushalkar, S., Maity, S., Pressler, M., Rendleman, J., Vitrinel, B., Carlock, M., Ross, T., Choi, H., & Vogel, C. (2022). Proteomic Signatures of the Serological Response to Influenza Vaccination in a Large Human Cohort Study. Viruses, 14(11), 2479. https://doi.org/10.3390/v14112479






