1. Introduction
In non-pandemic years, seasonal influenza generally imposes considerable mortality and morbidity burdens resulting in three to five million severe illnesses per year and 290,000 to 650,000 respiratory deaths worldwide [
1]. Public health initiatives to annually administer the seasonal influenza vaccine, targeting predominant circulating influenza strains, are an effective means of mitigating the impact of the virus. However, the effectiveness of the seasonal vaccine—defined as the percent reduction in the frequency of influenza illness among vaccinated individuals compared to the frequency among unvaccinated individuals [
2]—shows considerable variation between seasons and among individuals within a season. Between season variation in vaccine effectiveness is primarily attributed to mismatches between the vaccine strain selection and circulating viruses. By contrast, within season variation in vaccine effectiveness is impacted by both viral and host factors, including age, preexisting health conditions such as obesity [
3], and prior vaccination and infection history. Understanding the sources of interindividual variation in vaccine effectiveness is critical to the development of robust vaccination strategies.
Host biomolecular factors may be an underlying source of interindividual variation in vaccine effectiveness, specifically antibody repertoires. A number of observational studies provide evidence that pre-existing immunity, due to either prior infection or vaccination, may impact the immune response to vaccination, a phenomenon known as “original antigenic sin” [
4,
5]). The immune response to infection may be dominated by antibodies to previously encountered hemagglutinin (HA) epitopes, resulting in varying vaccine effectiveness that depends largely on an individual’s age and vaccination history. Additionally, antigenic similarity between consecutive vaccine strains may dampen an individual’s ability to generate antibodies following repeated vaccinations. Antibodies from the initial encounter may mask similar vaccine epitopes in the second encounter, resulting in a diminished response, a phenomenon known as the antigenic distance hypothesis [
6,
7].
Preexisting immunity and vaccine response can be assessed using the hemagglutination inhibition (HAI) assay, which detects the presence of antibodies that prevent the HA protein of the influenza virus from agglutinating red blood cells [
8]. Vaccine response is quantified on the basis of an increase in antibody titer, which can be inferred using the HAI assay for which a 4-fold rise in antibody titers is typically used to define seroconversion [
9]. Although not a direct measure of vaccine effectiveness, there is strong evidence that seroconversion is predictive of vaccine effectiveness [
10]. However, a caveat to the serological-based assessment of vaccine response using the HAI assay is that it may be inaccurate if most antibodies target the neuraminidase [
11]. HAI tests with oseltamivir are used to eliminate such effects [
12].
Prior studies have shown that there is considerable variation in the capacity to respond to vaccination [
13] with reduced responsiveness associated with biological variables such as sex [
14]. Differences in gene expression may be related to, and predictive of, variation in vaccine effectiveness. For example, one large-scale study identified nine genes and three gene modules that show significant expression variation associated with the magnitude of the antibody response [
15]. Other studies have identified age-associated differences in gene expression that are associated with differences in vaccine response [
16]. These studies indicate that gene expression profiles could be informative about how well an individual will respond to vaccination. However, whether these findings hold in other cohorts and the interactive effects of biological and molecular factors on the predictive value of gene expression in the context of the vaccine response have not previously been assessed.
In this study we sought to determine whether whole blood mRNA expression profiles are informative about pre-vaccination immunity and if they can be used to predict the response to influenza vaccination. We analyzed gene expression prior to vaccination and identified genes that are differentially expressed reflecting differing pre-vaccination immune states. We then tested whether gene expression differences were associated with differences in vaccine response among individuals. We find that a predictive model of vaccine response that uses only the gene expression state of individuals prior to vaccination shows comparable performance to a model that uses only physiological factors (e.g., body mass index (BMI), sex, and age) and knowledge of prior vaccination history. A model that incorporates both gene expression and physiological and demographic factors outperforms either of these models indicating that they contain complementary information. Our study demonstrates that gene expression states prior to vaccination show variation that reflects the preexisting immunological state of individuals. Furthermore, these profiles can enhance prediction of vaccine response when used in combination with physiological information and vaccination history.
3. Results
To investigate the utility of global gene expression profiling in predicting the response to seasonal influenza vaccination, we studied a cohort of participants recruited by the University of Georgia (UGA). The study included a total of 275 participants from a cohort herein referred to as UGA4 that was conducted during the 2019–2020 influenza season. Volunteers provided a blood sample for baseline serology prior to vaccination (Day 0). They were then given a commercially available, seasonal influenza split inactivated virus vaccine (Fluzone, Sanofi Pasteur), and subsequently provided a blood sample 28 days after vaccination to assess response serology. The vaccine was formulated with influenza A strains for H1N1 (A/Brisbane/2/2018) and H3N2 (A/Kansas/14/2017), in addition to influenza B strains for Victoria (B/Colorado/6/2017) and Yamagata (B/Phuket/3073/2013). Standard dose (SD) quadrivalent vaccines (0.5 mL dose with 15 µg HA/strain or 60 µg HA total) were administered to most participants (n = 214); however, those participants aged 65 years and older were given the option of a high-dose (HD) formulation (trivalent 0.5 mL dose with 60 µg HA/strain or 180 µg HA total; n = 61) in which the Yamagata component was excluded.
The 275 study participants included adult males (
n = 102) and females (
n = 173), ranging in age from 18–85 years (mean ± one standard deviation age = 50.3 ± 17.2 years). Self-declared race was recorded: study participants predominantly self-identified as white (
n = 220), with a small number of black (
n = 27), hispanic (
n = 17), asian (
n= 6), and Native American (
n = 2) participants. Body mass index (BMI) of participants ranged from 17.43 to 59.1 with 199 of the 275 study participants exceeding a BMI of 25 (
Table 1,
Table S1) which is classified as overweight using standard CDC definitions [
27]. Participants were assessed for risk factors including smoking (
n = 94 previous or current smokers) and comorbidities of which hypertension was the most common (
n = 49). Overall, health metrics are consistent with enrichment for high risk factors among study participants.
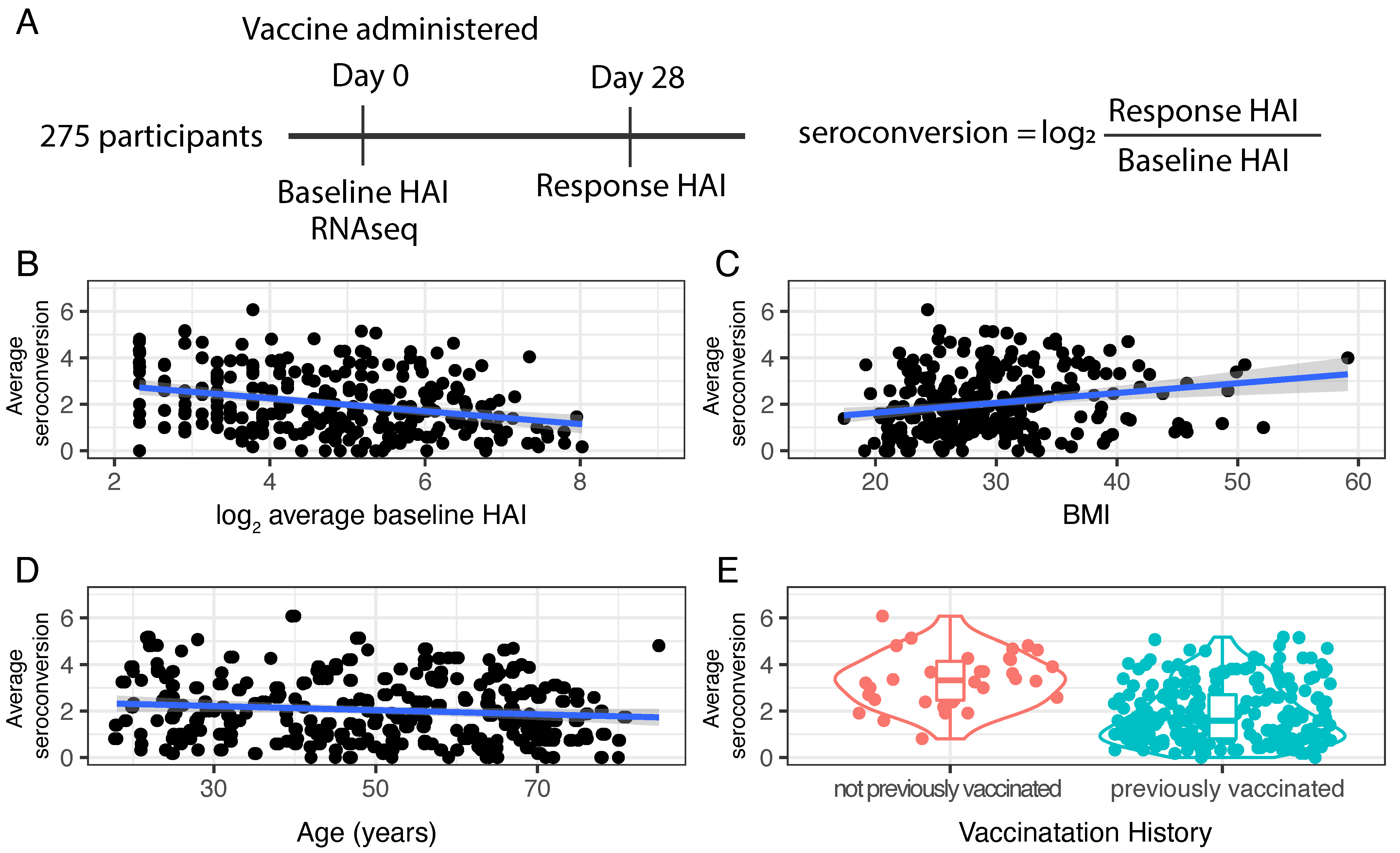
Whole blood samples were acquired from participants prior to and following vaccination (
Figure 1A). To assess immunogenicity to the influenza HA protein, a hemagglutination inhibition (HAI) assay was performed using two-fold serial dilutions of serum from each participant and turkey red blood cells (RBCs) (
Section 2). A numerical HAI value was assigned based on the reciprocal of the final serum dilution at which RBC agglutination inhibition was still observed. For example, if an individual’s serum sample inhibited viral-mediated agglutination of RBCs at a dilution of 1:40, but did not inhibit agglutination at a dilution of 1:80, an HAI score of 40 was assigned. The HAI assay was performed with serum diluted 1:5 using a two-fold dilution series of serum (i.e., 1:10, 1:20, 1:40, …). Thus, HAI values vary from 5 to 1280 with an HAI of 5 corresponding to no detectable antibody titer and higher values reflecting higher antibody titers.
To test the specificity of immunogenicity, HAI assays were performed with each of the four vaccine strains prior to vaccination (day 0, or “baseline HAI”) and four weeks post-vaccination (day 28, or “response HAI”) for all participants (
Section 2). However, because the Yamagata strain was not included in the HD formulation the day 28 HAI value was excluded in the serology metrics calculated for those individuals who received the HD vaccine. To account for the different number of strains used in the two formulations we computed average baseline and response serology metrics.
The distribution of baseline HAI values for the four different strains varies between individuals and strains (
Figure S1) reflecting significant variation in strain-specific pre-existing immunity among individuals. We observed an increase in the distribution of HAI values for all four strains 28 days after vaccine administration (
Figure S2). High response HAI values for each of the four strains reflect both an immunogenic response to the administered vaccine and pre-existing immunity. In general, baseline and response HAI values for each strain show positive correlation (
Figure S3). However, individuals with low baseline HAI values show extensive variation in response HAI.
To quantify the response to vaccination, a seroconversion score was computed for each strain by taking the ratio of the response HAI to the baseline HAI. Seroconversion scores were computed separately for each of the four influenza strains and expressed as log
2 value (i.e., a seroconversion score of 1 corresponds to a twofold increase in HAI value). We find that there is a negative correlation (ρ ≤ −0.2) between baseline HAI and seroconversion for each of the four strains (
Figure S4) as high seroconversion scores are generally only observed in those participants with low baseline HAI values. Seroconversion with respect to the Yamagata strain is significantly higher in those individuals who received the SD formulation compared with those receiving the HD formulation, which lacks the Yamagata component, consistent with a specific response to vaccine components (
Figure S5). Moreover, with a single exception, none of the participants receiving the HD vaccine had a seroconversion score greater than 2 (i.e., a fourfold increase in HAI) for the Yamagata strain supporting the use of a fourfold increase as indicative of a specific antibody response.
To define an average seroconversion score that incorporates information for each strain we computed the average untransformed seroconversion score for the four (SD) or three (HD) strains and expressed the average as a log
2 value (adapted from [
28]). In general, we treated seroconversion scores as continuous values; however, to increase the statistical power of some analyses, we defined a categorized seroconversion score. Therefore, for these analyses we categorized average seroconversion as “low” (<2, which corresponds to less than a fourfold average change in HAI) or “high” (≥2, which corresponds to a fourfold or higher average change in HAI), consistent with the US Food and Drug Administration Guidance [
29]
Average baseline HAI values, incorporating information from all strains, are negatively correlated with average seroconversion scores (ρ = −0.34,
p-value = 1.12 × 10
−8;
Figure 1B). Among our study participants we find a significant positive relationship between BMI and seroconversion (ρ = 0.21,
p-value = 0.0005) (
Figure 1C) and a slight negative correlation between age and seroconversion (ρ = −0.13,
p-value = 0.02;
Figure 1D). Seroconversion does not differ significantly between males and females (F statistic = 3.435,
p-value = 0.649).
The majority of study participants received at least one vaccine in the three years prior to the study. However, a small number of participants (
n = 34) reported no prior vaccination. We find that these individuals have significantly higher seroconversion scores (
t-statistic = −61.8,
p-value = 2.2 × 10
−16) (
Figure 1E) consistent with immunologically naive individuals mounting an enhanced response to vaccination.
3.1. Differential Gene Expression Analysis
We performed whole transcriptome analysis of whole blood samples for all study participants using RNA sequencing (RNAseq) and processed sequencing data using standard bioinformatic pipelines (
Section 2) to generate a table of gene counts (
Table S2). We obtained a median of 15 million reads per sample (
Figure S6). Within samples, the distribution of counts per gene is highly skewed as just three genes, encoding hemoglobin, account for almost 50% of the total transcript counts in each sample (
Figure S7). Therefore, these three genes were excluded from downstream analyses (
Section 2). To identify major sources of variation in the data we performed dimensionality reduction analysis. More than 50% of the variance in the data is captured by the first three principal components (
Figure S8). However, visual inspection failed to identify covariates that were non-randomly distributed across these principal components.
We sought to identify genes that show evidence for differences in expression as a function of study participant physiological and serological metrics (
Table 1). Therefore, we performed differential gene expression analysis to identify genes with statistically significant differences in expression (methods). Variables that had highly unbalanced categories (i.e., prior vaccination history, and self-reported race) were not considered. We performed differential gene expression analyses using either discrete groups (e.g., for sex) or linear models for continuous variables (e.g., age, BMI, HAI, and seroconversion). We assessed differential gene expression as a function of (1) log
2 transformed average baseline HAI, to identify genes that are differentially expressed with variation in pre-existing immunity, and (2) log
2 transformed average seroconversion, to identify genes whose expression prior to vaccination are predictive of the vaccine response.
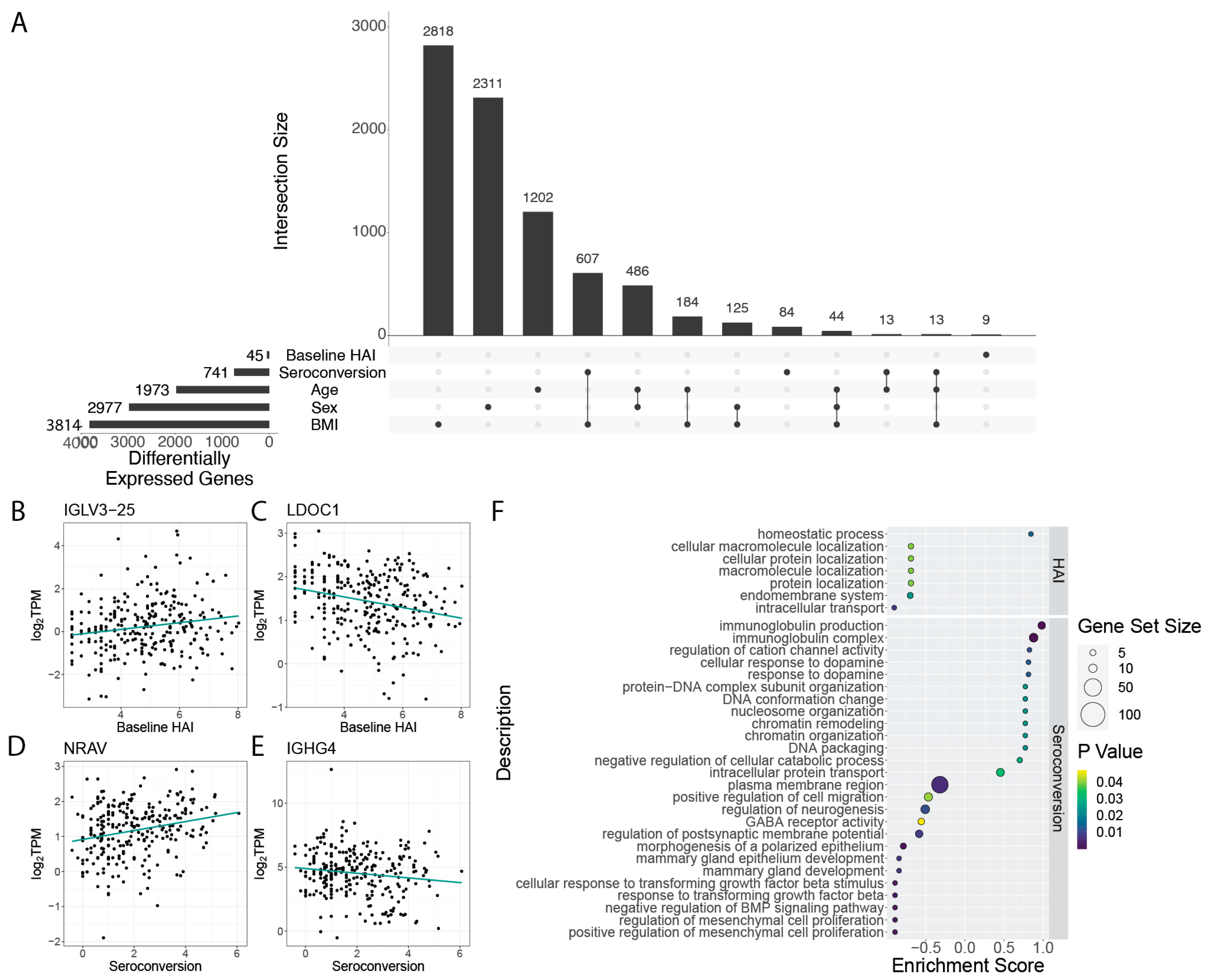
When considering the pre-existing immune status of individuals we find only 45 genes that are differentially expressed as a function of baseline HAI (
Figure 2A,
Table S3). 9 of these genes were uniquely differentially expressed as a function of baseline HAI, while the other 36 were also differentially expressed as a function of different physiological factors. Among the genes that are significantly increased in expression with higher baseline HAI levels are immunoglobulin genes, including
IGLV3-25 (
Figure 2B), whereas some genes, such as
LDOC1, show significant negative expression relationships with baseline HAI (
Figure 2C). Another immunoglobulin gene,
IGLV4-69 (logFC = 4.52, FDR = 7.91 × 10
−8), is also increased in expression with baseline HAI. Other immunoglobulin genes are also among the 45 differentially expressed genes, and include
IVLG8-61 (logFC = 3.88, FDR = 1.89 × 10
−5),
IGLV4-60 (logFC = 3.32, FDR = 1.59 × 10
−3),
IGLV3-
10 (logFC = 2.79, FDR = 4.63 × 10
−3), and
IGLV3-1 (logFC = 1.71, FDR = 3.1 × 10
−2) (
Figure S9). Among the genes that show the greatest negative relationship to baseline HAI are non-immunoglobulin genes that encode tryptase gamma 1 (
TPSG1, logFC = −5.82, FDR = 7.91 × 10
−8) and fibromodulin (
FMOD, logFC = −5.58, FDR = 1.63 × 10
−7). Immunoglobulins are expected to be associated with pre-existing immunity and their increased expression may reflect greater plasmablast cell populations in whole blood from individuals with higher baseline HAI. By contrast, the reduced expression of
TPSG1 with increasing baseline HAI may reflect the role of mast cell protease tryptase gamma 1 in mediation of IL-13/IL-4R/STAT6-dependent proinflammatory pathways via T-cell induction [
30]. Quenching of proinflammatory pathways may be required for maintaining a high level of immune response as lower levels of TPSG1 indicate a lower risk of COVID-19 hospitalization [
31].
FMOD encodes a member of the small interstitial proteoglycans which may play a role in the assembly of the extracellular matrix as well as in regulating TGFβ activity.
When considering differential expression as a function of seroconversion, we identified a set of 84 genes that are uniquely differentially expressed (i.e., show no significant gene expression differences with other participant metrics). A larger set of 741 genes are differentially expressed as a function of seroconversion, but also show significant association with at least one other factor (
Figure 2A; note that smaller subsets with fewer than 9 shared genes are omitted to improve visualization quality,
Table S4). These genes exhibit both increased (
Figure 2D) and decreased (
Figure 2E) expression with increasing seroconversion. The most significantly differentially expressed genes among this gene set include an uncharacterized transcript
ENSG00000273956 (FDR = 7.52 × 10
−22), and immunoglobulin genes
IGLV4-69 (FDR = 6.07 × 10
−11) and
IGLV8-61 (FDR = 5.43 × 10
−7) (
Figure S10). Interestingly,
IGLV4-69 has been identified as a component of a monoclonal antibody in a prior vaccination cohort and possesses a broad spectrum binding affinity against a broad range of H3N2 strains, including against the A/Hong Kong/4801/2014 (HK14) vaccine strain used in the 2019–2020 season [
32].
To determine the functions of genes that are differentially expressed depending on baseline HAI and seroconversion score, we performed gene set enrichment analysis (GSEA) of the set of significant genes, (
Figure 2F). Gene functions that are related to increased baseline HAI include those involved with homeostatic processes. Gene functions that are positively associated with increased seroconversion include immunoglobulin production, and chromatin organization. Conversely, we find gene functions related to growth factor beta responses and neurogenesis to be decreased in expression in individuals with high seroconversion scores. (
Figure 2F).
3.2. Interactive Effects on Differential Gene Expression
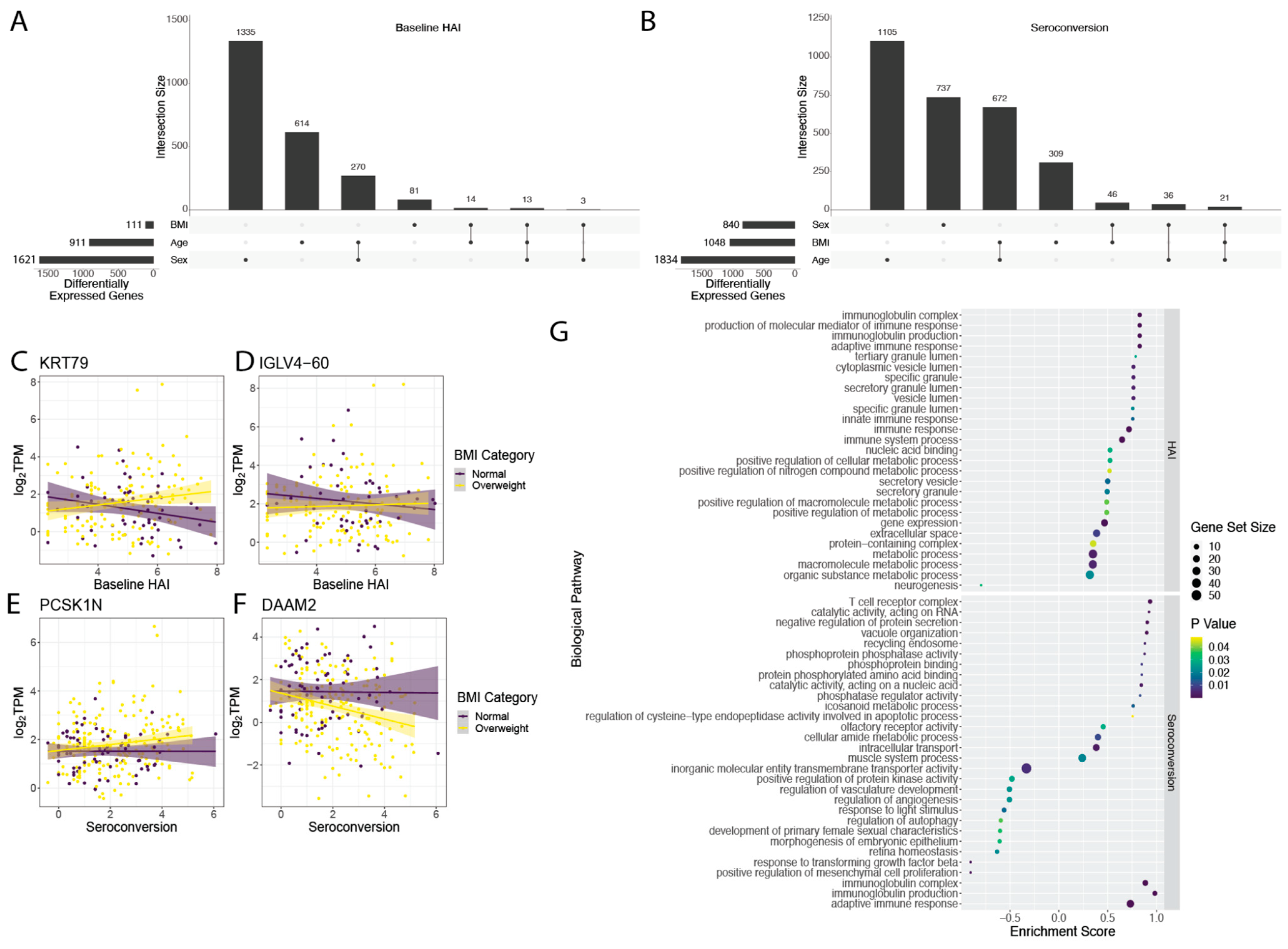
Genes that are differentially expressed as a function of immune status may show differing behaviors depending on other physiological factors. To test for interactive effects between immunological metrics and factors that vary among study participants such as age, BMI, and sex, we used bivariate linear models for both baseline HAI and seroconversion score. We found a number of genes with interactive effects between baseline HAI and sex (1621), age (911), and BMI (111) (
Figure 3A). In our bivariate analysis with seroconversion and other covariates, we found the greatest number of interactive effects between seroconversion and age (1834 genes) (
Figure 3B). We also found a set of 1048 genes that show significant interactive effects between seroconversion and BMI, and 840 genes that display significant interaction effects between seroconversion and sex (
Figure 3B).
We were particularly interested in the interaction between BMI and either baseline HAI or seroconversion as BMI has been identified as one of the major factors associated with increased influenza risk [
3]. The differential expression of 111 genes with baseline HAI is influenced by BMI (
Figure 3B and
Figure S11, Table S5). For example,
KRT79 increases in expression with higher baseline HAI in overweight individuals, but decreases in individuals with normal BMI (
Figure 3C). A similar effect of BMI on the relationship between gene expression and baseline HAI is observed for
IGLV4-60 (
Figure 3D). The differential expression of 1048 genes with seroconversion is influenced by BMI (
Figure 3B and
Figure S12, Table S6). For example,
PCSK1N increases in expression with seroconversion in overweight individuals, but not individuals with normal BMI (
Figure 3E). Conversely,
DAAM2 decreases in expression with seroconversion in overweight individuals but not in individuals with normal BMI (
Figure 3F).
We investigated enrichment of gene sets that show significant interaction between BMI and either baseline HAI or seroconversion. Genes that show interactive effects with BMI and baseline HAI are enriched for immunoglobulin production and immune responses (
Figure 3G upper panel). Genes that show interactive effects between BMI and seroconversion are positively enriched for diverse functions including metabolism, immunoglobulins and the adaptive immune response, and T cell receptor functions (
Figure 3G lower panel).
3.3. Identification of Gene Expression Modules Related to Serological Outcome
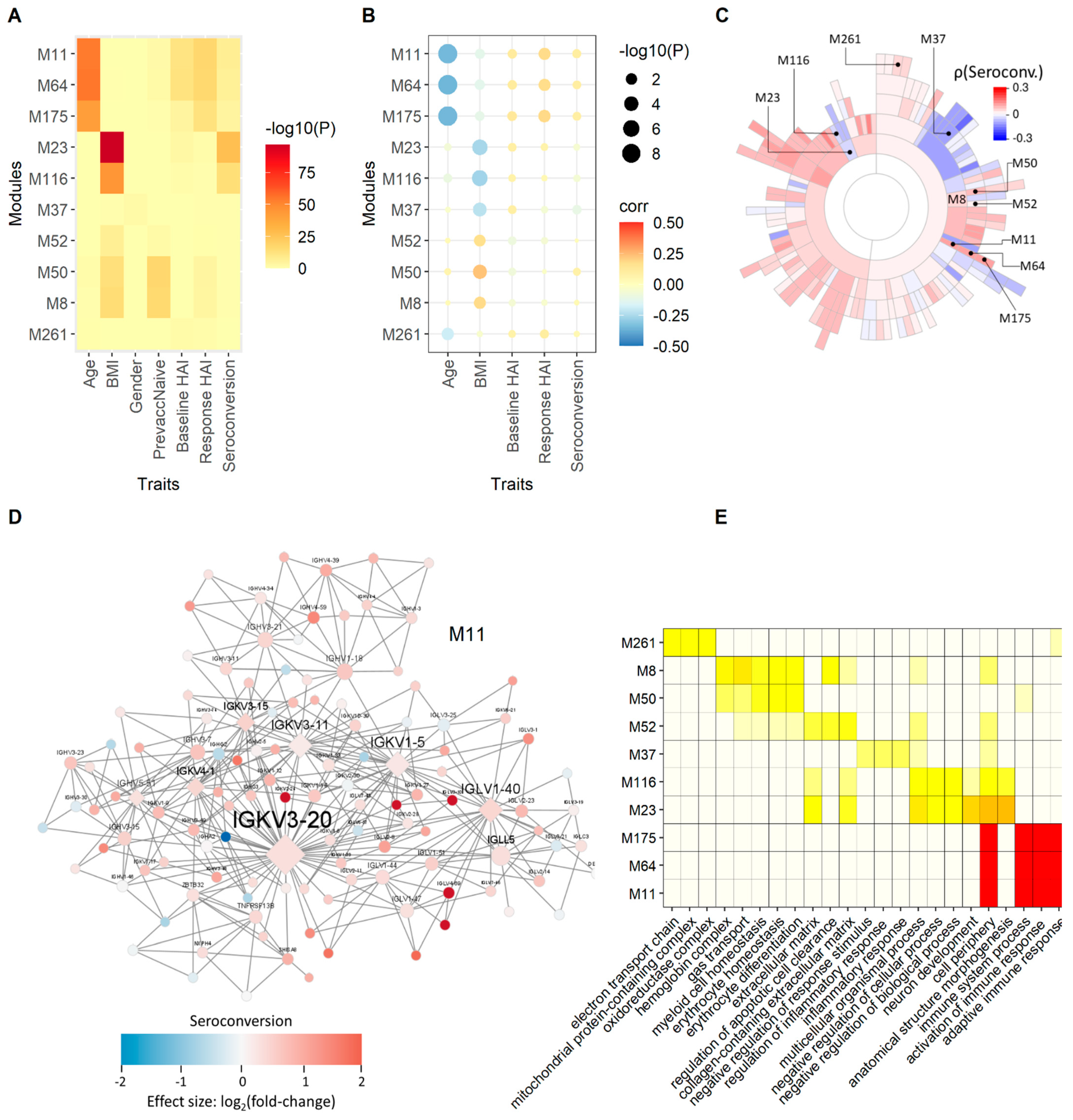
We sought to identify molecular processes that show coherent gene expression behavior among individuals. Therefore, we defined sets of genes that show correlated co-expression patterns across individuals using MEGENA (
Section 2). The MEGENA method identifies modules of correlated gene expression across samples and defines a hierarchical relationship between modules defined by different levels of stringency. Using the 275 transcriptomes, we identified 184 hierarchically ordered expression modules containing between 5 to 2450 genes.
We employed different measures based on correlation, differential gene expression enrichment, and a combination of both, to quantify the association between modules and physiological and serological metrics. We use the product of rank method (
Section 2) to compute an aggregated rank from individually ranked modules. The top ten highest ranked modules after enrichment of modules for baseline HAI, response HAI and seroconversion differentially expressed gene (DEG) signatures, are related to the adaptive immune response and immunoglobulin complexes (
Figure 4). These modules are strongly enriched for genes that are differentially expressed as a function of response HAI, but show only marginal enrichment for genes that are differentially expressed as a function of baseline HAI and seroconversion (
Figure 4A). However, we find significant positive correlation between these modules and all three serological scores (
Figure 4B). The hierarchical organization of five of the top ten modules is M11 → M64 → M175 and M23 → M116, where M11 is the parent module that includes each subset of smaller modules (
Figure 4C). Module M11 contains many immunoglobulin genes that show increased expression in subjects with high seroconversion (
Figure 4D). The most highly connected, or hub genes, of M11 are the variable chain immunoglobulin genes
IGHV5-51,
IGKV1-5,
IGKV3-11,
IGKV3-15,
IGKV3-20,
IGKV4-1, and
IGLV1-40. It is noteworthy that a proteomic analysis of influenza haemagglutinin-specific antibodies following H1N1pdm09 influenza A vaccination identified
IGKV3-20 as the dominant light chain variant [
33]. Modules defined by the M11 module family are enriched for functions related to the adaptive immune response (
Figure 4E). Interestingly, these modules are also strongly negatively correlated with age (
Figure 4B). This points to a potential indirect effect of age on serological outcome manifested in the expression state of these immunoglobulin-enriched modules that may underlie the observed negative relationship between age and seroconversion (
Figure 1D).
Additional highly ranked modules are suggestive of other relationships between gene expression and serology. For example, module M37 is enriched for inflammatory response and is negatively correlated with seroconversion (
Figure 4C) and positively correlated with baseline HAI. The module family M23 → M116, is enriched for mRNA 5′-splice site recognition, lyase activity regulation and extracellular matrix function and is positively correlated with baseline HAI. The most significant module relationships are found with age and BMI (
Figure S13). The module with the most significant correlation with age is M192 (ρ = −0.63, FDR = 4.58 × 10
−30), which contains 15 genes, including the type I transmembrane glycoprotein
CD248, Rho guanine nucleotide exchange factor 4 (
ARHGEF4), Wnt signaling factor regenerating family member 4 (REG4). M192 also has fibroblast growth factor 1 (
FGFR1) functionality (
PTK7,
CD248,
PHGDH,
FBLN2; FDR = 0.006).
With respect to age association, most modules are enriched for age-specific differential gene expression (
Figure S14A) and all are strongly correlated with age (
Figure S14B). Interestingly, the modules with the most significant enrichment for age-specific DEGs are the same as the highest ranked modules based on the serological response (
Figure 4). The module M11 → M64 → M175, which is enriched for immunoglobulin functions, is negatively correlated with age and positively correlated with baseline HAI, response HAI, and seroconversion. Although M192 is enriched for GO functions related to nervous system development, it involves hub-gene CD248 related to the promotion of CCL17 expression in pro-fibrotic macrophages (
Figure S14D). Another example of an age-related DEG enriched module is M311. This module is positively correlated with age and does not show any significant correlation with the serological responses. M311 is enriched for molecule transducer and signaling receptor activity with serine protease 23 (
PRSS23) as a key regulator (
Figure S14E).
With respect to BMI, the highest ranked module is M10 with basophil/mast cell regulation functions. M10 and related module M62 are weakly positively correlated with Baseline HAI values (
Figure S15B). Key regulators histidine decarboxylase (
HDC) and IgE-receptor β-subunit
MS4A2 modulate immune response (
Figure S15D). The IgE-receptor is expressed on basophils and mast cells, required for a robust vaccination response [
34]. A second set of key regulators involve A-kinase anchoring protein 12 (
AKAP12) and GATA binding protein
GATA2. Both genes are responsible for cell proliferation and migration. A second example of a module enriched for BMI DEGs is M13 (
Figure S15E). This module is positively correlated with both age and BMI but not with serological responses. M13 is enriched for immune effector process functions involving defensins including the key regulator, CEA cell adhesion molecule 8 (
CEACAM8), a cell-adhesion protein in neutrophils, and defensin
DEFA4, which is involved in host defense.
3.4. Prediction of Vaccine Response
We tested the predictive value of physiological information and gene expression with respect to seroconversion. For this purpose, we generated random forest models using different combinations of three distinct classes of features: (1) physiological metrics, (2) expression of genes that are differentially expressed as a function of seroconversion, and (3) co-expression modules. For our set of differentially expressed genes, we tested three subsets to account for the possibility of overfitting. We used gene sets consisting of (1) all 741 genes differentially expressed as a function of seroconversion, (2) the top 75% of seroconversion differentially expressed genes sorted by FDR, the same fraction used for our training set, and (3) a set of 84 genes differentially expressed exclusively as a function of seroconversion and no other physiological data. We initially attempted to predict quantitative seroconversion scores, but determined that all models performed poorly. Therefore, we classified individual seroconversion scores as high (average seroconversion ≥ 2, n = 124) or low (average seroconversion < 2, n = 151) for the purpose of prediction.
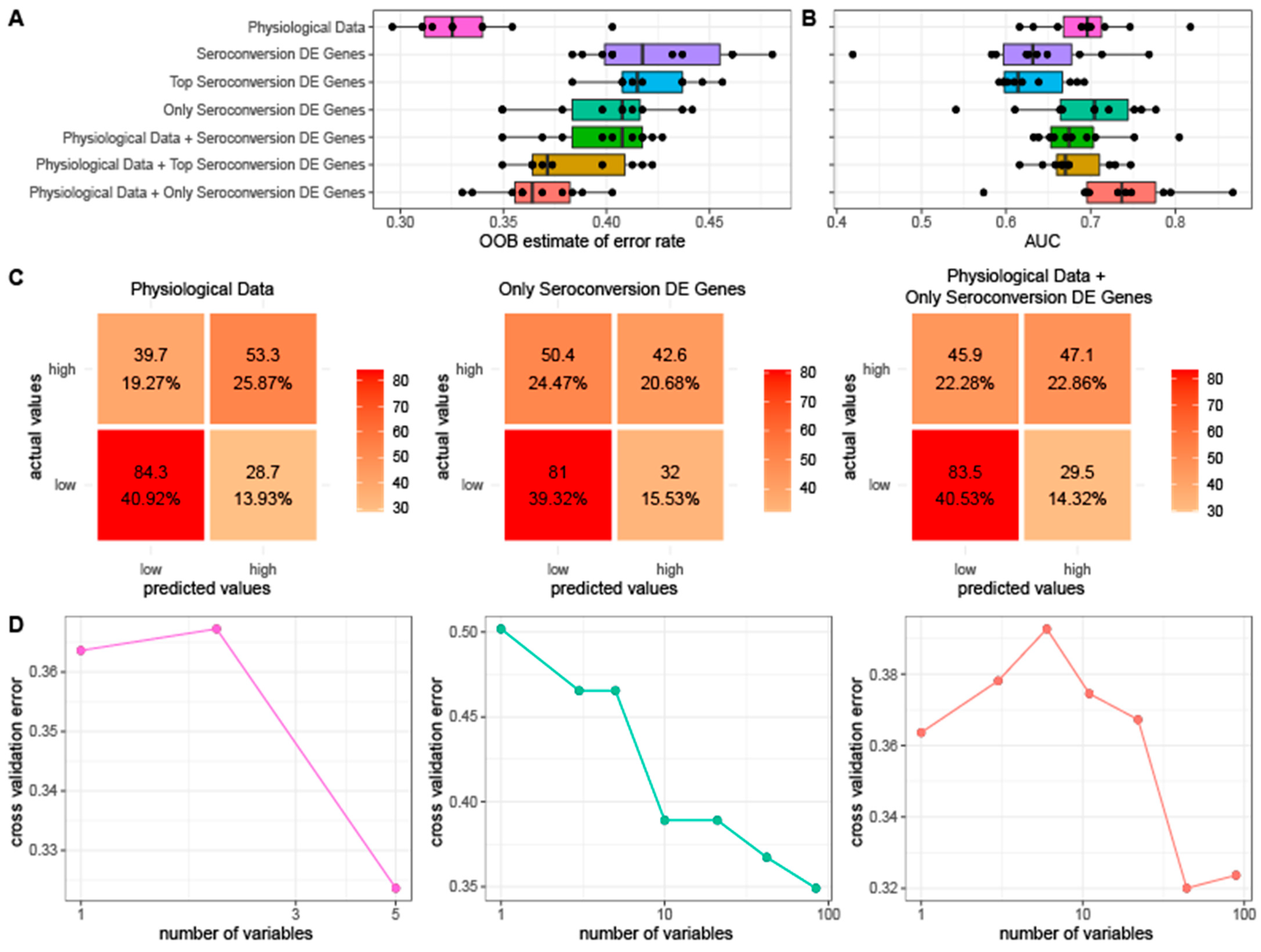
When used to predict seroconversion, we find that a random forest model generated using only physiological metrics results in the lowest out-of-bag (OOB) error rate. The random forest models generated using only sets of differentially expressed genes yield the highest OOB error rate whereas the combined data results in an intermediate error (
Figure 5A). We then evaluated the accuracy of each model’s average predictions by using a receiver operating characteristic (ROC) curve and calculating the resulting area under the curve (AUC). The random forest model generated using a combination of the physiological data and the expression data of the set of 84 genes exclusively differentially expressed as a function of seroconversion yielded the highest average AUC of 0.73 while all other models have AUCs less than 0.70 (
Figure 5B).
To confirm that our results are reproducible, we repeated our OOB and AUC tests varying the number of initial trees from 50 to 5000. We obtained similar results for each test in that the model generated using only physiological data had the lowest OOB error while the model generated using physiological data combined with the set of 84 DE genes had the best AUC regardless of the number of initial trees (
Figure S16). Of our data sets that only use expression data, the smallest set of 84 genes that were only differentially expressed as a function of seroconversion performs the best. Similarly, when combining expression data with physiological data for our models, using the set of 84 genes results in the best performance.
To identify the specific instances for which our random forest models fail, we generated confusion matrices using the average predictions of each of three of the datasets consisting of (1) only physiological data, (2) expression data of the 84 exclusively differentially expressed genes, and (3) the two data sets combined (
Figure 5C). All three random forest models have difficulty correctly categorizing high seroconversion, often mistakenly categorizing them as low. However, when assessed using the test dataset, the random forest model generated using only physiological data accurately classifies 53.3 of the 93 high seroconversion individuals on average compared to the models generated using only expression data (42.6 of 93 correctly assigned) and clinical data paired with expression data (47.1 of 93 correctly assigned), respectively.
We performed cross validation analyses for each of the three random forest models to assess the reproducibility of the model on unseen data as well as to identify the optimal number of features for each model. We find that for models that contain physiological data the error is lowest when less than 5 features are used (
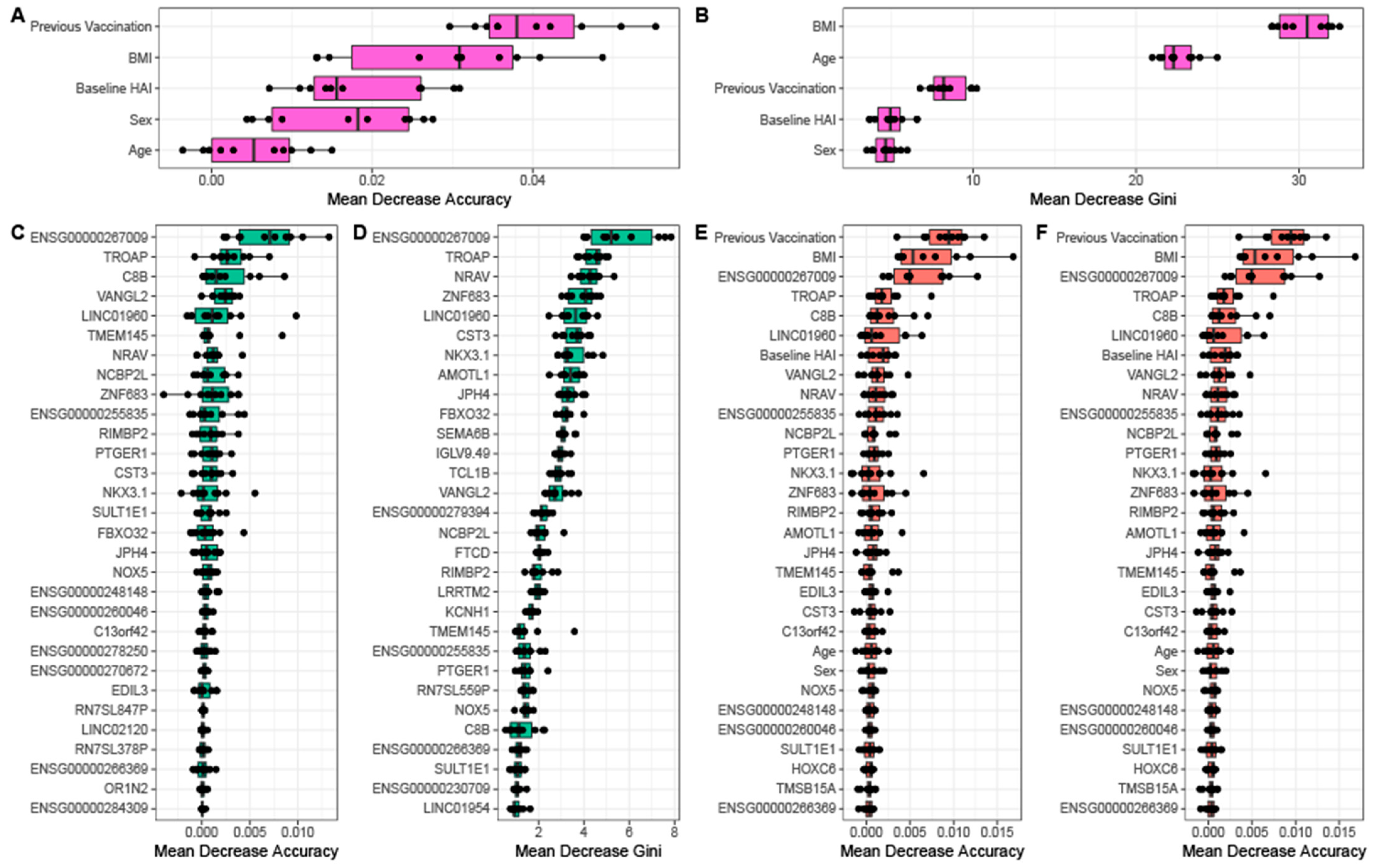
Figure 5D). By comparison, the random forest generated using only gene expression data requires almost all features to obtain a similar predictive error. This suggests that physiological information is more informative about serological response than baseline gene expression. For each random forest model, we calculated and ranked the average importance value for each feature (
Figure 6). When using a model with only physiological metrics to predict seroconversion, prior vaccination status is the most important variable (
Figure 6A), but BMI, baseline HAI, sex and age also contribute to model performance and reproducibility as measured using the GINI coefficient (
Figure 6B). When using only the set of 84 differentially expressed genes we find that removal of individual genes has incremental effects on model performance as measured by the decrease in model accuracy (
Figure 6C) and GINI coefficient (
Figure 6D) as do models that combine physiological metrics and differentially expressions genes (
Figure 6E,F). This is consistent with no single gene having an expression value that is especially information of seroconversion. However, we do find that the non coding RNAs
ENSG00000267009,
LINC01960, negative regulator of antiviral response
NRAV as well as complement C8B, novel protein
ENSG00000255835, trophinin associated protein
TROAP, and planar cell polarity protein 2
VANGL2 consistently make the most significant contribution to seroconversion prediction.
We further investigated whether prediction of seroconversion outcome would improve with the inclusion of the aggregated module data generated using MEGENA. Combination of one of principal components 1–5 of the module data from the top 20 ranked modules with the physiological data and DEGs yields a comparable OOB error rate but a decreased AUC compared to random forest models generated with physiological data with DEGs (
Figure S17). An exception is the contribution by the principal component 2 of the top ranked modules, which results in a similar AUC compared to the physiological data plus DEG only models. However, we did not observe any improvement in prediction by including module contributions. Similarly, ranking variables by importance in this set of random forest models consistently indicates previous vaccination status to be the most important variable in predicting seroconversion category (
Figure S18). Of the clinical variables, baseline HAI, BMI, and previous vaccination status consistently are in the top 10 variables for this set of random forest models. While no modules appear in the top 10 most important features across multiple random forest models in this set, similar to the models without module inclusion (
Figure 6) we do see the genes
C8B,
ENSG00000267009,
LINC01960,
NRAV, and
TROAP show up in the top 10 features in multiple random forest models in this set (
Figure S18).
When combining physiological features with gene expression, previous vaccination status, BMI, and baseline HAI remain highly predictive along with above mentioned genes and lncRNAs in addition to ENSG00000255835,
NCBP2L,
NKX3.1,
PTGER1, and
VANGL2. Complement factor
C8B,
NRAV and
PTGER1 have immune system relevant functions.
C8B is the β-chain of the membrane attack complex, which mediates cell lysis, and initiates membrane penetration of the complex. The lncRNA
NRAV modulates antiviral responses through suppression of interferon-stimulated gene transcription [
35]. Prostaglandin E receptor 1 (
PTGER1), a member of the G protein-coupled receptor family, plays a role in inflammatory response and modulates inflammation by down-regulation of COX-2 [
36]. Overall, the main biological functions of these genes are related to immune processes.
4. Discussion
The immunological response to the seasonal influenza vaccine exhibits significant heterogeneity among individuals. In this study, we addressed two key questions with respect to the observed variation: (1) to what extent do whole blood gene expression profiles and physiological variables relate to serological status prior to, and following, vaccination, and (ii) are gene expression and physiological metrics predictive of vaccine response.
We first assessed the effect of physiological factors on serological status. As expected, the response to the vaccine is strongly impacted by existing immunity. We quantified existing immunity to each of the four influenza strains included in the vaccine and observed significant variation among individuals. Among those individuals with higher existing immunity we detect a muted increase in immune status following vaccination. Individuals who had not been vaccinated in the prior three years show a much greater response than those who received at least one influenza vaccine in the prior three years. Surprisingly, we detect a trend of higher BMI associated with increased vaccine response. This could reflect either systematically decreased pre-existing immunity, or a greater degree of immunological priming, in higher BMI individuals.
Gene expression profiling revealed a large number of genes that are differentially expressed with physiological factors including BMI (3814 genes), sex (2977 genes), and age (2977 genes). By contrast, only 45 genes are differentially expressed as a function of baseline HAI. Surprisingly, a much larger number of genes are differentially expressed with seroconversion (741) although only 11% (84) of these are not associated with one of the physiological factors. This discrepancy suggests that whole blood gene expression profiles obtained prior to vaccination are more informative of the response to vaccination than the pre-existing immunological state of an individual.
Among genes that are increased in expression with increasing seroconversion are several immunoglobulin genes. As gene expression is assayed prior to vaccination this may indicate that those individuals who will mount the greatest serological response to the vaccine are predisposed to do so as a result of increased pre-existing immunological activity [
37,
38]. It is possible that these expression values may be influenced by imprecise mapping of RNAseq reads to variable gene segments of immunoglobulins. However, we believe that this is unlikely as visual inspection of aligned reads for a representative example (
IGLV4-69) indicates high quality mapping and BLAST searches of
IGLV4-69 mapped reads against the reference genome identify this feature as the most likely source. Our findings suggest the interesting possibility that enhanced vaccine response may result from either increased plasmablast subpopulations or enhanced expression of specific immunoglobulins. Testing these hypotheses requires additional analyses, possibly through variable sequence reconstruction using RNAseq reads [
39,
40], and cell profiling of participants.
Using a network-based approach we identified co-expression modules that were significantly associated with serological status. As with our gene-level analysis, we identified modules involving immunoglobulins. Module M11 with hub genes IGHV5-51, IGKV1-5, IGKV3-11, IGKV3-15, IGKV3-20, IGKV4-1, and IGLV1-40, together with modules M64 and M175 are positively correlated with baseline HAI, response HAI and seroconversion. These modules are functionally enriched for antigen binding, immunoglobulin complex, and activation of adaptive immune response. We further observed negative correlations between these immunoglobulin modules and age, which may reflect reduced immunity with age.
Central to our analysis was the identification and assessment of factors for the prediction of seasonal influenza vaccine response. For this purpose we employed a random forest approach for both feature selection and modeling. Consistently, the most important physiological predictive features are baseline HAI, BMI, and prior vaccination status. When combining physiological features with gene expression, these three features remain highly predictive along with genes and lncRNAs, such as
C8B,
ENSG00000267009,
ENSG00000255835,
LINC01960,
NCBP2L,
NKX3.1,
NRAV,
PTGER1,
TROAP, and
VANGL2. Complement factor C8B, NRAV, and PTGER1 have immune system relevant functions.
C8B is the β-chain of the membrane attack complex, which mediates cell lysis, and initiates membrane penetration of the complex. Complement activation is associated with vaccine efficacy, although the exact mechanisms are still unclear [
41]. A recent study suggested active modulation of glycosylation of serum glycoproteins by the immune system to establish effective post vaccine protection via complement activation [
42]. The lncRNA
NRAV modulates antiviral responses through suppression of interferon-stimulated gene transcription [
35]. Prostaglandin E receptor 1 (
PTGER1), a member of the G protein-coupled receptor family, plays a role in inflammatory response and modulates inflammation by down-regulation of COX-2 [
36]. Among the other, not directly immune-system related genes are three with unknown functions, two lncRNA,
ENSG00000267009 and
LINC01960, and one protein coding gene,
ENSG0000025583 with hypothetical pyrroline-5-carboxylate reductase function. These predictive but unknown molecular factors may require further investigation in their role affecting vaccination outcome. However, despite the predictive value of gene expression we find that response prediction using exclusively physiological information performs better than models that include gene expression, either exclusively or additionally.
Overall, our study further highlights the importance of physiological variables on the vaccine response, and identifies key genes and co-regulatory networks associated with the individual vaccine response. Whereas physiological factors, such as prior vaccination status, age, and BMI are major determinants of vaccination outcome, we demonstrate that an individual’s baseline gene expression also impacts their response to vaccination. This finding has important implications for the prediction of vaccine response, and may point to biological pathways that are critical for mounting an effective immune response to influenza vaccination.