A Short 5′triphosphate RNA nCoV-L Induces a Broad-Spectrum Antiviral Response by Activating RIG-I
Abstract
1. Introduction
2. Materials and Methods
2.1. Biosafety and Ethics Approval
2.2. Cell Culture
2.3. Viruses
2.4. Mice
2.5. In Vitro Transcription and Gel Analysis
- 5′-GGUUUAAUACCUAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA
- UCCCUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUAGGUAUUAAA
- CC-3′ (100 nt).
2.6. Transfections
2.7. Luciferase Assays
2.8. Protein Extraction and Western Blot
2.9. RNA Extraction
2.10. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.11. In Vitro Virus Infection
2.12. In Vivo Virus Infection
2.13. Histopathological Examination
2.14. ELISA
2.15. Statistical Analyses
3. Results
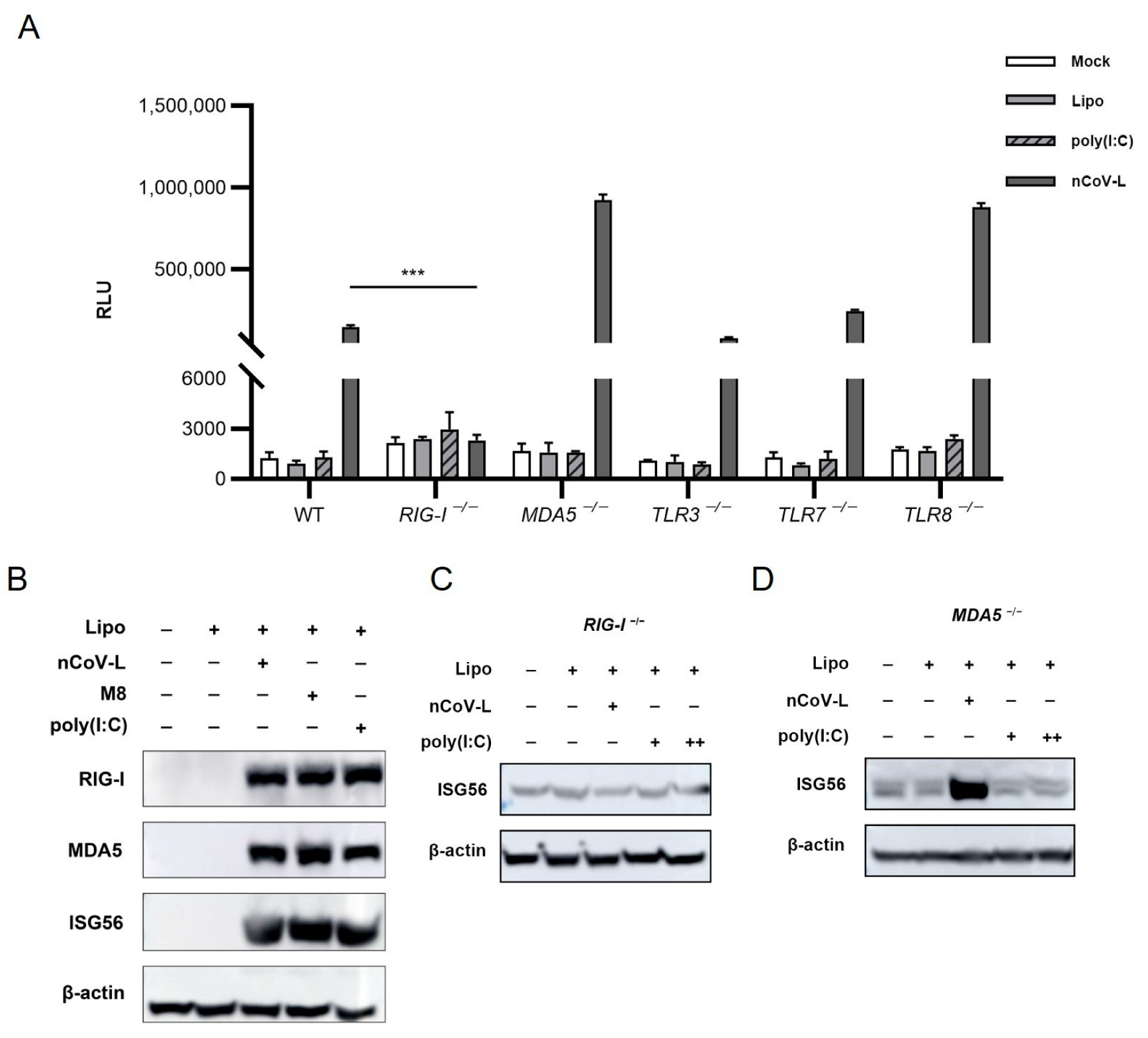
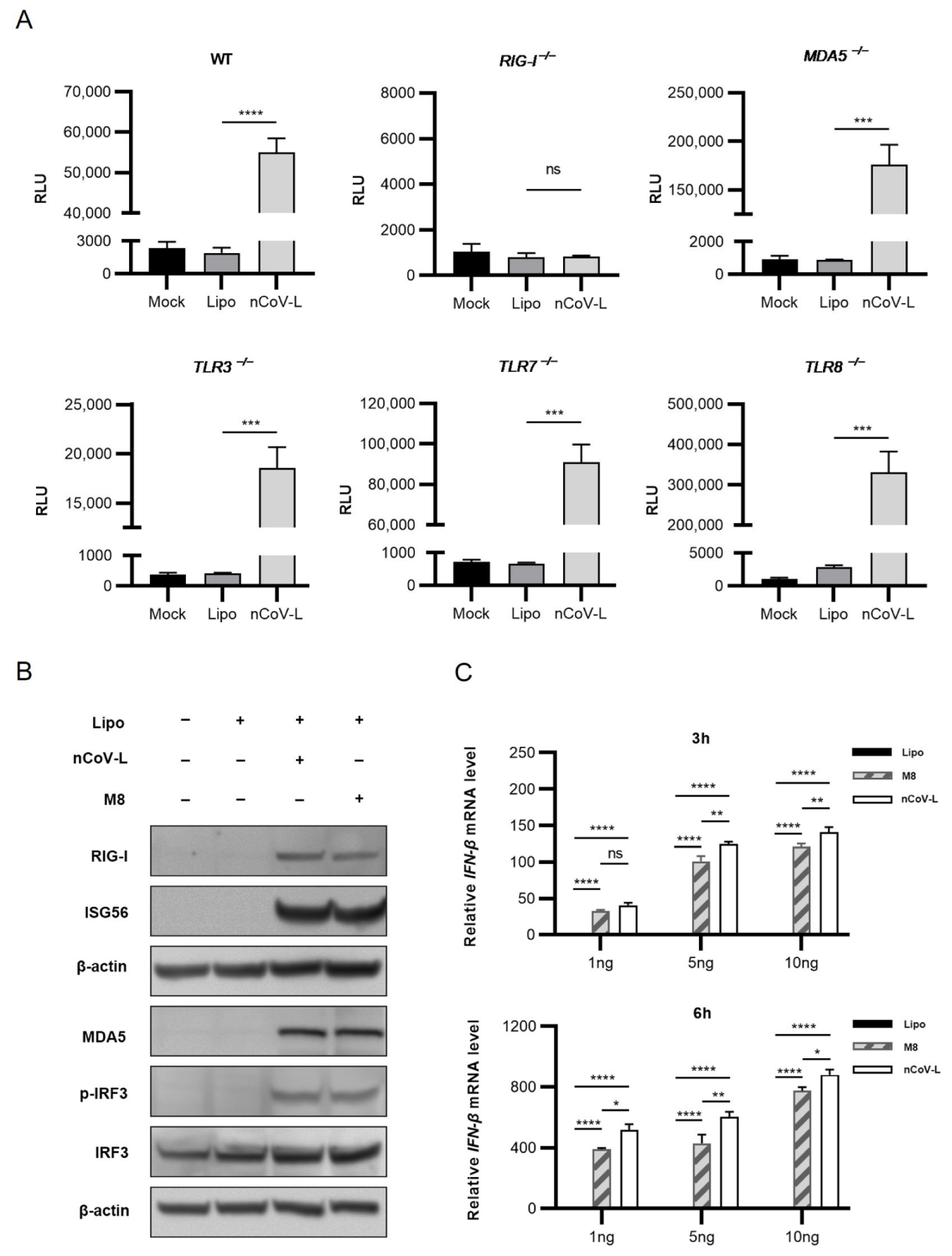
3.1. nCoV-L Induce Interferon Response via the RIG-I Receptor
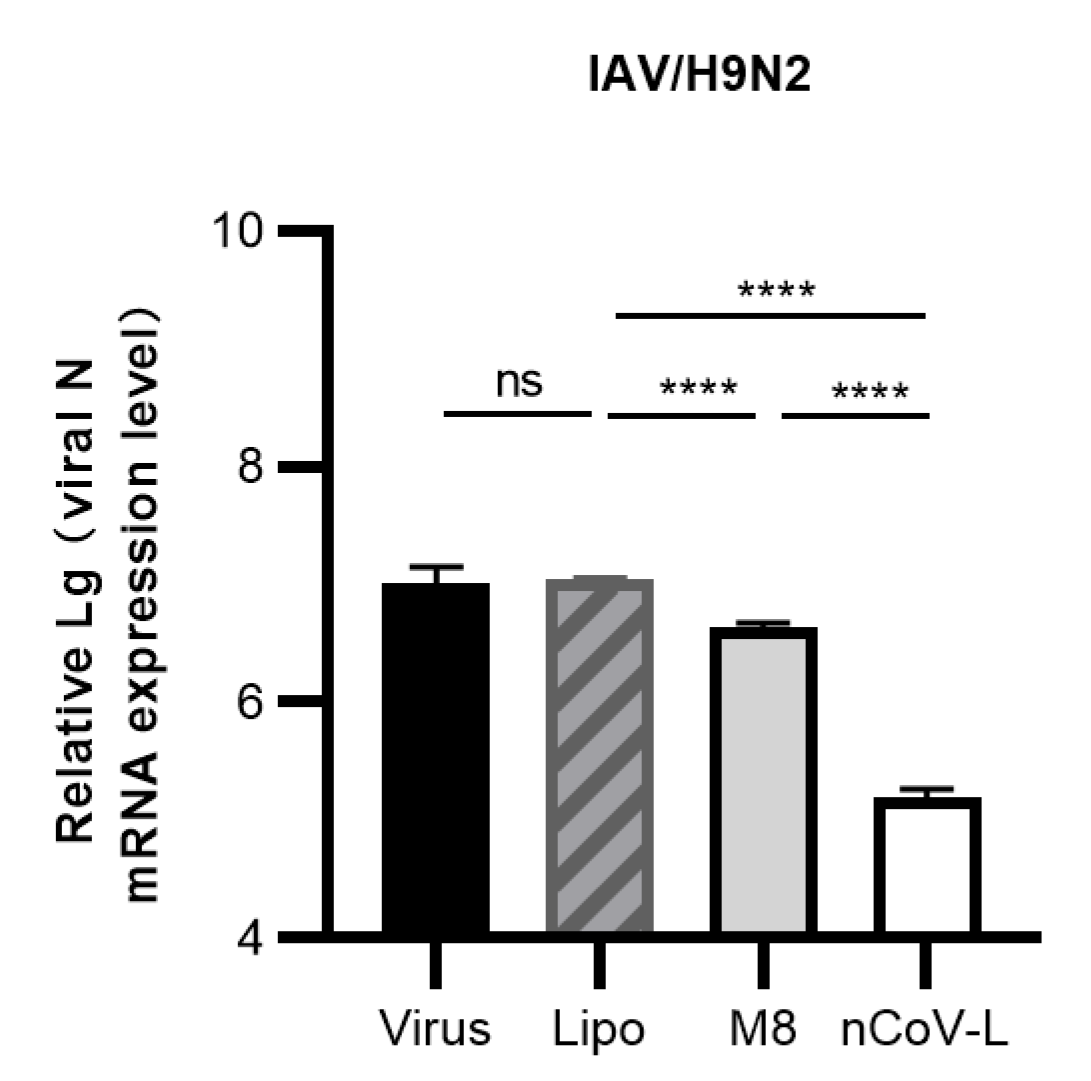
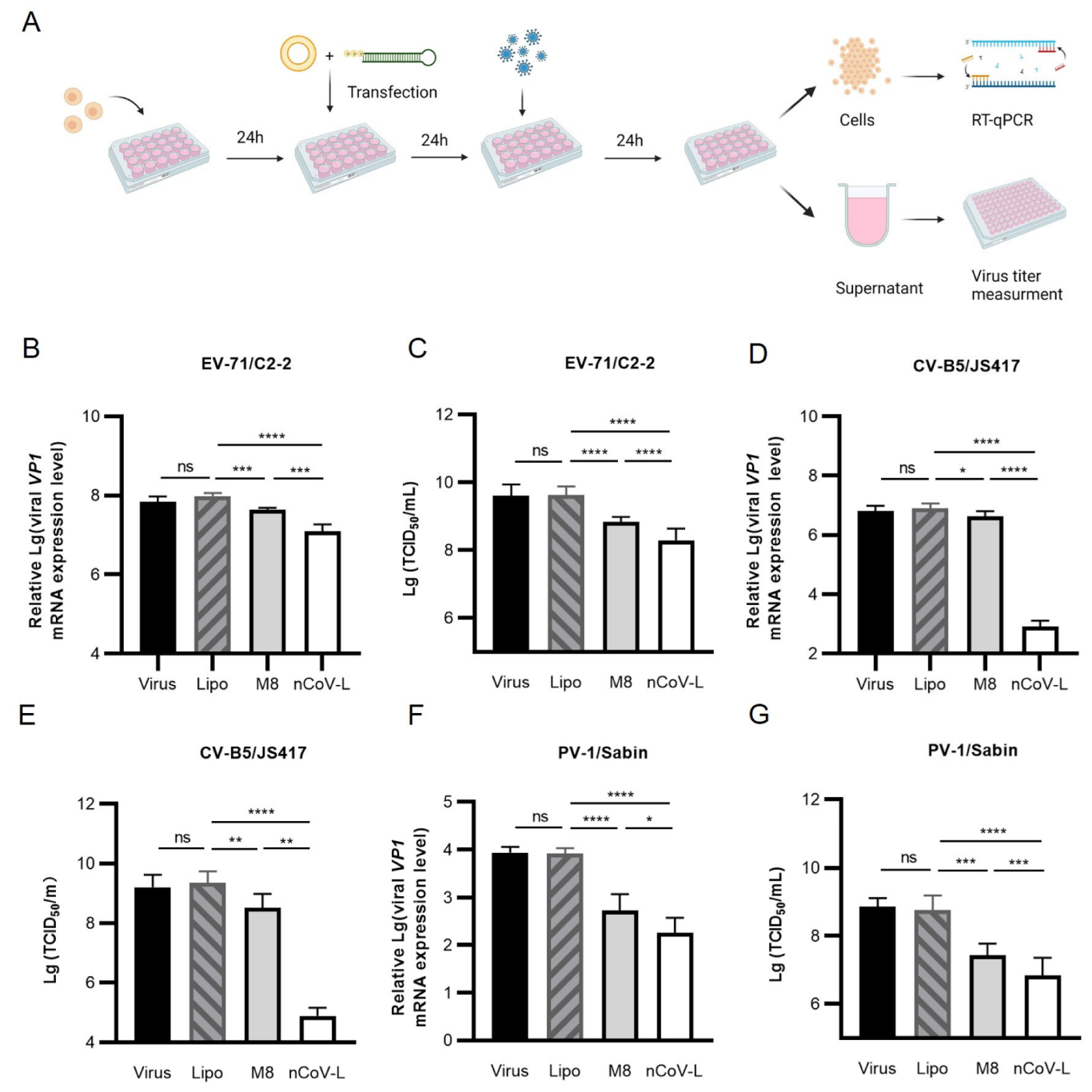
3.2. In Vitro Antiviral Effects of nCoV-L against RNA Viruses EV-71, CV-B5, PV-1 and IAV
3.3. nCoV-L Protected Suckling Mice from Lethal EV71 Virus Infection
3.4. Prophylactic Administration of nCoV-L Protects Mice from Lethal SARS-CoV-2 Virus Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Wild-Type Sequence | Post-Editing Sequence | Mutation Type |
|---|---|---|---|
| TLR3 | CAACTTTCTTGGGACTA----AAGTGGACAAATCTCAC | CAACTTTCTTGGGACTAATTTAAGTGGACAAATCTCAC | frame shift |
| TLR7 | AACTCTCTTCAGCATGTGCCCCCAAGATGGTTT | AACTCTCTT-------------CAAGATGGTTT | frame shift |
| TLR8 | ATTTAGTGGGAGAAATAGC--CTCTGGGGCATTTTT | ATTTAGTGGGAGAAATAGCGCCTCTGGGGCATTTTT | frame shift |
| RIG-I | AAAAAATTCCCACAAGGACAAAAGGGGAAAG | AAAAAATTCCCACAAGGACAA--GGGGAAAG | frame shift |
| MDA5 | CCCCGGAGCCAGAACTCCAGCTCAGGCCTTACCAA | CCCCGGAGCCAGAACTCC----CAGGCCTTACCAA | frame shift |


References
- Kanneganti, T.-D. Intracellular innate immune receptors: Life inside the cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Barbalat, R.; Ewald, S.E.; Mouchess, M.L.; Barton, G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 2011, 29, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Eidenschenk, C.; Crozat, K.; Imler, J.L.; Takeuchi, O.; Hoffmann, J.A.; Akira, S. Genetic analysis of resistance to viral infection. Nat. Rev. Immunol. 2007, 7, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Hartmann, G. The chase for the RIG-I ligand--recent advances. Mol. Ther. 2010, 18, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Schlee, M.; Deddouche, S.; Pruijssers, A.J.; Zillinger, T.; Goldeck, M.; Schuberth, C.; Van der Veen, A.G.; Fujimura, T.; Rehwinkel, J.; et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 2014, 514, 372–375. [Google Scholar] [CrossRef]
- Okamoto, M.; Kouwaki, T.; Fukushima, Y.; Oshiumi, H. Regulation of RIG-I Activation by K63-Linked Polyubiquitination. Front. Immunol. 2017, 8, 1942. [Google Scholar] [CrossRef]
- Fujita, T. A nonself RNA pattern: Tri-p to panhandle. Immunity 2009, 31, 4–5. [Google Scholar] [CrossRef]
- Bamming, D.; Horvath, C.M. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J. Biol. Chem. 2009, 284, 9700–9712. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Akira, S. Cell type specific involvement of RIG-I in antiviral responses. Nihon Rinsho 2006, 64, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Onomoto, K.; Onoguchi, K.; Yoneyama, M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell. Mol. Immunol. 2021, 18, 539–555. [Google Scholar] [CrossRef] [PubMed]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.C.; Young, H.A. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Deddouche, S.; Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 2013, 38, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasomes and anti-viral immunity. J. Clin. Immunol. 2010, 30, 632–637. [Google Scholar] [CrossRef]
- Linehan, M.M.; Dickey, T.H.; Molinari, E.S.; Fitzgerald, M.E.; Potapova, O.; Iwasaki, A.; Pyle, A.M. A minimal RNA ligand for potent RIG-I activation in living mice. Sci. Adv. 2018, 4, e1701854. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Giovannoni, G.; Munschauer, F.E., 3rd; Deisenhammer, F. Neutralising antibodies to interferon beta during the treatment of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 465–469. [Google Scholar] [CrossRef][Green Version]
- Matsuda, F.; Torii, Y.; Enomoto, H.; Kuga, C.; Aizawa, N.; Iwata, Y.; Saito, M.; Imanishi, H.; Shimomura, S.; Nakamura, H.; et al. Anti-interferon-α neutralizing antibody is associated with nonresponse to pegylated interferon-α plus ribavirin in chronic hepatitis C. J. Viral Hepat. 2012, 19, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Beljanski, V.; Yin, K.; Olagnier, D.; Ben Yebdri, F.; Steel, C.; Goulet, M.L.; DeFilippis, V.R.; Streblow, D.N.; Haddad, E.K.; et al. Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists. J. Virol. 2015, 89, 8011–8025. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.K.; Schlee, M.; et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, S.; Yang, Z.; Lin, H.; Zhu, J.; Liu, L.; Wang, W.; Liu, S.; Liu, W.; Ma, Y.; et al. Self-Recognition of an Inducible Host lncRNA by RIG-I Feedback Restricts Innate Immune Response. Cell 2018, 173, 906–919.e913. [Google Scholar] [CrossRef]
- Brisse, M.; Ly, H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef]
- Schlee, M.; Roth, A.; Hornung, V.; Hagmann, C.A.; Wimmenauer, V.; Barchet, W.; Coch, C.; Janke, M.; Mihailovic, A.; Wardle, G.; et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 2009, 31, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Langereis, M.A.; Olagnier, D.; Chiang, C.; van de Winkel, R.; van Essen, P.; Zoll, J.; Hiscott, J.; van Kuppeveld, F.J. Coxsackievirus cloverleaf RNA containing a 5′ triphosphate triggers an antiviral response via RIG-I activation. PLoS ONE 2014, 9, e95927. [Google Scholar] [CrossRef] [PubMed]
- Goulet, M.L.; Olagnier, D.; Xu, Z.; Paz, S.; Belgnaoui, S.M.; Lafferty, E.I.; Janelle, V.; Arguello, M.; Paquet, M.; Ghneim, K.; et al. Systems analysis of a RIG-I agonist inducing broad spectrum inhibition of virus infectivity. PLoS Pathog. 2013, 9, e1003298. [Google Scholar] [CrossRef]
- Jia, J.; Fu, J.; Tang, H. Activation and Evasion of RLR Signaling by DNA Virus Infection. Front. Microbiol. 2021, 12, 804511. [Google Scholar] [CrossRef]
- Konno, Y.; Kimura, I.; Uriu, K.; Fukushi, M.; Irie, T.; Koyanagi, Y.; Sauter, D.; Gifford, R.J.; Nakagawa, S.; Sato, K. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep. 2020, 32, 108185. [Google Scholar] [CrossRef]
- Setaro, A.C.; Gaglia, M.M. All hands on deck: SARS-CoV-2 proteins that block early anti-viral interferon responses. Curr. Res. Virol. Sci. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Tao, J.; Guo, D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes 2011, 42, 37–45. [Google Scholar] [CrossRef]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021, 22, 820–828. [Google Scholar] [CrossRef]
- Xu, D.; Biswal, M.; Neal, A.; Hai, R. Review Devil’s tools: SARS-CoV-2 antagonists against innate immunity. Curr. Res. Virol. Sci. 2021, 2, 100013. [Google Scholar] [CrossRef]
- Bojkova, D.; Widera, M.; Ciesek, S.; Wass, M.N.; Michaelis, M.; Cinatl, J., Jr. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates. Cell Res. 2022, 32, 319–321. [Google Scholar] [CrossRef]
- Mao, T.; Israelow, B.; Lucas, C.; Vogels, C.B.F.; Gomez-Calvo, M.L.; Fedorova, O.; Breban, M.I.; Menasche, B.L.; Dong, H.; Linehan, M.; et al. A stem-loop RNA RIG-I agonist protects against acute and chronic SARS-CoV-2 infection in mice. J. Exp. Med. 2022, 219, e20211818. [Google Scholar] [CrossRef] [PubMed]

| Gene | sgRNA |
|---|---|
| TLR3 | CAACTTTCTTGGGACTAAAG |
| TLR7 | TTCAGCATGTGCCCCCAAGA |
| TLR8 | TTAGTGGGAGAAATAGCCTC |
| MDA5 | GCTCAGGCCTTACCAAATGG |
| RIG-I | AATTCCCACAAGGACAAAAG |
| Gene | Direction | Primer Sequence (5′-3′) |
|---|---|---|
| EV71/C2-2 | Forward | TAG TTT CTT CAG CAG GGC GG |
| Reverse | ACC ATT GGT AAG CAC TCG CA | |
| CV-B5/JS417 | Forward | GGT GTC CGT GTT TCC TTT TAT TCC TAC |
| Reverse | CAA GTA GAT AAT AGC TCT GTT TGT CAC CG | |
| PV-1/Sabin | Forward | TGA TCA CAA CCC GAC CAA GG |
| Reverse | TAA TCC ACT CCA GGG CCG TA | |
| IAV/H9N2 | Forward | CCG GAA TTT CTG GAG AGG CG |
| Reverse | TAC ACA AGC AGG CAA GCA GG | |
| IFN-β | Forward | GCT TGG ATT CCT ACA AAG AAG CA |
| Reverse | ATA GAT GGT CAA TGC GGC GTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Wang, Q.; Bian, L.; An, C.; Cui, B.; Mao, Q.; Wu, X.; He, Q.; Bai, Y.; Liu, J.; et al. A Short 5′triphosphate RNA nCoV-L Induces a Broad-Spectrum Antiviral Response by Activating RIG-I. Viruses 2022, 14, 2451. https://doi.org/10.3390/v14112451
Song Z, Wang Q, Bian L, An C, Cui B, Mao Q, Wu X, He Q, Bai Y, Liu J, et al. A Short 5′triphosphate RNA nCoV-L Induces a Broad-Spectrum Antiviral Response by Activating RIG-I. Viruses. 2022; 14(11):2451. https://doi.org/10.3390/v14112451
Chicago/Turabian StyleSong, Ziyang, Qian Wang, Lianlian Bian, Chaoqiang An, Bopei Cui, Qunying Mao, Xing Wu, Qian He, Yu Bai, Jianyang Liu, and et al. 2022. "A Short 5′triphosphate RNA nCoV-L Induces a Broad-Spectrum Antiviral Response by Activating RIG-I" Viruses 14, no. 11: 2451. https://doi.org/10.3390/v14112451
APA StyleSong, Z., Wang, Q., Bian, L., An, C., Cui, B., Mao, Q., Wu, X., He, Q., Bai, Y., Liu, J., Song, L., Liu, D., Zhang, J., Gao, F., Li, X., & Liang, Z. (2022). A Short 5′triphosphate RNA nCoV-L Induces a Broad-Spectrum Antiviral Response by Activating RIG-I. Viruses, 14(11), 2451. https://doi.org/10.3390/v14112451





