Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus–Host Interactions
Abstract
1. Epidemiology of PEDV
1.1. The Morbidity and Mortality of PEDV
1.2. Transmission of PEDV
1.3. Genotyping, Distribution, and Origin of PEDV
1.4. Virion Structure and Function of PEDV
2. Factors Affecting Pathogenicity
2.1. Variation in Structural/Accessory/Non-Structural Proteins
2.2. Sequence Heterogeneity of S, ORF3, E, M, and N Genes
2.3. Potential Key Amino Acids
3. Virus-Host Interaction
3.1. PEDV Proteins That Interact with Host Factors
3.2. Proviral Host Factor
3.3. Antiviral Host Factor
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCoV | Bovine coronavirus |
| CHIKV | Chikungunya virus |
| DENV | Dengue virus |
| EBV | Epstein–Barr Virus |
| FMDV | Foot-and-mouth disease virus |
| HAV | Hepatitis A virus |
| HCMV | Human cytomegalovirus |
| HCoV-NL63 | Human coronavirus NL63 |
| HCoV-OC43 | Human coronavirus OC43 |
| HCoV-229E | Human coronavirus 229E |
| HCV | Hepatitis C virus |
| HIV-1 | Human immunodeficiency virus type 1 |
| HPV | Human papillomavirus |
| HRV | Human rhinovirus |
| HSV-1 | Herpes simplex virus 1 |
| IAV | Influenza A virus |
| IBDV | Infectious bursal disease virus |
| IBV | Human infectious bronchitis coronavirus |
| JEV | Japanese encephalitis virus |
| LASV | Lassa virus |
| MARV | Marburg virus |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MHV | Mouse hepatitis virus |
| PED | Porcine epidemic diarrhea |
| PEDV | Porcine epidemic diarrhea virus |
| PCV2 | Porcine circovirus types 2 |
| PCV3 | Porcine circovirus types 3 |
| PoRV | Porcine rotavirus |
| PRCV | Porcine respiratory coronavirus |
| PRV | Porcine pseudorabies virus |
| RBDs | Receptor binding domains |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| RABV | Rabies virus |
| RSV | Human respiratory syncytial virus |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SBV | Schmallenberg virus |
| TGEV | Transmissible gastroenteritis virus |
| VSV | Vesicular stomatitis virus |
| YFV | Yellow fever virus |
References
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Have, P.; Moving, V.; Svansson, V.; Uttenthal, A.; Bloch, B. Coronavirus infection in mink (Mustela vison). Serological evidence of infection with a coronavirus related to transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Vet. Microbiol. 1992, 31, 1–10. [Google Scholar] [CrossRef]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef]
- Gallien, S.; Moro, A.; Lediguerher, G.; Catinot, V.; Paboeuf, F.; Bigault, L.; Berri, M.; Gauger, P.C.; Pozzi, N.; Authie, E.; et al. Evidence of porcine epidemic diarrhea virus (PEDV) shedding in semen from infected specific pathogen-free boars. Vet. Res. 2018, 49, 7. [Google Scholar] [CrossRef]
- Si, F.; Jiang, L.; Yu, R.; Wei, W.; Li, Z. Study on the Characteristic Codon Usage Pattern in Porcine Epidemic Diarrhea Virus Genomes and Its Host Adaptation Phenotype. Front. Microbiol. 2021, 12, 738082. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Wang, X.M.; Niu, B.B.; Yan, H.; Gao, D.S.; Yang, X.; Chen, L.; Chang, H.T.; Zhao, J.; Wang, C.Q. Genetic properties of endemic Chinese porcine epidemic diarrhea virus strains isolated since 2010. Arch. Virol. 2013, 158, 2487–2494. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect. Dis 2012, 18, 1350–1353. [Google Scholar] [CrossRef]
- Cima, G. PED virus reinfecting U.S. herds. Virus estimated to have killed 7 million-plus pigs. J. Am. Vet. Med. Assoc. 2014, 245, 166–167. [Google Scholar] [PubMed]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013, 25, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Hanke, D.; Jenckel, M.; Petrov, A.; Ritzmann, M.; Stadler, J.; Akimkin, V.; Blome, S.; Pohlmann, A.; Schirrmeier, H.; Beer, M.; et al. Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerg. Infect. Dis. 2015, 21, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Reveles-Felix, S.; Carreon-Napoles, R.; Mendoza-Elvira, S.; Quintero-Ramirez, V.; Garcia-Sanchez, J.; Martinez-Bautista, R.; Saavedra-Montanez, M.; Mosqueda Gualito, J.J.; Sanchez-Betancourt, J.I. Emerging strains of porcine epidemic diarrhoea virus (PEDv) in Mexico. Transbound Emerg. Dis. 2020, 67, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Huang, L.; Yuan, C.; Wang, J.; Yang, Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat. Commun. 2018, 9, 3811. [Google Scholar] [CrossRef]
- Bowman, A.S.; Krogwold, R.A.; Price, T.; Davis, M.; Moeller, S.J. Investigating the introduction of porcine epidemic diarrhea virus into an Ohio swine operation. BMC Vet. Res. 2015, 11, 38. [Google Scholar] [CrossRef]
- Lowe, J.; Gauger, P.; Harmon, K.; Zhang, J.; Connor, J.; Yeske, P.; Loula, T.; Levis, I.; Dufresne, L.; Main, R. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg. Infect. Dis. 2014, 20, 872–874. [Google Scholar] [CrossRef]
- Guo, J.; Fang, L.; Ye, X.; Chen, J.; Xu, S.; Zhu, X.; Miao, Y.; Wang, D.; Xiao, S. Evolutionary and genotypic analyses of global porcine epidemic diarrhea virus strains. Transbound Emerg. Dis. 2019, 66, 111–118. [Google Scholar] [CrossRef]
- Park, S.J.; Moon, H.J.; Luo, Y.; Kim, H.K.; Kim, E.M.; Yang, J.S.; Song, D.S.; Kang, B.K.; Lee, C.S.; Park, B.K. Cloning and further sequence analysis of the ORF3 gene of wild- and attenuated-type porcine epidemic diarrhea viruses. Virus Genes 2008, 36, 95–104. [Google Scholar] [CrossRef]
- Puranaveja, S.; Poolperm, P.; Lertwatcharasarakul, P.; Kesdaengsakonwut, S.; Boonsoongnern, A.; Urairong, K.; Kitikoon, P.; Choojai, P.; Kedkovid, R.; Teankum, K.; et al. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009, 15, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Jiao, D.; Zhang, R.; Zhou, J.; Guo, R.; Yu, Z.; Shi, D.; Zhao, Y.; Gu, J.; Niu, B.; et al. Origin and epidemic status of porcine epidemic diarrhea virus variants in China. Transbound Emerg. Dis. 2020, 67, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Shang, Y.; Tan, R.; Ji, M.; Yue, X.; Wang, N.; Liu, J.; Wang, C.; Li, Y.; et al. Emergence and evolution of highly pathogenic porcine epidemic diarrhea virus by natural recombination of a low pathogenic vaccine isolate and a highly pathogenic strain in the spike gene. Virus Evol. 2020, 6, veaa049. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Song, D.S.; Ha, G.W.; Park, B.K. Cloning and further sequence analysis of the spike gene of attenuated porcine epidemic diarrhea virus DR13. Virus Genes 2007, 35, 55–64. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, M.; Ju, D.; Wu, B.; Ning, C.; Song, N.; Feng, T.; Chen, F.; Wang, X.; Wu, Y.; et al. Isolation, molecular characterization and an artificial infection model for a variant porcine epidemic diarrhea virus strain from Jiangsu Province, China. Arch. Virol. 2017, 162, 3611–3618. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Saif, L.J.; Marthaler, D.; Wang, Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016, 226, 20–39. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Zhang, L.; Zhou, P.; Yang, J.; Fang, Y.; Dong, Z.; Zhao, D.; Li, W.; Feng, J.; et al. A newly isolated Chinese virulent genotype GIIb porcine epidemic diarrhea virus strain: Biological characteristics, pathogenicity and immune protective effects as an inactivated vaccine candidate. Virus Res. 2019, 259, 18–27. [Google Scholar] [CrossRef]
- Cui, J.T.; Qiao, H.; Hou, C.Y.; Zheng, H.H.; Li, X.S.; Zheng, L.L.; Chen, H.Y. Characteristics of the spike and ORF3 genes of porcine epidemic diarrhea virus in Henan and Shanxi provinces of China. Arch. Virol. 2020, 165, 2323–2333. [Google Scholar] [CrossRef]
- Wang, L.; Hayes, J.; Byrum, B.; Zhang, Y. US variant porcine epidemic diarrhea virus: Histological lesions and genetic characterization. Virus Genes 2016, 52, 578–581. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, M.; Zhan, J.; Liu, Q.Y.; Fang, L.L.; Zhao, C.Y.; Jiang, P.; Li, Y.F.; Bai, J. Pathogenicity and immunogenicity of a new strain of porcine epidemic diarrhea virus containing a novel deletion in the N gene. Vet. Microbiol. 2020, 240, 108511. [Google Scholar] [CrossRef]
- Li, R.; Qiao, S.; Yang, Y.; Guo, J.; Xie, S.; Zhou, E.; Zhang, G. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China. Virus Genes 2016, 52, 91–98. [Google Scholar] [CrossRef]
- Pensaert, M.B.; Martelli, P. Porcine epidemic diarrhea: A retrospect from Europe and matters of debate. Virus Res. 2016, 226, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Y.; Han, X.; Yu, Z.; Wei, Y.; Zhang, G. Porcine epidemic diarrhea virus in Asia: An alarming threat to the global pig industry. Infect. Genet. Evol. 2019, 70, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, G.; Tramacere, M.; Borghetti, A.F. Cell-density control of amino acid transport in simian virus 40-transformed 3T3 cells. Biochem. Soc. Trans. 1979, 7, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, Z.; Zhang, Y.; Wang, G.; Wang, H.; Yang, F.; Tian, F.; Jiang, S. Complete genome sequence of a Vero cell-adapted isolate of porcine epidemic diarrhea virus in eastern China. J. Virol. 2012, 86, 13858–13859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, X.; Hu, H.; Chen, F.; Li, Z.; Ye, S.; Cheng, S.; Zhang, M.; He, Q. iTRAQ-based comparative proteomic analysis of Vero cells infected with virulent and CV777 vaccine strain-like strains of porcine epidemic diarrhea virus. J. Proteom. 2016, 130, 65–75. [Google Scholar] [CrossRef]
- Fan, B.; Yu, Z.; Pang, F.; Xu, X.; Zhang, B.; Guo, R.; He, K.; Li, B. Characterization of a pathogenic full-length cDNA clone of a virulent porcine epidemic diarrhea virus strain AH2012/12 in China. Virology 2017, 500, 50–61. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, J.; Liu, X.; Guo, J.; Gu, X.; Ruan, W. Isolation and recombinant analysis of variants of porcine epidemic diarrhea virus strains from Beijing, China. Virusdisease 2019, 30, 294–301. [Google Scholar] [CrossRef]
- Wang, X.M.; Niu, B.B.; Yan, H.; Gao, D.S.; Huo, J.Y.; Chen, L.; Chang, H.T.; Wang, C.Q.; Zhao, J. Complete genome sequence of a variant porcine epidemic diarrhea virus strain isolated in central china. Genome Announc. 2013, 1, e00243-12. [Google Scholar] [CrossRef]
- Song, D.; Chen, Y.; Peng, Q.; Huang, D.; Zhang, T.; Huang, T.; Zhang, F.; Zhou, X.; Tang, Y. Full-Length Genome Sequence of a Variant Porcine Epidemic Diarrhea Virus Strain, CH/GDZQ/2014, Responsible for a Severe Outbreak of Diarrhea in Piglets in Guangdong, China, 2014. Genome Announc. 2014, 2, e01239-14. [Google Scholar] [CrossRef]
- Murakami, S.; Miyazaki, A.; Takahashi, O.; Hashizume, W.; Hase, Y.; Ohashi, S.; Suzuki, T. Complete Genome Sequence of the Porcine Epidemic Diarrhea Virus Variant Tottori2/JPN/2014. Genome Announc. 2015, 3, e00877-15. [Google Scholar] [CrossRef] [PubMed]
- Marthaler, D.; Jiang, Y.; Otterson, T.; Goyal, S.; Rossow, K.; Collins, J. Complete Genome Sequence of Porcine Epidemic Diarrhea Virus Strain USA/Colorado/2013 from the United States. Genome Announc. 2013, 1, e00555-13. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Wang, Q.; Scheuer, K.A.; Lu, Z.; Zhang, Y.; Saif, L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014, 20, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lin, C.M.; Yokoyama, M.; Yount, B.L.; Marthaler, D.; Douglas, A.L.; Ghimire, S.; Qin, Y.; Baric, R.S.; Saif, L.J.; et al. Deletion of a 197-Amino-Acid Region in the N-Terminal Domain of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Piglets. J. Virol. 2017, 91, e00555-13. [Google Scholar] [CrossRef]
- Bi, J.; Zeng, S.; Xiao, S.; Chen, H.; Fang, L. Complete genome sequence of porcine epidemic diarrhea virus strain AJ1102 isolated from a suckling piglet with acute diarrhea in China. J. Virol. 2012, 86, 10910–10911. [Google Scholar] [CrossRef]
- Chen, F.; Pan, Y.; Zhang, X.; Tian, X.; Wang, D.; Zhou, Q.; Song, Y.; Bi, Y. Complete genome sequence of a variant porcine epidemic diarrhea virus strain isolated in China. J. Virol. 2012, 86, 12448. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.; Wu, M.; Ku, X.; Ye, S.; Li, Z.; Guo, X.; He, Q. Comparative Genomic Analysis of Classical and Variant Virulent Parental/Attenuated Strains of Porcine Epidemic Diarrhea Virus. Viruses 2015, 7, 5525–5538. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, X.; Li, W.; Zhou, Q.; Wang, D.; Bi, Y.; Chen, F.; Song, Y. Isolation and characterization of a variant porcine epidemic diarrhea virus in China. Virol. J. 2012, 9, 195. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Lu, W.H.; Li, Z.L.; Mo, J.Y.; Zeng, X.D.; Zeng, Z.L.; Sun, B.L.; Chen, F.; Xie, Q.M.; Bee, Y.Z.; et al. Complete genome sequence of novel porcine epidemic diarrhea virus strain GD-1 in China. J. Virol. 2012, 86, 13824–13825. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, J.; Ye, Y.; Tong, T.; Xie, K.; Liao, M. Complete genome sequence of a novel porcine epidemic diarrhea virus in south China. J. Virol. 2012, 86, 10248–10249. [Google Scholar] [CrossRef]
- Wang, L.; Byrum, B.; Zhang, Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014, 20, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Byrum, B.; Zhang, Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg Infect. Dis 2014, 20, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Theuns, S.; Conceicao-Neto, N.; Christiaens, I.; Zeller, M.; Desmarets, L.M.; Roukaerts, I.D.; Acar, D.D.; Heylen, E.; Matthijnssens, J.; Nauwynck, H.J. Complete genome sequence of a porcine epidemic diarrhea virus from a novel outbreak in belgium, january 2015. Genome Announc. 2015, 3, e00506-15. [Google Scholar] [CrossRef]
- Sun, M.; Ma, J.; Wang, Y.; Wang, M.; Song, W.; Zhang, W.; Lu, C.; Yao, H. Genomic and epidemiological characteristics provide new insights into the phylogeographical and spatiotemporal spread of porcine epidemic diarrhea virus in Asia. J. Clin. Microbiol. 2015, 53, 1484–1492. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Jung, J.; Kim, I.J.; Hyun, B.H.; Kim, H.I.; Park, C.K.; Oem, J.K.; Kim, Y.H.; Lee, M.H.; et al. Genetic characterization of porcine epidemic diarrhea virus in Korea from 1998 to 2013. Arch. Virol. 2015, 160, 1055–1064. [Google Scholar] [CrossRef]
- Masuda, T.; Murakami, S.; Takahashi, O.; Miyazaki, A.; Ohashi, S.; Yamasato, H.; Suzuki, T. New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Arch. Virol. 2015, 160, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Vui, D.T.; Tung, N.; Inui, K.; Slater, S.; Nilubol, D. Complete genome sequence of porcine epidemic diarrhea virus in Vietnam. Genome Announc. 2014, 2, e00753-14. [Google Scholar] [CrossRef] [PubMed]
- Cheun-Arom, T.; Temeeyasen, G.; Srijangwad, A.; Tripipat, T.; Sangmalee, S.; Vui, D.T.; Chuanasa, T.; Tantituvanont, A.; Nilubol, D. Complete Genome Sequences of Two Genetically Distinct Variants of Porcine Epidemic Diarrhea Virus in the Eastern Region of Thailand. Genome Announc. 2015, 3, e00634-15. [Google Scholar] [CrossRef]
- Paraguison-Alili, R.; Domingo, C.Y. Phylogenetic tracking of current porcine epidemic diarrhea virus (PEDV) strains in the Philippines. Arch. Virol. 2016, 161, 2601–2604. [Google Scholar] [CrossRef]
- Diep, N.V.; Sueyoshi, M.; Izzati, U.; Fuke, N.; Teh, A.P.P.; Lan, N.T.; Yamaguchi, R. Appearance of US-like porcine epidemic diarrhoea virus (PEDV) strains before US outbreaks and genetic heterogeneity of PEDVs collected in Northern Vietnam during 2012-2015. Transbound Emerg. Dis. 2018, 65, e83–e93. [Google Scholar] [CrossRef]
- Huang, Y.W.; Dickerman, A.W.; Pineyro, P.; Li, L.; Fang, L.; Kiehne, R.; Opriessnig, T.; Meng, X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio 2013, 4, e00737-13. [Google Scholar] [CrossRef] [PubMed]
- Ojkic, D.; Hazlett, M.; Fairles, J.; Marom, A.; Slavic, D.; Maxie, G.; Alexandersen, S.; Pasick, J.; Alsop, J.; Burlatschenko, S. The first case of porcine epidemic diarrhea in Canada. Can. Vet. J. 2015, 56, 149–152. [Google Scholar] [PubMed]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Zoels, S.; Fux, R.; Hanke, D.; Pohlmann, A.; Blome, S.; Weissenbock, H.; Weissenbacher-Lang, C.; Ritzmann, M.; Ladinig, A. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet. Res. 2015, 11, 142. [Google Scholar] [CrossRef]
- Dortmans, J.; Li, W.; van der Wolf, P.J.; Buter, G.J.; Franssen, P.J.M.; van Schaik, G.; Houben, M.; Bosch, B.J. Porcine epidemic diarrhea virus (PEDV) introduction into a naive Dutch pig population in 2014. Vet. Microbiol. 2018, 221, 13–18. [Google Scholar] [CrossRef]
- Grasland, B.; Bigault, L.; Bernard, C.; Quenault, H.; Toulouse, O.; Fablet, C.; Rose, N.; Touzain, F.; Blanchard, Y. Complete genome sequence of a porcine epidemic diarrhea s gene indel strain isolated in france in december 2014. Genome Announc. 2015, 3, e00535-15. [Google Scholar] [CrossRef]
- Mesquita, J.R.; Hakze-van der Honing, R.; Almeida, A.; Lourenco, M.; van der Poel, W.H.; Nascimento, M.S. Outbreak of Porcine Epidemic Diarrhea Virus in Portugal, 2015. Transbound Emerg. Dis. 2015, 62, 586–588. [Google Scholar] [CrossRef]
- Steinrigl, A.; Fernandez, S.R.; Stoiber, F.; Pikalo, J.; Sattler, T.; Schmoll, F. First detection, clinical presentation and phylogenetic characterization of Porcine epidemic diarrhea virus in Austria. BMC Vet. Res. 2015, 11, 310. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, Y.; Yang, S.; Zhang, G.; Xiao, S.; Fu, Z.F.; Peng, G. Structural and Biological Basis of Alphacoronavirus nsp1 Associated with Host Proliferation and Immune Evasion. Viruses 2020, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, J.; Ma, Y.; Liang, X.; Yang, Y.; Peng, G.; Qi, Q.; Jiang, S.; Li, J.; Du, L.; et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015, 89, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; van Kuppeveld, F.J.M.; He, Q.; Rottier, P.J.M.; Bosch, B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016, 226, 117–127. [Google Scholar] [CrossRef]
- Su, Y.; Hou, Y.; Wang, Q. The enhanced replication of an S-intact PEDV during coinfection with an S1 NTD-del PEDV in piglets. Vet. Microbiol. 2019, 228, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Santiago, C.; Mudgal, G.; Ordono, D.; Enjuanes, L.; Casasnovas, J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012, 8, e1002859. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Li, Y.; Gao, S.; Xiao, S. Porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and vaccines. Microb. Pathog. 2020, 149, 104553. [Google Scholar] [CrossRef]
- Eckert, D.M.; Kim, P.S. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001, 70, 777–810. [Google Scholar] [CrossRef]
- Sun, D.; Feng, L.; Shi, H.; Chen, J.; Cui, X.; Chen, H.; Liu, S.; Tong, Y.; Wang, Y.; Tong, G. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet. Microbiol. 2008, 131, 73–81. [Google Scholar] [CrossRef]
- Van Diep, N.; Choijookhuu, N.; Fuke, N.; Myint, O.; Izzati, U.Z.; Suwanruengsri, M.; Hishikawa, Y.; Yamaguchi, R. New tropisms of porcine epidemic diarrhoea virus (PEDV) in pigs naturally coinfected by variants bearing large deletions in the spike (S) protein and PEDVs possessing an intact S protein. Transbound Emerg. Dis. 2020, 67, 2589–2601. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Y.; Yang, Y.; Zheng, Y.; Shang, J.; Zhou, Y.; Jiang, S.; Du, L.; Li, J.; Li, F. Cell Entry of Porcine Epidemic Diarrhea Coronavirus Is Activated by Lysosomal Proteases. J. Biol. Chem. 2016, 291, 24779–24786. [Google Scholar] [CrossRef]
- Lee, D.K.; Park, C.K.; Kim, S.H.; Lee, C. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus Res. 2010, 149, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wicht, O.; Li, W.; Willems, L.; Meuleman, T.J.; Wubbolts, R.W.; van Kuppeveld, F.J.; Rottier, P.J.; Bosch, B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014, 88, 7952–7961. [Google Scholar] [CrossRef] [PubMed]
- Jantraphakorn, Y.; Viriyakitkosol, R.; Jongkaewwattana, A.; Kaewborisuth, C. Interaction Between PEDV and Its Hosts: A Closer Look at the ORF3 Accessory Protein. Front. Vet. Sci. 2021, 8, 744276. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, W.; Chen, J.; Xie, S.; Shi, H.; Hsu, H.; Yu, W.; Xu, K.; Bian, C.; Fischer, W.B.; et al. PEDV ORF3 encodes an ion channel protein and regulates virus production. FEBS Lett. 2012, 586, 384–391. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, B.J.; Xu, K.; Schwarz, W.; Du, L.; Wong, C.K.; Chen, J.; Duan, S.; Deubel, V.; Sun, B. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc. Natl. Acad. Sci. USA 2006, 103, 12540–12545. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Li, Z.; Chen, F.; Li, W.; Guo, X.; Hu, H.; He, Q. Porcine epidemic diarrhea virus ORF3 gene prolongs S-phase, facilitates formation of vesicles and promotes the proliferation of attenuated PEDV. Virus Genes 2015, 51, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Zhang, Q.; Dong, J.; Liang, Y.; Huang, Y.; Liu, H.J.; Tong, D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Maheswari, U.; Parthasarathy, K.; Ng, L.; Liu, D.X.; Gong, X. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007, 16, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, X.; Guo, D.; Cao, J.; Cheng, L.; Li, X.; Zou, D.; Zhang, Y.; Xu, J.; Wu, X.; et al. Porcine epidemic diarrhea virus E protein suppresses RIG-I signaling-mediated interferon-beta production. Vet. Microbiol. 2021, 254, 108994. [Google Scholar] [CrossRef] [PubMed]
- Vennema, H.; Godeke, G.J.; Rossen, J.W.; Voorhout, W.F.; Horzinek, M.C.; Opstelten, D.J.; Rottier, P.J. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 1996, 15, 2020–2028. [Google Scholar] [CrossRef]
- Fischer, F.; Stegen, C.F.; Masters, P.S.; Samsonoff, W.A. Analysis of constructed E gene mutants of mouse hepatitis virus confirms a pivotal role for E protein in coronavirus assembly. J. Virol. 1998, 72, 7885–7894. [Google Scholar] [CrossRef] [PubMed]
- Raamsman, M.J.; Locker, J.K.; de Hooge, A.; de Vries, A.A.; Griffiths, G.; Vennema, H.; Rottier, P.J. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein E. J. Virol. 2000, 74, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Hogue, B.G. Protein interactions during coronavirus assembly. J. Virol. 1997, 71, 9278–9284. [Google Scholar] [CrossRef] [PubMed]
- De Haan, C.A.; Kuo, L.; Masters, P.S.; Vennema, H.; Rottier, P.J. Coronavirus particle assembly: Primary structure requirements of the membrane protein. J. Virol. 1998, 72, 6838–6850. [Google Scholar] [CrossRef]
- Vennema, H.; de Groot, R.J.; Harbour, D.A.; Horzinek, M.C.; Spaan, W.J. Primary structure of the membrane and nucleocapsid protein genes of feline infectious peritonitis virus and immunogenicity of recombinant vaccinia viruses in kittens. Virology 1991, 181, 327–335. [Google Scholar] [CrossRef]
- Woods, R.D.; Wesley, R.D.; Kapke, P.A. Neutralization of porcine transmissible gastroenteritis virus by complement-dependent monoclonal antibodies. Am. J. Vet. Res. 1988, 49, 300–304. [Google Scholar]
- Zhang, Q.; Shi, K.; Yoo, D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology 2016, 489, 252–268. [Google Scholar] [CrossRef]
- Xu, X.G.; Zhang, H.L.; Zhang, Q.; Dong, J.; Huang, Y.; Tong, D.W. Porcine epidemic diarrhea virus M protein blocks cell cycle progression at S-phase and its subcellular localization in the porcine intestinal epithelial cells. Acta. Virol. 2015, 59, 265–275. [Google Scholar] [CrossRef]
- McBride, R.; van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Zuniga, S.; Cruz, J.L.; Sola, I.; Mateos-Gomez, P.A.; Palacio, L.; Enjuanes, L. Coronavirus nucleocapsid protein facilitates template switching and is required for efficient transcription. J. Virol. 2010, 84, 2169–2175. [Google Scholar] [CrossRef]
- Hurst, K.R.; Ye, R.; Goebel, S.J.; Jayaraman, P.; Masters, P.S. An interaction between the nucleocapsid protein and a component of the replicase-transcriptase complex is crucial for the infectivity of coronavirus genomic RNA. J. Virol. 2010, 84, 10276–10288. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Fang, L.; Jing, H.; Zeng, S.; Wang, D.; Liu, L.; Zhang, H.; Luo, R.; Chen, H.; Xiao, S. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J. Virol. 2014, 88, 8936–8945. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Zhang, Q.; Huang, Y.; Dong, J.; Liang, Y.; Liu, H.J.; Tong, D. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet. Microbiol. 2013, 164, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Shi, H.; Sun, D.; Chen, J.; Zhang, X.; Wang, X.; Zhang, J.; Ji, Z.; Liu, J.; Cao, L.; et al. Nucleocapsid Interacts with NPM1 and Protects it from Proteolytic Cleavage, Enhancing Cell Survival, and is Involved in PEDV Growth. Sci. Rep. 2017, 7, 39700. [Google Scholar] [CrossRef]
- Liwnaree, B.; Narkpuk, J.; Sungsuwan, S.; Jongkaewwattana, A.; Jaru-Ampornpan, P. Growth enhancement of porcine epidemic diarrhea virus (PEDV) in Vero E6 cells expressing PEDV nucleocapsid protein. PLoS ONE 2019, 14, e0212632. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Hofmann, H.; Hattermann, K.; Marzi, A.; Gramberg, T.; Geier, M.; Krumbiegel, M.; Kuate, S.; Uberla, K.; Niedrig, M.; Pohlmann, S. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004, 78, 6134–6142. [Google Scholar] [CrossRef]
- Hulswit, R.J.; de Haan, C.A.; Bosch, B.J. Coronavirus Spike Protein and Tropism Changes. Adv. Virus Res. 2016, 96, 29–57. [Google Scholar] [CrossRef]
- Hou, Y.; Ke, H.; Kim, J.; Yoo, D.; Su, Y.; Boley, P.; Chepngeno, J.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Engineering a Live Attenuated Porcine Epidemic Diarrhea Virus Vaccine Candidate via Inactivation of the Viral 2′-O-Methyltransferase and the Endocytosis Signal of the Spike Protein. J. Virol. 2019, 93, e00406-19. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, X.; Zhou, S.; Zhou, T.; Tan, X.; Wu, X.; Tong, W.; Gao, F.; Yu, L.; Jiang, Y.; et al. A Virulent PEDV Strain FJzz1 with Genomic Mutations and Deletions at the High Passage Level Was Attenuated in Piglets via Serial Passage In Vitro. Virol. Sin. 2021, 36, 1052–1065. [Google Scholar] [CrossRef]
- Chen, P.; Wang, K.; Hou, Y.; Li, H.; Li, X.; Yu, L.; Jiang, Y.; Gao, F.; Tong, W.; Yu, H.; et al. Genetic evolution analysis and pathogenicity assessment of porcine epidemic diarrhea virus strains circulating in part of China during 2011-2017. Infect. Genet. Evol. 2019, 69, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Annamalai, T.; Liu, X.; Gao, X.; Lu, Z.; El-Tholoth, M.; Hu, H.; Saif, L.J.; Wang, Q. Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet. Res. 2015, 46, 134. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.J.; Deng, M.C.; Wang, F.I.; Tsai, S.H.; Chang, C.; Chang, C.Y.; Huang, Y.L. Deletion in the S1 Region of Porcine Epidemic Diarrhea Virus Reduces the Virulence and Influences the Virus-Neutralizing Activity of the Antibody Induced. Viruses 2020, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Meulia, T.; Gao, X.; Saif, L.J.; Wang, Q. Deletion of both the Tyrosine-Based Endocytosis Signal and the Endoplasmic Reticulum Retrieval Signal in the Cytoplasmic Tail of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Pigs. J. Virol. 2019, 93, e01758-18. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Wang, D.; Tian, X.; Song, Y.; Cao, Y. Identification and pathogenicity of a variant porcine epidemic diarrhea virus field strain with reduced virulence. Virol. J. 2015, 12, 88. [Google Scholar] [CrossRef]
- Lee, S.; Son, K.Y.; Noh, Y.H.; Lee, S.C.; Choi, H.W.; Yoon, I.J.; Lee, C. Genetic characteristics, pathogenicity, and immunogenicity associated with cell adaptation of a virulent genotype 2b porcine epidemic diarrhea virus. Vet. Microbiol. 2017, 207, 248–258. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, W.; Zhong, L.; Qin, Y.; Liu, X.; Yang, C.; Wang, R.; Su, X.; Du, C.; Mi, X.; et al. Comparative Characterization and Pathogenicity of a Novel Porcine Epidemic Diarrhea Virus (PEDV) with a Naturally Occurring Truncated ORF3 Gene Coinfected with PEDVs Possessing an Intact ORF3 Gene in Piglets. Viruses 2021, 13, 1562. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, H.X.; Chen, X.M.; Zhang, L.H.; Zhao, Y.Y.; Luo, A.F.; Yang, Y.R.; Zheng, L.L.; Chen, H.Y. Genetic Characteristics and Pathogenicity of a Novel Porcine Epidemic Diarrhea Virus with a Naturally Occurring Truncated ORF3 Gene. Viruses 2022, 14, 487. [Google Scholar] [CrossRef]
- Suzuki, T.; Terada, Y.; Enjuanes, L.; Ohashi, S.; Kamitani, W. S1 Subunit of Spike Protein from a Current Highly Virulent Porcine Epidemic Diarrhea Virus Is an Important Determinant of Virulence in Piglets. Viruses 2018, 10, 467. [Google Scholar] [CrossRef]
- Wang, D.; Ge, X.; Chen, D.; Li, J.; Cai, Y.; Deng, J.; Zhou, L.; Guo, X.; Han, J.; Yang, H. The S Gene Is Necessary but Not Sufficient for the Virulence of Porcine Epidemic Diarrhea Virus Novel Variant Strain BJ2011C. J. Virol. 2018, 92, e00603-18. [Google Scholar] [CrossRef]
- Song, D.S.; Yang, J.S.; Oh, J.S.; Han, J.H.; Park, B.K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine 2003, 21, 1833–1842. [Google Scholar] [CrossRef]
- Si, F.; Chen, B.; Hu, X.; Yu, R.; Dong, S.; Wang, R.; Li, Z. Porcine Epidemic Diarrhea Virus ORF3 Protein Is Transported through the Exocytic Pathway. J. Virol. 2020, 94, e00808-20. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Gralinski, L.E.; Mitchell, H.D.; Dinnon, K.H., 3rd; Leist, S.R.; Yount, B.L., Jr.; McAnarney, E.T.; Graham, R.L.; Waters, K.M.; Baric, R.S. Combination Attenuation Offers Strategy for Live Attenuated Coronavirus Vaccines. J. Virol. 2018, 92, e00710-18. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Li, C.; Qi, S.; Yang, D.; Jiang, N.; Yin, B.; Guo, D.; Kong, F.; Yuan, D.; Feng, L.; et al. A molecular epidemiological investigation of PEDV in China: Characterization of co-infection and genetic diversity of S1-based genes. Transbound Emerg. Dis. 2020, 67, 1129–1140. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wang, J.; Man, K.; Yang, Q. Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine 2017, 35, 7033–7041. [Google Scholar] [CrossRef]
- Wen, F.; Yang, J.; Li, A.; Gong, Z.; Yang, L.; Cheng, Q.; Wang, C.; Zhao, M.; Yuan, S.; Chen, Y.; et al. Genetic characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Guangdong, China, between 2018 and 2019. PLoS ONE 2021, 16, e0253622. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, X.; Li, H.; Ma, B.; Guan, R.; Yang, J.; Chen, D.; Han, X.; Zhou, L.; Song, Z.; et al. Molecular characterization of porcine epidemic diarrhea virus associated with outbreaks in southwest China during 2014–2018. Transbound Emerg. Dis. 2021, 68, 3482–3497. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, L.; Yang, J.; Yu, F.; Ge, J.; Guo, Q.; Gao, X.; Song, T. Sequence heterogeneity of the ORF3 gene of porcine epidemic diarrhea viruses field samples in Fujian, China, 2010—2012. Viruses 2013, 5, 2375–2383. [Google Scholar] [CrossRef]
- Escors, D.; Camafeita, E.; Ortego, J.; Laude, H.; Enjuanes, L. Organization of two transmissible gastroenteritis coronavirus membrane protein topologies within the virion and core. J. Virol. 2001, 75, 12228–12240. [Google Scholar] [CrossRef]
- Chen, J.F.; Sun, D.B.; Wang, C.B.; Shi, H.Y.; Cui, X.C.; Liu, S.W.; Qiu, H.J.; Feng, L. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes 2008, 36, 355–364. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Lang, H.; Wang, Z.; Shi, D.; Shi, H.; Zhang, X.; Feng, L. Genetic variation of nucleocapsid genes of porcine epidemic diarrhea virus field strains in China. Arch. Virol. 2013, 158, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, F.; Yuan, Y.; Zeng, X.; Wei, Z.; Zhu, L.; Sun, B.; Xie, Q.; Cao, Y.; Xue, C.; et al. Sequence and phylogenetic analysis of nucleocapsid genes of porcine epidemic diarrhea virus (PEDV) strains in China. Arch. Virol. 2013, 158, 1267–1273. [Google Scholar] [CrossRef][Green Version]
- Park, J.Y.; Pak, S.I.; Sung, H.W.; Kim, J.H.; Song, C.S.; Lee, C.W.; Kwon, H.M. Variations in the nucleocapsid protein gene of infectious bronchitis viruses isolated in Korea. Virus Genes 2005, 31, 153–162. [Google Scholar] [CrossRef] [PubMed]
- de Haan, C.A.; Rottier, P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005, 64, 165–230. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zeng, Y.; Zhang, R.; Peng, K.; Jiang, C.; Xu, Z.; Zhu, L. Genetic variations in S gene of porcine epidemic diarrhoea virus from 2018 in Sichuan Province, China. Vet. Med. Sci. 2020, 6, 910–918. [Google Scholar] [CrossRef]
- Kim, S.J.; Nguyen, V.G.; Huynh, T.M.; Park, Y.H.; Park, B.K.; Chung, H.C. Molecular Characterization of Porcine Epidemic Diarrhea Virus and Its New Genetic Classification Based on the Nucleocapsid Gene. Viruses 2020, 12, 790. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q. Emerging Highly Virulent Porcine Epidemic Diarrhea Virus: Molecular Mechanisms of Attenuation and Rational Design of Live Attenuated Vaccines. Int. J. Mol. Sci. 2019, 20, 5478. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Chen, J.; Zhang, X.; Ji, Z.; Yuan, J.; Shi, D.; Cao, L.; Zhu, X.; Dong, H.; et al. Neutralization of genotype 2 porcine epidemic diarrhea virus strains by a novel monoclonal antibody. Virology 2017, 507, 257–262. [Google Scholar] [CrossRef]
- Lin, C.M.; Hou, Y.; Marthaler, D.G.; Gao, X.; Liu, X.; Zheng, L.; Saif, L.J.; Wang, Q. Attenuation of an original US porcine epidemic diarrhea virus strain PC22A via serial cell culture passage. Vet. Microbiol. 2017, 201, 62–71. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Zhou, Q.; Li, Q.; Xu, Z.; Shen, H.; Chen, F. Characterization and pathogenicity of Vero cell-attenuated porcine epidemic diarrhea virus CT strain. Virol. J. 2019, 16, 121. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kao, C.F.; Chang, C.Y.; Jeng, C.R.; Tsai, P.S.; Pang, V.F.; Chiou, H.Y.; Peng, J.Y.; Cheng, I.C.; Chang, H.W. Evaluation and Comparison of the Pathogenicity and Host Immune Responses Induced by a G2b Taiwan Porcine Epidemic Diarrhea Virus (Strain Pintung 52) and Its Highly Cell-Culture Passaged Strain in Conventional 5-Week-Old Pigs. Viruses 2017, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Thavorasak, T.; Chulanetra, M.; Glab-Ampai, K.; Teeranitayatarn, K.; Songserm, T.; Yodsheewan, R.; Sae-Lim, N.; Lekcharoensuk, P.; Sookrung, N.; Chaicumpa, W. Novel Neutralizing Epitope of PEDV S1 Protein Identified by IgM Monoclonal Antibody. Viruses 2022, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, W.; Lucio de Esesarte, E.; Guo, H.; van den Elzen, P.; Aarts, E.; van den Born, E.; Rottier, P.J.M.; Bosch, B.J. Cell Attachment Domains of the Porcine Epidemic Diarrhea Virus Spike Protein Are Key Targets of Neutralizing Antibodies. J. Virol. 2017, 91, e00273-17. [Google Scholar] [CrossRef] [PubMed]
- Okda, F.A.; Lawson, S.; Singrey, A.; Nelson, J.; Hain, K.S.; Joshi, L.R.; Christopher-Hennings, J.; Nelson, E.A.; Diel, D.G. The S2 glycoprotein subunit of porcine epidemic diarrhea virus contains immunodominant neutralizing epitopes. Virology 2017, 509, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Cruz, D.J.; Shin, H.J. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011, 156, 1749–1756. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, L.; Wang, G.; Shi, Y.; Dong, W.; Fu, Y.; Fu, Z.; Chen, H.; Peng, G. The trypsin-enhanced infection of porcine epidemic diarrhea virus is determined by the S2 subunit of the spike glycoprotein. J. Virol. 2021, 95, e02453-e20. [Google Scholar] [CrossRef]
- Belouzard, S.; Chu, V.C.; Whittaker, G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA 2009, 106, 5871–5876. [Google Scholar] [CrossRef]
- Ducatelle, R.; Coussement, W.; Pensaert, M.B.; Debouck, P.; Hoorens, J. In vivo morphogenesis of a new porcine enteric coronavirus, CV 777. Arch. Virol. 1981, 68, 35–44. [Google Scholar] [CrossRef]
- Ducatelle, R.; Coussement, W.; Debouck, P.; Hoorens, J. Pathology of experimental CV777 coronavirus enteritis in piglets. II. Electron microscopic study. Vet. Pathol. 1982, 19, 57–66. [Google Scholar] [CrossRef]
- Debouck, P.; Pensaert, M.; Coussement, W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV 777. Vet. Microbiol. 1981, 6, 157–165. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, H.J. Porcine epidemic diarrhea virus infects and replicates in porcine alveolar macrophages. Virus Res. 2014, 191, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Gu, H.; Qu, H.; Bao, W.; Li, Y.; Cai, D. Aberrant Cholesterol Metabolic Genes Regulation in a Negative Feedback Loop Induced by an Alphacoronavirus. Front. Nutr. 2022, 9, 870680. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Cruz, D.J.; Shin, H.J. Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells. Virus Res. 2014, 191, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R Soc. Lond. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Raftery, N.; Stevenson, N.J. Advances in anti-viral immune defence: Revealing the importance of the IFN JAK/STAT pathway. Cell Mol. Life Sci. 2017, 74, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Liu, D.X. Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells. J. Virol. 2009, 83, 8744–8758. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Y.; Yang, Q. Transferrin receptor 1 levels at the cell surface influence the susceptibility of newborn piglets to PEDV infection. PLoS Pathog. 2020, 16, e1008682. [Google Scholar] [CrossRef]
- Xia, S.; Yan, L.; Xu, W.; Agrawal, A.S.; Algaissi, A.; Tseng, C.K.; Wang, Q.; Du, L.; Tan, W.; Wilson, I.A.; et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019, 5, eaav4580. [Google Scholar] [CrossRef]
- Li, F. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J. Virol. 2015, 89, 1954–1964. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; van der Hoek, L.; Geier, M.; Berkhout, B.; Pohlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Xiao, X.; Chakraborti, S.; Dimitrov, A.S.; Gramatikoff, K.; Dimitrov, D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003, 312, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Raj, V.S.; van Kuppeveld, F.J.; Rottier, P.J.; Haagmans, B.L.; Bosch, B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 2013, 87, 9379–9383. [Google Scholar] [CrossRef] [PubMed]
- Godet, M.; Grosclaude, J.; Delmas, B.; Laude, H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994, 68, 8008–8016. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Sun, D.; Rajashankar, K.R.; Qian, Z.; Holmes, K.V.; Li, F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 10696–10701. [Google Scholar] [CrossRef]
- Wong, A.H.M.; Tomlinson, A.C.A.; Zhou, D.; Satkunarajah, M.; Chen, K.; Sharon, C.; Desforges, M.; Talbot, P.J.; Rini, J.M. Receptor-binding loops in alphacoronavirus adaptation and evolution. Nat. Commun. 2017, 8, 1735. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Delmas, B.; Gelfi, J.; L’Haridon, R.; Vogel, L.K.; Sjostrom, H.; Noren, O.; Laude, H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 1992, 357, 417–420. [Google Scholar] [CrossRef]
- Kubo, H.; Yamada, Y.K.; Taguchi, F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J. Virol. 1994, 68, 5403–5410. [Google Scholar] [CrossRef]
- Krempl, C.; Schultze, B.; Laude, H.; Herrler, G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997, 71, 3285–3287. [Google Scholar] [CrossRef]
- Peng, G.; Xu, L.; Lin, Y.L.; Chen, L.; Pasquarella, J.R.; Holmes, K.V.; Li, F. Crystal structure of bovine coronavirus spike protein lectin domain. J. Biol. Chem. 2012, 287, 41931–41938. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Ye, G.; Liu, Q.; Navid, M.T.; Zhong, X.; Li, Y.; Wan, C.; Xiao, S.; He, Q.; Fu, Z.F.; et al. Identification and Comparison of Receptor Binding Characteristics of the Spike Protein of Two Porcine Epidemic Diarrhea Virus Strains. Viruses 2016, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, J.; Guo, L.; Guo, T.; Zhang, L.; Feng, L.; Chen, H.; Wang, Y. Porcine Epidemic Diarrhea Virus-Induced Epidermal Growth Factor Receptor Activation Impairs the Antiviral Activity of Type I Interferon. J. Virol. 2018, 92, e02095-17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, Z.; Yang, F.; Cao, W.; Yang, J.; Ma, C.; Zhao, Z.; Tian, H.; Liu, X.; Ma, J.; et al. Porcine Epidemic Diarrhea Virus Membrane Protein Interacted with IRF7 to Inhibit Type I IFN Production during Viral Infection. J. Immunol. 2021, 206, 2909–2923. [Google Scholar] [CrossRef]
- Dong, S.; Wang, R.; Yu, R.; Chen, B.; Si, F.; Xie, C.; Li, Z. Identification of cellular proteins interacting with PEDV M protein through APEX2 labeling. J. Proteom. 2021, 240, 104191. [Google Scholar] [CrossRef]
- Su, M.; Shi, D.; Xing, X.; Qi, S.; Yang, D.; Zhang, J.; Han, Y.; Zhu, Q.; Sun, H.; Wang, X.; et al. Coronavirus Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Interacts with p53 To Induce Cell Cycle Arrest in S-Phase and Promotes Viral Replication. J. Virol 2021, 95, e0018721. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Smith, R. Nuclear functions of heterogeneous nuclear ribonucleoproteins A/B. Cell Mol. Life Sci. 2009, 66, 1239–1256. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, W.; Ye, S.; Lv, J.; Nie, A.; Zhang, B.; Sun, Y.; Han, H.; He, Q. Cellular hnRNP A1 Interacts with Nucleocapsid Protein of Porcine Epidemic Diarrhea Virus and Impairs Viral Replication. Viruses 2018, 10, 127. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Z.Q.; Li, G.W.; Chen, C.; Luo, H.; Liu, Y.J.; Zhuo, X.H.; Shi, X.F.; Fang, W.H.; Li, X.L. Nucleocapsid protein from porcine epidemic diarrhea virus isolates can antagonize interferon-lambda production by blocking the nuclear factor-kappaB nuclear translocation. J. Zhejiang Univ. Sci. B 2018, 19, 570–580. [Google Scholar] [CrossRef]
- Xu, J.; Mao, J.; Han, X.; Shi, F.; Gao, Q.; Wang, T.; Zhang, Z.; Shan, Y.; Fang, W.; Li, X. Porcine Epidemic Diarrhea Virus Inhibits HDAC1 Expression To Facilitate Its Replication via Binding of Its Nucleocapsid Protein to Host Transcription Factor Sp1. J. Virol. 2021, 95, e0085321. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Karagiannis, T.C. Regulation of immune responses by histone deacetylase inhibitors. ISRN Hematol. 2012, 2012, 690901. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Xu, J.; Duan, X.; Xu, X.; Li, P.; Cheng, L.; Zheng, L.; Li, X.; Zhang, Y.; Wang, X.; et al. Porcine epidemic diarrhea virus ORF3 protein causes endoplasmic reticulum stress to facilitate autophagy. Vet. Microbiol. 2019, 235, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; He, Q.; Jongkaewwattana, A. The Accessory Protein ORF3 Contributes to Porcine Epidemic Diarrhea Virus Replication by Direct Binding to the Spike Protein. Viruses 2018, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, L.; Xu, J.; Li, P.; Li, X.; Zou, D.; Zhang, Y.; Wang, X.; Wu, X.; Shen, Y.; et al. The accessory protein ORF3 of porcine epidemic diarrhea virus inhibits cellular interleukin-6 and interleukin-8 productions by blocking the nuclear factor-kappaB p65 activation. Vet. Microbiol. 2020, 251, 108892. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; Koonpaew, S.; Srisutthisamphan, K.; Viriyakitkosol, R.; Jaru-Ampornpan, P.; Jongkaewwattana, A. PEDV ORF3 Independently Regulates IkappaB Kinase beta-Mediated NF-kappaB and IFN-beta Promoter Activities. Pathogens 2020, 9, 376. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Senanayake, S.D.; Brian, D.A. A translation-attenuating intraleader open reading frame is selected on coronavirus mRNAs during persistent infection. Proc. Natl. Acad. Sci. USA 1993, 90, 11733–11737. [Google Scholar] [CrossRef]
- Zhang, Q.; Ke, H.; Blikslager, A.; Fujita, T.; Yoo, D. Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling. J. Virol. 2018, 92, e01677-17. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, J.; Yoo, D. Inhibition of NF-kappaB activity by the porcine epidemic diarrhea virus nonstructural protein 1 for innate immune evasion. Virology 2017, 510, 111–126. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Chen, J.; Shi, H.; Ji, Z.; Zhang, X.; Shi, D.; Liu, J.; Tian, J.; Wang, X.; et al. Innate Immune Evasion of Porcine Epidemic Diarrhea Virus through Degradation of the FBXW7 Protein via the Ubiquitin-Proteasome Pathway. J. Virol. 2022, 96, e0088921. [Google Scholar] [CrossRef]
- Yu, L.; Dong, J.; Wang, Y.; Zhang, P.; Liu, Y.; Zhang, L.; Liang, P.; Wang, L.; Song, C. Porcine epidemic diarrhea virus nsp4 induces pro-inflammatory cytokine and chemokine expression inhibiting viral replication in vitro. Arch. Virol. 2019, 164, 1147–1157. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Wei, D.; Zhang, H.; Luo, R.; Chen, H.; Li, K.; Xiao, S. Hepatitis A virus 3C protease cleaves NEMO to impair induction of beta interferon. J. Virol. 2014, 88, 10252–10258. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.; Li, K.; Zhong, H.; Fan, J.; Ouyang, C.; Zhang, H.; Duan, E.; Luo, R.; Zhang, Z.; et al. Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling. J. Virol. 2012, 86, 9311–9322. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Q.; Guo, X.K.; Yu, Z.B.; Xu, A.T.; Tang, J.; Feng, W.H. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-kappaB essential modulator. J. Virol. 2014, 88, 10934–10945. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.; Shi, Y.; Zhang, H.; Gao, L.; Peng, G.; Chen, H.; Li, K.; Xiao, S. Porcine Epidemic Diarrhea Virus 3C-Like Protease Regulates Its Interferon Antagonism by Cleaving NEMO. J. Virol. 2016, 90, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, B.; Liu, M.; Zhou, H.; He, K.; Fan, H. Nonstructural protein 6 of porcine epidemic diarrhea virus induces autophagy to promote viral replication via the PI3K/Akt/mTOR axis. Vet. Microbiol. 2020, 244, 108684. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.; Fry, E.; Carter, L.; Sainsbury, S.; Walter, T.; Nettleship, J.; Berrow, N.; Owens, R.; Gilbert, R.; Davidson, A.; et al. The nsp9 replicase protein of SARS-coronavirus, structure and functional insights. Structure 2004, 12, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Luo, X.; Li, R.; Xu, Y.; Zhang, J.; Ge, J.; Bu, Z.; Feng, L.; Wang, Y. Porcine Epidemic Diarrhea Virus Infection Inhibits Interferon Signaling by Targeted Degradation of STAT1. J. Virol. 2016, 90, 8281–8292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, S.; Peng, Q.; Ding, Z.; Hao, W.; Peng, G.; Xiao, S.; Fang, L. Porcine Epidemic Diarrhea Virus nsp7 Inhibits Interferon-Induced JAK-STAT Signaling through Sequestering the Interaction between KPNA1 and STAT1. J. Virol. 2022, 96, e0040022. [Google Scholar] [CrossRef]
- Ren, J.; Ding, Z.; Fang, P.; Xiao, S.; Fang, L. ATPase and helicase activities of porcine epidemic diarrhea virus nsp13. Vet. Microbiol. 2021, 257, 109074. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, H.; Lu, M.; Ma, Y.; Li, A.; Gao, Y.; Zhou, J.; Gu, H.; Li, J.; Gu, J. Porcine Epidemic Diarrhea Virus Deficient in RNA Cap Guanine-N-7 Methylation Is Attenuated and Induces Higher Type I and III Interferon Responses. J. Virol. 2020, 94, e00447-20. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Shi, Z.; Chen, J.; Li, M.; Shi, H.; Shi, D.; Guo, L.; Feng, L. Porcine Epidemic Diarrhea Virus nsp15 Antagonizes Interferon Signaling by RNA Degradation of TBK1 and IRF3. Viruses 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Su, Y.; Li, R.; Liang, Z.; Dong, S.; Huang, J. PEDV nsp16 negatively regulates innate immunity to promote viral proliferation. Virus Res. 2019, 265, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Ge, J.W.; Li, Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology 2007, 365, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef]
- Luo, X.; Guo, L.; Zhang, J.; Xu, Y.; Gu, W.; Feng, L.; Wang, Y. Tight Junction Protein Occludin Is a Porcine Epidemic Diarrhea Virus Entry Factor. J. Virol 2017, 91, e00202-17. [Google Scholar] [CrossRef]
- Sawada, N. Tight junction-related human diseases. Pathol. Int. 2013, 63, 1–12. [Google Scholar] [CrossRef]
- Yun, J.P.; Chew, E.C.; Liew, C.T.; Chan, J.Y.; Jin, M.L.; Ding, M.X.; Fai, Y.H.; Li, H.K.; Liang, X.M.; Wu, Q.L. Nucleophosmin/B23 is a proliferate shuttle protein associated with nuclear matrix. J. Cell Biochem. 2003, 90, 1140–1148. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, J.; Park, J.E.; Hong, E.J.; Shin, H.J. Heat shock protein 70 could enhance porcine epidemic diarrhoea virus replication by interacting with membrane proteins. Vet. Res. 2021, 52, 138. [Google Scholar] [CrossRef]
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014, 24, 26–34. [Google Scholar] [CrossRef]
- Kong, N.; Shan, T.; Wang, H.; Jiao, Y.; Zuo, Y.; Li, L.; Tong, W.; Yu, L.; Jiang, Y.; Zhou, Y.; et al. BST2 suppresses porcine epidemic diarrhea virus replication by targeting and degrading virus nucleocapsid protein with selective autophagy. Autophagy 2020, 16, 1737–1752. [Google Scholar] [CrossRef]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Teis, D. The ESCRT machinery. Curr. Biol. 2012, 22, R116–R120. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; Yingchutrakul, Y.; Roytrakul, S.; Jongkaewwattana, A. Porcine Epidemic Diarrhea Virus (PEDV) ORF3 Interactome Reveals Inhibition of Virus Replication by Cellular VPS36 Protein. Viruses 2019, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, J.; Li, M.; Chen, M.; Sun, C. Multifaceted Functions of CH25H and 25HC to Modulate the Lipid Metabolism, Immune Responses, and Broadly Antiviral Activities. Viruses 2020, 12, 727. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.; Wang, M.; Lan, M.; Zhang, K.; Jiang, P.; Li, Y.; Bai, J.; Wang, X. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Vet. Microbiol. 2019, 231, 129–138. [Google Scholar] [CrossRef]
- Sun, L.; Chen, H.; Ming, X.; Bo, Z.; Shin, H.J.; Jung, Y.S.; Qian, Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021, 95, e02344-20. [Google Scholar] [CrossRef]
- Pandey, K.; Zhong, S.; Diel, D.G.; Hou, Y.; Wang, Q.; Nelson, E.; Wang, X. GTPase-activating protein-binding protein 1 (G3BP1) plays an antiviral role against porcine epidemic diarrhea virus. Vet. Microbiol. 2019, 236, 108392. [Google Scholar] [CrossRef]
- Ramezani-Rad, P.; Leung, C.R.; Apgar, J.R.; Rickert, R.C. E3 Ubiquitin Ligase Fbw7 Regulates the Survival of Mature B Cells. J. Immunol. 2020, 204, 1535–1542. [Google Scholar] [CrossRef]
- Wang, Y.; Robertson, J.D.; Walcheck, B. Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation. J. Biol. Chem. 2011, 286, 38980–38988. [Google Scholar] [CrossRef]
- Masutani, M.; Sonenberg, N.; Yokoyama, S.; Imataka, H. Reconstitution reveals the functional core of mammalian eIF3. EMBO J. 2007, 26, 3373–3383. [Google Scholar] [CrossRef]
- Wang, R.; Yu, R.; Chen, B.; Si, F.; Wang, J.; Xie, C.; Men, C.; Dong, S.; Li, Z. Identification of host cell proteins that interact with the M protein of porcine epidemic diarrhea virus. Vet. Microbiol. 2020, 246, 108729. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.R.; Shum, L.; Glaunsinger, B.A. Importin alpha-mediated nuclear import of cytoplasmic poly(A) binding protein occurs as a direct consequence of cytoplasmic mRNA depletion. Mol. Cell Biol. 2011, 31, 3113–3125. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, X.; Wu, Q.; Yang, Q. Role of intestinal extracellular matrix-related signaling in porcine epidemic diarrhea virus infection. Virulence 2021, 12, 2352–2365. [Google Scholar] [CrossRef]
- Garbers, C.; Scheller, J. Interleukin-6 and interleukin-11: Same same but different. Biol. Chem. 2013, 394, 1145–1161. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Gao, Y.; Xu, X.; Fu, K.; Wang, S.; Li, Y.; Peng, R. The protective role of interleukin-11 against neutron radiation injury in mouse intestines via MEK/ERK and PI3K/Akt dependent pathways. Dig. Dis. Sci. 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Uemura, T.; Nakayama, T.; Kusaba, T.; Yakata, Y.; Yamazumi, K.; Matsuu-Matsuyama, M.; Shichijo, K.; Sekine, I. The protective effect of interleukin-11 on the cell death induced by X-ray irradiation in cultured intestinal epithelial cell. J. Radiat. Res. 2007, 48, 171–177. [Google Scholar] [CrossRef][Green Version]
- Agthe, M.; Garbers, Y.; Putoczki, T.; Garbers, C. Interleukin-11 classic but not trans-signaling is essential for fertility in mice. Placenta 2017, 57, 13–16. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Jin, Y.; Yang, Q. Antiviral activity of interleukin-11 as a response to porcine epidemic diarrhea virus infection. Vet. Res. 2019, 50, 111. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008, 29, 947–957. [Google Scholar] [CrossRef]
- Basu, R.; O’Quinn, D.B.; Silberger, D.J.; Schoeb, T.R.; Fouser, L.; Ouyang, W.; Hatton, R.D.; Weaver, C.T. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 2012, 37, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.S.A.; Melo, E.O. Mucin 2 (MUC2) promoter characterization: An overview. Cell Tissue Res. 2018, 374, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhou, Y.; Sun, S.; Wang, H.; Wu, S.; Bao, W. Effect of Promoter Methylation on the Expression of Porcine MUC2 Gene and Resistance to PEDV Infection. Front. Vet. Sci. 2021, 8, 646408. [Google Scholar] [CrossRef]
- Yeager, C.L.; Ashmun, R.A.; Williams, R.K.; Cardellichio, C.B.; Shapiro, L.H.; Look, A.T.; Holmes, K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 1992, 357, 420–422. [Google Scholar] [CrossRef]
- Kamau, A.N.; Park, J.E.; Park, E.S.; Yu, J.E.; Rho, J.; Shin, H.J. Porcine amino peptidase N domain VII has critical role in binding and entry of porcine epidemic diarrhea virus. Virus Res. 2017, 227, 150–157. [Google Scholar] [CrossRef]
- Li, W.; Luo, R.; He, Q.; van Kuppeveld, F.J.M.; Rottier, P.J.M.; Bosch, B.J. Aminopeptidase N is not required for porcine epidemic diarrhea virus cell entry. Virus Res. 2017, 235, 6–13. [Google Scholar] [CrossRef]
- Park, J.E.; Park, E.S.; Yu, J.E.; Rho, J.; Paudel, S.; Hyun, B.H.; Yang, D.K.; Shin, H.J. Development of transgenic mouse model expressing porcine aminopeptidase N and its susceptibility to porcine epidemic diarrhea virus. Virus Res. 2015, 197, 108–115. [Google Scholar] [CrossRef]
- Shirato, K.; Maejima, M.; Islam, M.T.; Miyazaki, A.; Kawase, M.; Matsuyama, S.; Taguchi, F. Porcine aminopeptidase N is not a cellular receptor of porcine epidemic diarrhea virus, but promotes its infectivity via aminopeptidase activity. J. Gen. Virol. 2016, 97, 2528–2539. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef]
- Burzynska, P.; Sobala, L.F.; Mikolajczyk, K.; Jodlowska, M.; Jaskiewicz, E. Sialic Acids as Receptors for Pathogens. Biomolecules 2021, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, I.N.; de Vries, R.P.; Grone, A.; de Haan, C.A.; Verheije, M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011, 85, 8903–8912. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; Fouchier, R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hulswit, R.J.G.; Widjaja, I.; Raj, V.S.; McBride, R.; Peng, W.; Widagdo, W.; Tortorici, M.A.; van Dieren, B.; Lang, Y.; et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA 2017, 114, E8508–E8517. [Google Scholar] [CrossRef]
- Schultze, B.; Enjuanes, L.; Cavanagh, D.; Herrler, G. N-acetylneuraminic acid plays a critical role for the haemagglutinating activity of avian infectious bronchitis virus and porcine transmissible gastroenteritis virus. Adv. Exp. Med. Biol. 1993, 342, 305–310. [Google Scholar] [CrossRef]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular organization of tricellular tight junctions. Tissue Barriers 2014, 2, e28960. [Google Scholar] [CrossRef]
- Torres-Flores, J.M.; Arias, C.F. Tight Junctions Go Viral! Viruses 2015, 7, 5145–5154. [Google Scholar] [CrossRef]
- Ploss, A.; Evans, M.J.; Gaysinskaya, V.A.; Panis, M.; You, H.; de Jong, Y.P.; Rice, C.M. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 2009, 457, 882–886. [Google Scholar] [CrossRef]
- Coyne, C.B.; Shen, L.; Turner, J.R.; Bergelson, J.M. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2007, 2, 181–192. [Google Scholar] [CrossRef]
- Zong, Q.F.; Huang, Y.J.; Wu, L.S.; Wu, Z.C.; Wu, S.L.; Bao, W.B. Effects of porcine epidemic diarrhea virus infection on tight junction protein gene expression and morphology of the intestinal mucosa in pigs. Pol J. Vet. Sci. 2019, 22, 345–353. [Google Scholar] [CrossRef]
- Fankhauser, C.; Izaurralde, E.; Adachi, Y.; Wingfield, P.; Laemmli, U.K. Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: Dissociation by the Rev response element. Mol. Cell Biol. 1991, 11, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Mori, Y.; Abe, T.; Yamashita, T.; Okamoto, T.; Ichimura, T.; Moriishi, K.; Matsuura, Y. Nucleolar protein B23 interacts with Japanese encephalitis virus core protein and participates in viral replication. Microbiol. Immunol. 2006, 50, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.A.; Okuwaki, M.; Haruki, H.; Nagata, K. Physical and functional interaction between a nucleolar protein nucleophosmin/B23 and adenovirus basic core proteins. FEBS Lett. 2007, 581, 3283–3288. [Google Scholar] [CrossRef][Green Version]
- Lymberopoulos, M.H.; Bourget, A.; Ben Abdeljelil, N.; Pearson, A. Involvement of the UL24 protein in herpes simplex virus 1-induced dispersal of B23 and in nuclear egress. Virology 2011, 412, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.D.; Chen, Y.L.; Min, Y.L.; Zhao, B.; Cheng, C.P.; Kang, M.S.; Chiu, S.J.; Kieff, E.; Peng, C.W. The nuclear chaperone nucleophosmin escorts an Epstein-Barr Virus nuclear antigen to establish transcriptional cascades for latent infection in human B cells. PLoS Pathog. 2012, 8, e1003084. [Google Scholar] [CrossRef] [PubMed]
- Passos-Castilho, A.M.; Marchand, C.; Archambault, D. B23/nucleophosmin interacts with bovine immunodeficiency virus Rev protein and facilitates viral replication. Virology 2018, 515, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Li, H.; Zhang, Y.; Dong, W.; Jin, Y.; Yan, Y.; Gu, J.; Zhou, J. The serine-48 residue of nucleolar phosphoprotein nucleophosmin-1 plays critical role in subcellular localization and interaction with porcine circovirus type 3 capsid protein. Vet. Res. 2021, 52, 4. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hou, L.; Wang, D.; Wei, L.; Zhu, S.; Wang, J.; Quan, R.; Jiang, H.; Shi, R.; Liu, J. Nucleolar Phosphoprotein NPM1 Interacts With Porcine Circovirus Type 3 Cap Protein and Facilitates Viral Replication. Front. Microbiol. 2021, 12, 679341. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, Y.; Lin, C.; Zhang, Y.; Feng, Z.; Dong, W.; Jin, Y.; Yan, Y.; Zhou, J.; Gu, J. Nucleolar protein NPM1 is essential for circovirus replication by binding to viral capsid. Virulence 2020, 11, 1379–1393. [Google Scholar] [CrossRef]
- Abraham, R.; Singh, S.; Nair, S.R.; Hulyalkar, N.V.; Surendran, A.; Jaleel, A.; Sreekumar, E. Nucleophosmin (NPM1)/B23 in the Proteome of Human Astrocytic Cells Restricts Chikungunya Virus Replication. J. Proteome Res. 2017, 16, 4144–4155. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, C.; Braakman, I. Synthesis and quality control of viral membrane proteins. Curr Top. Microbiol. Immunol. 2005, 285, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.D.; Wang, R.Y.; Pogany, J.; Hafren, A.; Makinen, K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 2011, 411, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, E.; Atagi, H.; Hiraki, A.; Kim, J. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J. Virol. 2004, 78, 1263–1270. [Google Scholar] [CrossRef]
- De Marco, A.; Santoro, M.G. Antiviral effect of short hyperthermic treatment at specific stages of vesicular stomatitis virus replication cycle. J. Gen. Virol 1993, 74 Pt 8, 1685–1690. [Google Scholar] [CrossRef]
- Lupberger, J.; Zeisel, M.B.; Xiao, F.; Thumann, C.; Fofana, I.; Zona, L.; Davis, C.; Mee, C.J.; Turek, M.; Gorke, S.; et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011, 17, 589–595. [Google Scholar] [CrossRef]
- Diao, J.; Pantua, H.; Ngu, H.; Komuves, L.; Diehl, L.; Schaefer, G.; Kapadia, S.B. Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J. Virol. 2012, 86, 10935–10949. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, S.; Shen, Y.; Yang, Q. Epidermal growth factor receptor is a co-factor for transmissible gastroenteritis virus entry. Virology 2018, 521, 33–43. [Google Scholar] [CrossRef]
- Eierhoff, T.; Hrincius, E.R.; Rescher, U.; Ludwig, S.; Ehrhardt, C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010, 6, e1001099. [Google Scholar] [CrossRef]
- Klann, K.; Bojkova, D.; Tascher, G.; Ciesek, S.; Munch, C.; Cinatl, J. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Mol. Cell 2020, 80, 164–174.e164. [Google Scholar] [CrossRef]
- Wang, S.M.; Huang, K.J.; Wang, C.T. BST2/CD317 counteracts human coronavirus 229E productive infection by tethering virions at the cell surface. Virology 2014, 449, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.B.; Qu, X.W.; Jiang, D.; Zhao, X.L.; Han, J.C.; Wei, L. BST2/Tetherin inhibits hepatitis C virus production in human hepatoma cells. Antiviral. Res. 2013, 98, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.B.; Han, J.C.; Cong, X.; Wei, L. BST2/tetherin inhibits dengue virus release from human hepatoma cells. PLoS ONE 2012, 7, e51033. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, P.; Zheng, Z.; Hu, K.; Zhang, M.; Guan, X.; Fu, M.; Zhang, D.; Wang, W.; Xiao, G.; et al. Japanese encephalitis virus counteracts BST2 restriction via its envelope protein E. Virology 2017, 510, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Le Tortorec, A.; Neil, S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009, 83, 11966–11978. [Google Scholar] [CrossRef]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef]
- Jouvenet, N.; Neil, S.J.; Zhadina, M.; Zang, T.; Kratovac, Z.; Lee, Y.; McNatt, M.; Hatziioannou, T.; Bieniasz, P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009, 83, 1837–1844. [Google Scholar] [CrossRef]
- Sakuma, T.; Noda, T.; Urata, S.; Kawaoka, Y.; Yasuda, J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009, 83, 2382–2385. [Google Scholar] [CrossRef]
- Blondeau, C.; Pelchen-Matthews, A.; Mlcochova, P.; Marsh, M.; Milne, R.S.; Towers, G.J. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein M. J. Virol. 2013, 87, 13124–13133. [Google Scholar] [CrossRef]
- Viswanathan, K.; Smith, M.S.; Malouli, D.; Mansouri, M.; Nelson, J.A.; Fruh, K. BST2/Tetherin enhances entry of human cytomegalovirus. PLoS Pathog. 2011, 7, e1002332. [Google Scholar] [CrossRef]
- Teo, H.; Perisic, O.; Gonzalez, B.; Williams, R.L. ESCRT-II, an endosome-associated complex required for protein sorting: Crystal structure and interactions with ESCRT-III and membranes. Dev. Cell. 2004, 7, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e116. [Google Scholar] [CrossRef]
- Wang, R.; Simoneau, C.R.; Kulsuptrakul, J.; Bouhaddou, M.; Travisano, K.A.; Hayashi, J.M.; Carlson-Stevermer, J.; Zengel, J.R.; Richards, C.M.; Fozouni, P.; et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell 2021, 184, 106–119.e114. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Gale, M., Jr. Sterol-izing innate immunity. Immunity 2013, 38, 3–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Z.; Tian, B.; Zhou, M.; Fu, Z.F.; Zhao, L. Cholesterol 25-hydroxylase suppresses rabies virus infection by inhibiting viral entry. Arch. Virol. 2019, 164, 2963–2974. [Google Scholar] [CrossRef]
- Shrivastava-Ranjan, P.; Bergeron, E.; Chakrabarti, A.K.; Albarino, C.G.; Flint, M.; Nichol, S.T.; Spiropoulou, C.F. 25-Hydroxycholesterol Inhibition of Lassa Virus Infection through Aberrant GP1 Glycosylation. mBio 2016, 7, e01808-16. [Google Scholar] [CrossRef]
- Magoro, T.; Dandekar, A.; Jennelle, L.T.; Bajaj, R.; Lipkowitz, G.; Angelucci, A.R.; Bessong, P.O.; Hahn, Y.S. IL-1beta/TNF-alpha/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. J. Biol. Chem. 2019, 294, 14591–14602. [Google Scholar] [CrossRef]
- Roulin, P.S.; Lotzerich, M.; Torta, F.; Tanner, L.B.; van Kuppeveld, F.J.; Wenk, M.R.; Greber, U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe 2014, 16, 677–690. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Zhang, L.; Guo, Z.Z.; Lu, S.F.; Ming, S.L.; Li, G.L.; Wan, B.; Tian, K.G.; Yang, G.Y.; et al. Cholesterol 25-hydroxylase acts as a host restriction factor on pseudorabies virus replication. J. Gen. Virol. 2017, 98, 1467–1476. [Google Scholar] [CrossRef]
- Ke, W.; Fang, L.; Jing, H.; Tao, R.; Wang, T.; Li, Y.; Long, S.; Wang, D.; Xiao, S. Cholesterol 25-Hydroxylase Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication through Enzyme Activity-Dependent and -Independent Mechanisms. J. Virol. 2017, 91, e00827-17. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Antiviral effect of 25-hydroxycholesterol against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther 2018, 23, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Jin, J.; Li, J.; Ye, M.; Jin, X. The roles of G3BP1 in human diseases (review). Gene 2022, 821, 146294. [Google Scholar] [CrossRef] [PubMed]
- Tourriere, H.; Chebli, K.; Zekri, L.; Courselaud, B.; Blanchard, J.M.; Bertrand, E.; Tazi, J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 2003, 160, 823–831. [Google Scholar] [CrossRef]
- McCormick, C.; Khaperskyy, D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017, 17, 647–660. [Google Scholar] [CrossRef]
- Yang, W.; Ru, Y.; Ren, J.; Bai, J.; Wei, J.; Fu, S.; Liu, X.; Li, D.; Zheng, H. G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell Death Dis. 2019, 10, 946. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, Q.; Yin, M.; Qin, L.; Hu, J.; Chen, H.; Li, X.; Qian, P. Seneca Valley Virus 3C Protease Inhibits Stress Granule Formation by Disrupting eIF4GI-G3BP1 Interaction. Front. Immunol. 2020, 11, 577838. [Google Scholar] [CrossRef]
- Humoud, M.N.; Doyle, N.; Royall, E.; Willcocks, M.M.; Sorgeloos, F.; van Kuppeveld, F.; Roberts, L.O.; Goodfellow, I.G.; Langereis, M.A.; Locker, N. Feline Calicivirus Infection Disrupts Assembly of Cytoplasmic Stress Granules and Induces G3BP1 Cleavage. J. Virol. 2016, 90, 6489–6501. [Google Scholar] [CrossRef]
- Ng, C.S.; Jogi, M.; Yoo, J.S.; Onomoto, K.; Koike, S.; Iwasaki, T.; Yoneyama, M.; Kato, H.; Fujita, T. Encephalomyocarditis virus disrupts stress granules, the critical platform for triggering antiviral innate immune responses. J. Virol. 2013, 87, 9511–9522. [Google Scholar] [CrossRef]
- Visser, L.J.; Medina, G.N.; Rabouw, H.H.; de Groot, R.J.; Langereis, M.A.; de Los Santos, T.; van Kuppeveld, F.J.M. Foot-and-Mouth Disease Virus Leader Protease Cleaves G3BP1 and G3BP2 and Inhibits Stress Granule Formation. J. Virol. 2019, 93, e00922-18. [Google Scholar] [CrossRef]
- Panas, M.D.; Varjak, M.; Lulla, A.; Eng, K.E.; Merits, A.; Karlsson Hedestam, G.B.; McInerney, G.M. Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection. Mol. Biol. Cell 2012, 23, 4701–4712. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, Z.; Zhang, Q.; Fan, S.; Zhong, Y.; Guo, D.; Qin, Y.; Chen, M. SG formation relies on eIF4GI-G3BP interaction which is targeted by picornavirus stress antagonists. Cell Discov. 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Cardenas, A.M.; Marissen, W.E.; Lloyd, R.E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2007, 2, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Nabeel-Shah, S.; Lee, H.; Ahmed, N.; Burke, G.L.; Farhangmehr, S.; Ashraf, K.; Pu, S.; Braunschweig, U.; Zhong, G.; Wei, H.; et al. SARS-CoV-2 nucleocapsid protein binds host mRNAs and attenuates stress granules to impair host stress response. iScience 2022, 25, 103562. [Google Scholar] [CrossRef]
- Khaperskyy, D.A.; Emara, M.M.; Johnston, B.P.; Anderson, P.; Hatchette, T.F.; McCormick, C. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS Pathog. 2014, 10, e1004217. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Wang, Y.; Li, X.; Gao, L.; Cao, H.; Zheng, S.J. Critical role for G3BP1 in infectious bursal disease virus (IBDV)-induced stress granule formation and viral replication. Vet. Microbiol. 2020, 248, 108806. [Google Scholar] [CrossRef]
- Choudhury, P.; Bussiere, L.D.; Miller, C.L. Mammalian Orthoreovirus Factories Modulate Stress Granule Protein Localization by Interaction with G3BP1. J. Virol. 2017, 91, e01298-17. [Google Scholar] [CrossRef]
- Scholte, F.E.; Tas, A.; Albulescu, I.C.; Zusinaite, E.; Merits, A.; Snijder, E.J.; van Hemert, M.J. Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication. J. Virol. 2015, 89, 4457–4469. [Google Scholar] [CrossRef]
- Wang, Z.; Inuzuka, H.; Fukushima, H.; Wan, L.; Gao, D.; Shaik, S.; Sarkar, F.H.; Wei, W. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 2011, 13, 36–43. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Chen, S.; Chen, S.; Xiang, N.; Chen, Y.; Guo, D. Ubiquitin ligase Fbw7 restricts the replication of hepatitis C virus by targeting NS5B for ubiquitination and degradation. Biochem. Biophys. Res. Commun. 2016, 470, 697–703. [Google Scholar] [CrossRef]
- Mikulicic, S.; Finke, J.; Boukhallouk, F.; Wustenhagen, E.; Sons, D.; Homsi, Y.; Reiss, K.; Lang, T.; Florin, L. ADAM17-dependent signaling is required for oncogenic human papillomavirus entry platform assembly. eLife 2019, 8, e44345. [Google Scholar] [CrossRef] [PubMed]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Li, D.; Li, C.; Zhang, Y.; Song, H.; Li, S.; Deng, H.; Gao, G.F.; Zheng, A. ADAM17 is an essential attachment factor for classical swine fever virus. PLoS Pathog. 2021, 17, e1009393. [Google Scholar] [CrossRef] [PubMed]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pohlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol 2014, 88, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Niu, J.; Yu, H.; Gu, W.; Li, R.; Luo, X.; Huang, M.; Tian, Z.; Feng, L.; Wang, Y. Modulation of CD163 expression by metalloprotease ADAM17 regulates porcine reproductive and respiratory syndrome virus entry. J. Virol. 2014, 88, 10448–10458. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, L.; Yang, L.; Xu, J.; Zhang, L.; Feng, L.; Chen, H.; Wang, Y. Metalloprotease ADAM17 regulates porcine epidemic diarrhea virus infection by modifying aminopeptidase N. Virology 2018, 517, 24–29. [Google Scholar] [CrossRef]
- Valente, S.T.; Gilmartin, G.M.; Mott, C.; Falkard, B.; Goff, S.P. Inhibition of HIV-1 replication by eIF3f. Proc. Natl. Acad. Sci. USA 2009, 106, 4071–4078. [Google Scholar] [CrossRef]
- Morais, A.; Terzian, A.C.; Duarte, D.V.; Bronzoni, R.V.; Madrid, M.C.; Gavioli, A.F.; Gil, L.H.; Oliveira, A.G.; Zanelli, C.F.; Valentini, S.R.; et al. The eukaryotic translation initiation factor 3 subunit L protein interacts with Flavivirus NS5 and may modulate yellow fever virus replication. Virol. J. 2013, 10, 205. [Google Scholar] [CrossRef]
- Holcomb, D.; Alexaki, A.; Hernandez, N.; Hunt, R.; Laurie, K.; Kames, J.; Hamasaki-Katagiri, N.; Komar, A.A.; DiCuccio, M.; Kimchi-Sarfaty, C. Gene variants of coagulation related proteins that interact with SARS-CoV-2. PLoS Comput. Biol. 2021, 17, e1008805. [Google Scholar] [CrossRef]
- Jiao, Y.; Kong, N.; Wang, H.; Sun, D.; Dong, S.; Chen, X.; Zheng, H.; Tong, W.; Yu, H.; Yu, L.; et al. PABPC4 Broadly Inhibits Coronavirus Replication by Degrading Nucleocapsid Protein through Selective Autophagy. Microbiol. Spectr. 2021, 9, e0090821. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Sleeman, J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv. Cancer Res. 2014, 123, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Heldin, P.; Basu, K.; Kozlova, I.; Porsch, H. HAS2 and CD44 in breast tumorigenesis. Adv. Cancer Res. 2014, 123, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.J.; Racaniello, V.R. CD44 is not required for poliovirus replication. J. Virol. 1997, 71, 2793–2798. [Google Scholar] [CrossRef]
- Goujon, C.; Rebendenne, A.; Roy, P.; Bonaventure, B.; Valadao, A.C.; Desmarets, L.; Rouille, Y.; Tauziet, M.; Arnaud-Arnould, M.; Giovannini, D.; et al. Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal HCoVs. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Liu, A.; Pan, Q.; Wang, S.; Zhang, Y.; Li, Y.; Wang, Y.; Qi, X.; Gao, L.; Liu, C.; Zhang, Y.; et al. Identification of Chicken CD44 as a Novel B Lymphocyte Receptor for Infectious Bursal Disease Virus. J. Virol. 2022, 96, e0011322. [Google Scholar] [CrossRef]
- Favors, S.E.; Curd, L.M.; Gregg, R.K. Use of the anti-inflammatory cytokine interleukin-11 to reverse HIV-1gp120 repression of a natural killer cell line. Cell Immunol. 2012, 276, 1–5. [Google Scholar] [CrossRef]
- Xue, M.; Zhao, J.; Ying, L.; Fu, F.; Li, L.; Ma, Y.; Shi, H.; Zhang, J.; Feng, L.; Liu, P. IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway. Antiviral. Res. 2017, 142, 68–75. [Google Scholar] [CrossRef]
- Das, S.; St Croix, C.; Good, M.; Chen, J.; Zhao, J.; Hu, S.; Ross, M.; Myerburg, M.M.; Pilewski, J.M.; Williams, J.; et al. Interleukin-22 Inhibits Respiratory Syncytial Virus Production by Blocking Virus-Mediated Subversion of Cellular Autophagy. iScience 2020, 23, 101256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wan, S.; Sun, N.; Sun, P.; Sun, Y.; Khan, A.; Guo, J.; Zheng, X.; Fan, K.; Yin, W.; et al. Damage to intestinal barrier integrity in piglets caused by porcine reproductive and respiratory syndrome virus infection. Vet. Res. 2021, 52, 93. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Liu, Y.; Li, C.; Zhang, L.; Wang, T.; Zhao, D.; Xu, X.; Zhang, Y. MicroRNA-221-5p Inhibits Porcine Epidemic Diarrhea Virus Replication by Targeting Genomic Viral RNA and Activating the NF-kappaB Pathway. Int. J. Mol. Sci. 2018, 19, 3381. [Google Scholar] [CrossRef]
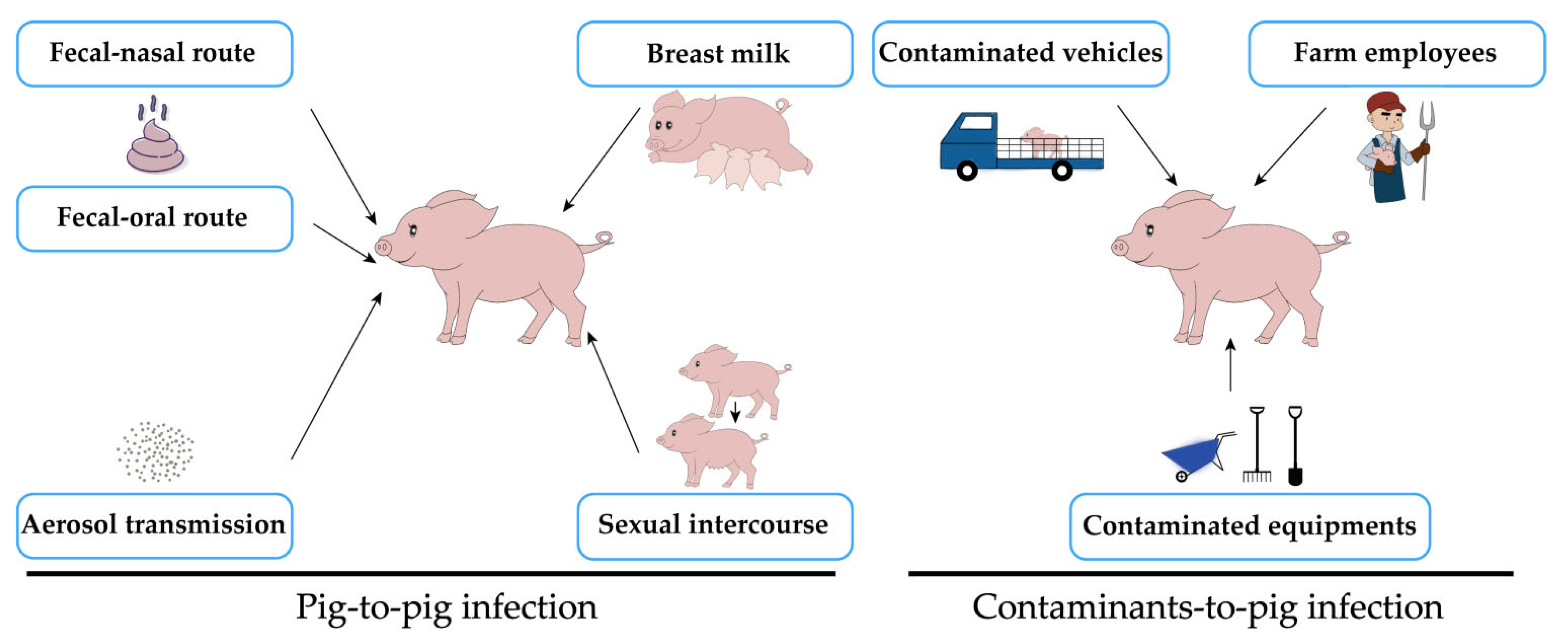

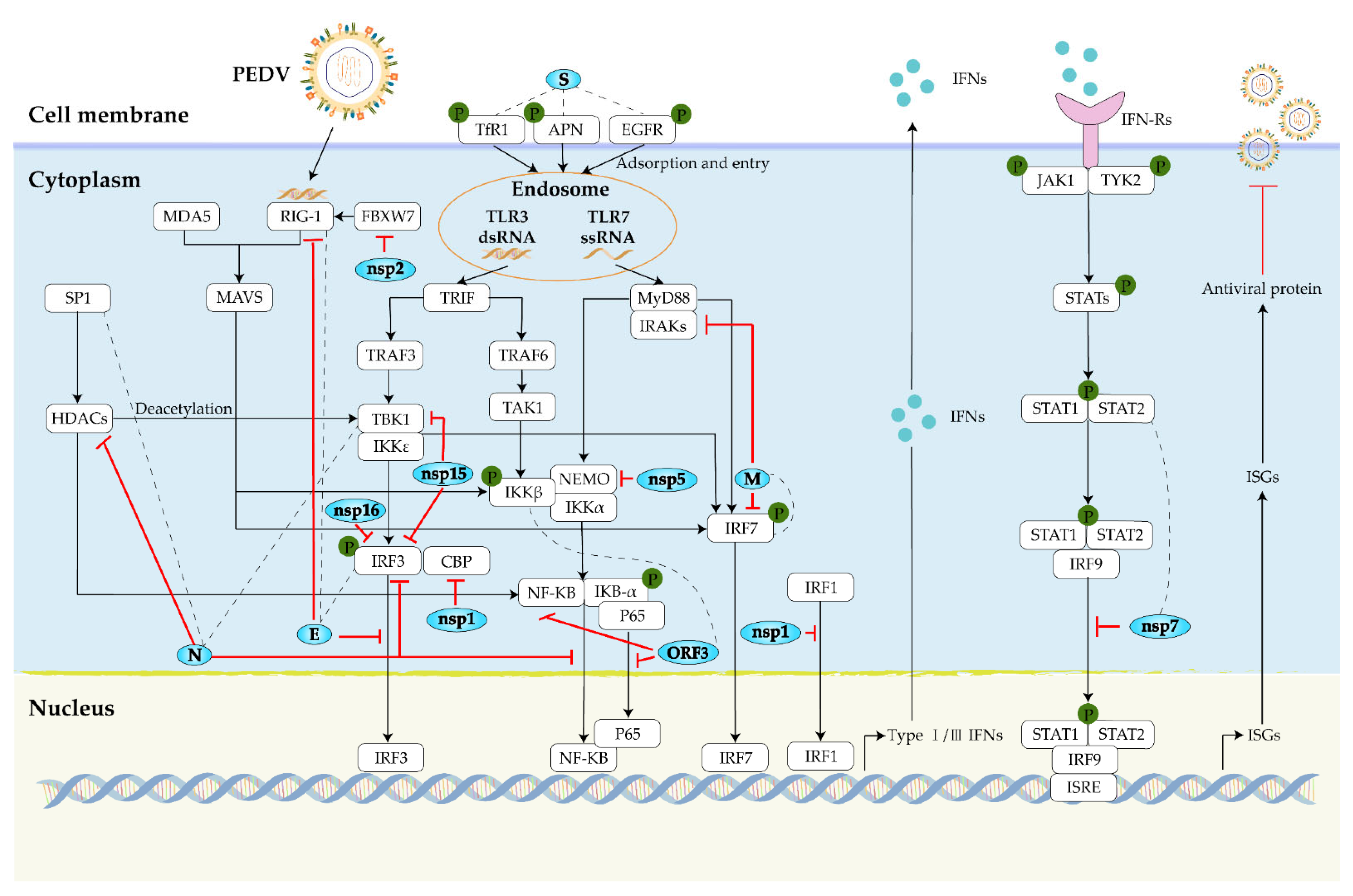
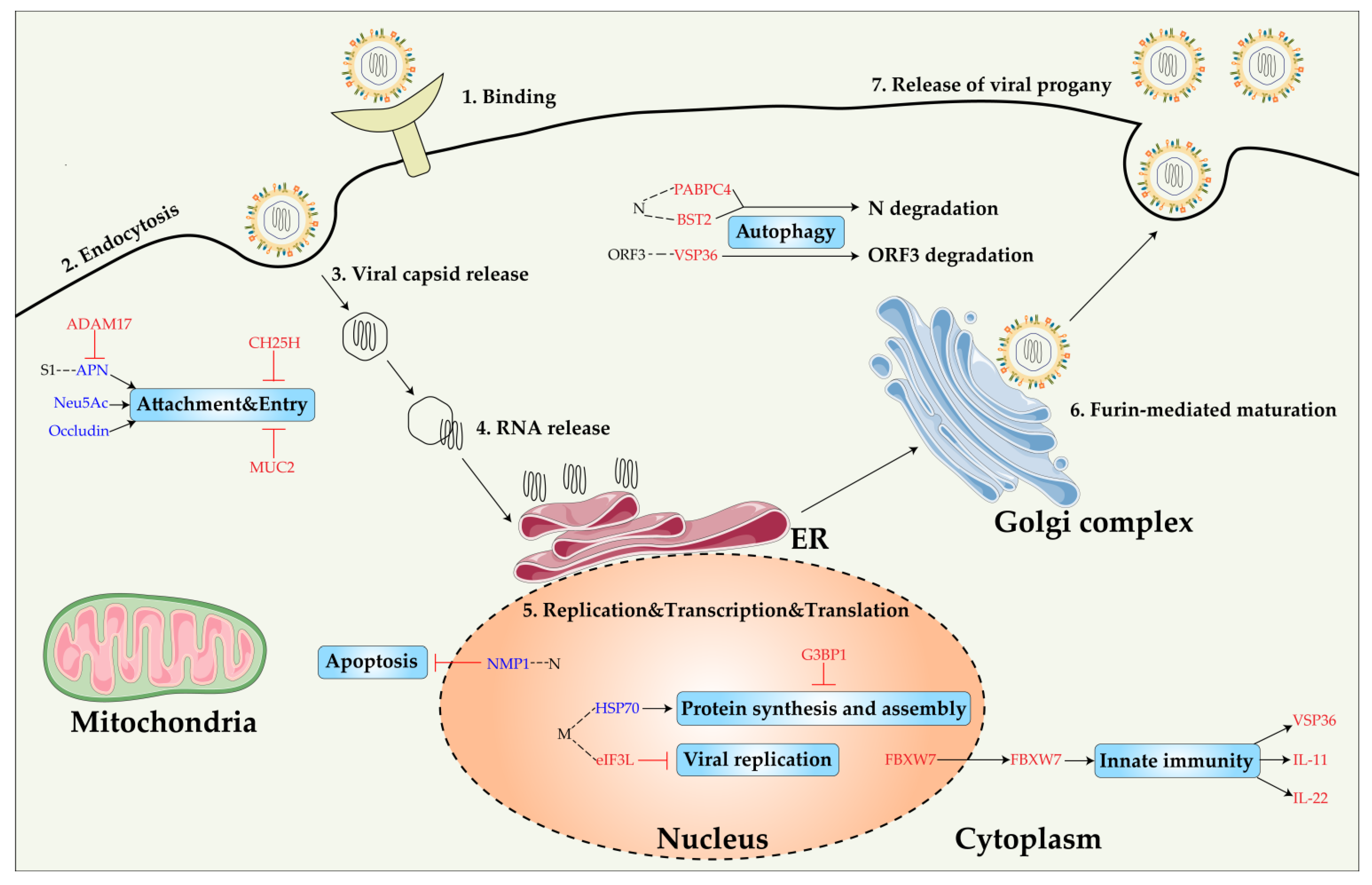
| Genotype | Variation | Representative Strains | Distribution | Reference |
|---|---|---|---|---|
| GIa | Classical strains | CV777; DR13; LZC; CH-S; AVCT12; SM98; CHM2013 | Asia–China, Korea, Thailand; Europe–Belgium, Russia | [19,20,21,22] |
| GIb | S-Indel vaccine strains relative to GIa | JS-2004-2; attenuated CV777; attenuated DR13; SD-M; SC1402; ZJ08; SQ2014; AH-M | Asia–China, Korea, Japan, Thailand | [1,19,23,24,25] |
| GIIa | Recombined non-S INDEL variant strains | AH2012; LZW; ZMDZY; GDZQ; FJZZ-9; GD-B; KNU-1305; Tottori2; MYG-1; Colorado; PC21A; PC22A; PC177; TC-PC177; MN; Kansas-166; Minnesota79; MEX/104/2013 | Asia–China, Korea, Japan; North America–USA, Mexico | [6,19,22,23,26,27,28] |
| GIIb | Recombined non-S INDEL variant strains | AJ1102; LC; AH2012-12; YN1; CHGD-01; GD-1; GDS01; GD-A; ZJCZ4; HNPJ | Asia–China | [6,19,26,27,29] |
| GIIc | S-INDEL strains recombined between GIa and GIIa | ZL29; CH/HNQX-3/14; KNU-1406-1; OH851; Iowa106; Minnesota211; IL20697; GER/L00862/2014; 15V010; FR/001/2014 | Asia–China, Korea; North America–USA; Europe–Germany, Romania, Austria, Belgium, Italy, France, Hungary, Slovenija | [1,6,19,22,25,29,30,31] |
| Genotype | Strain | ORFs | Major Variation | Pathogenicity | Reference |
|---|---|---|---|---|---|
| GIIb | icPC22A-KDKE4A-SYA | Nsp16, S | Nsp16 (quadruple alanine substitutions[K45A-D129A-K169A-E202A]), S (Y1378A) | Attenuated | [109] |
| GII | FJzz1-F200 (FJzz1) | S1-NTD | 55I56G57E → 55K56Δ57Δ, 877–878SG →877–878RR | Attenuated | [110,111] |
| GII | Iowa106 (PC21A) | S1 | Deletion at nt 176–186 and nt 416–418, 6-nt insertion at nt 474–475 | Attenuated | [63,112] |
| GII | 5-17-V (KF452323) | S1-NTD | Deletion at aa 23–229 | Attenuated | [113] |
| GIIa | TC-PC177, icPC22A-S1Δ197 (PC21A, icPC22A) | S1-NTD | Deletion at aa 34–230 | Attenuated | [44,74] |
| GIIb | icPC22A-icΔ10aa (icPC22A) | S-CTs | ΔYxxΦEKVHVQ | Attenuated | [114] |
| GIIb | FL2013 (AJ1102) | S-CTs | Deletion at aa 1385–1391 | Attenuated | [1,115] |
| GIIb | KNU-141112-S DEL2/ORF3 (KNU-141112) | S, ORF3 | S N-DEL2 (a combination of S C-DEL5/ORF3 N-DEL70, and ORF3 C-DEL88) | Attenuated | [116] |
| GIa | CHM2013, SM98, AVCT12 (CV777) | S-CTs, ORF3, M | S (deletion at nt 4144–4165), ΔORF3 (deletion at nt 1–30), M (4–aa [MLVL]) insertion at nt 36–37) | Mild | [55,117,118] |
| GIIb | 17GXCZ-1ORF3d (17GXCZ-1ORF3c) | ORF3 | Deletion at nt 172–554 | Virulent | [119] |
| GIIa | HN2021 (HNCADC-2017) | ORF3 | Deletion at nt 207–373 | Virulent | [117] |
| GIb | attenuated DR13 (DR13) | ORF3, E | ORF3 (Deletion at nt 245–293), E (Deletion at nt 67–87) | Attenuated | [20,120,121] |
| GIIb | SH (LZW) | N | Deletion at aa 399–410 | Virulent | [30] |
| ORFs | PC22A-P120 [139] | CT-P120 [140] | PT-P96 [141] | YN144 [47] | FJzz1-F200 [110] | OH851 [51,64] | |
|---|---|---|---|---|---|---|---|
| S1 | domains 0 | 55–57IGE→K–, I166V | T144I | ∆144–145TG | 55–57IGE→K–, F128Y | S88A, N130D, N132K | |
| domains A | Q454K, D466G, ^477H | D265A | D405G, G428A | D265A, D378N, T491R | T361A | ||
| domains B/COE | F636R | F555S | |||||
| domains CD | S723R | A694D | |||||
| S2 | V881F, Q893K, A971V, G1009V, F1015L, E1379 stop | S888R, C1363G | S888R, S969A, I1022S, K1027R, L1253R, C1355F, C1359F | T780N, Q826H, I1011V, I1305L, C1355F | K774N, 888–889SG →RR, L901V, N1010D, I1340T, C1355F | G1162S, V1242L | |
| Host Factors | Function | Role in VIRAL Infection | Reference | |
|---|---|---|---|---|
| Proviral factors | pAPN | Enzymatic cleavage of peptides; endocytosis; signal transduction | PEDV binds pAPN domain VII for entry into cells | [203,204] |
| Neu5Ac | As a cell surface glycoprotein; immune regulation and recognition; viral interactions | As a sugar coreceptor for PEDV entry cells | [73,205,206] | |
| Occludin | A tight junction protein; signal transduction; innate immune regulation | As a PEDV entry cofactor | [207,208] | |
| NPM1 | Ribosome assembly and chromatin remodeling; nuclear export; cell growth regulation | Interacts with N protein to promote PEDV growth | [104,209] | |
| HSP70 | Protein folding; participates in cellular processes; promotes viral replication. | Interacts with M protein to regulate PEDV replication, viral protein synthesis, and assembly | [210] | |
| EGFR | Regulates endocytic transport; regulates sorting after internalization and endocytosis | Inhibits I-IFN response through STAT3-mediated signaling | [211] | |
| Antiviral factors | BST2 | Regulates the transport of secreted cytokines; IFN-inducing markers | Binds and degrades the N protein of PEDV to inhibit PEDV replication | [212,205] |
| VPS36 | Regulation of protein sorting and MVB biogenesis | Promotes degradation of ORF3 by interacting with ORF3 | [206,213] | |
| CH25H | Regulates lipid metabolism, cholesterol homeostasis, inflammation, and immune responses | Inhibits entry of PEDV virions | [214,215] | |
| G3BP1 | Involves RNA recognition, host mRNA turnover and translation, SG formation | Induces antiviral SG formation and impairs PEDV replication | [216,217] | |
| FBXW7 | Regulates immune cells; tumor suppressor | Promotes host IFN-mediated antiviral response | [218] | |
| ADAM17 | Mediates cleavage and cleavage of cell surface proteins | Inhibits PEDV infection by regulating APN expression | [219] | |
| eIF3L | Regulates the physical stability of eIF3 assembly | Inhibits PEDV replication by interacting with M protein | [220,221] | |
| PABPC4 | An RNA processing protein that enhances translation and mRNA stability | Promotes degradation of N protein by interacting with N protein | [222] | |
| CD44 | Regulates signal transduction and tumor growth; cell adhesion | Activates NF-κB nuclear translocation and enhances protective cytokine release | [223,224] | |
| IL-11 | Regulates inflammation, apoptosis, epithelial regeneration and fertility | Anti-PEDV infection by activating the STAT3 signaling pathway | [225,226,227,228,229] | |
| IL-22 | Inhibits pro-inflammatory responses; protects the host gut barrier; maintains tissue integrity | Anti-PEDV infection by activating the STAT3 signaling pathway | [230,231,232] | |
| MUC2 | Regulates intestinal homeostasis | Regulates the replication of PEDV | [233,234] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, Y.; Zhou, J.; Wang, X.; Ma, L.; Li, J.; Yang, L.; Yuan, H.; Pang, D.; Ouyang, H. Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus–Host Interactions. Viruses 2022, 14, 2434. https://doi.org/10.3390/v14112434
Zhang Y, Chen Y, Zhou J, Wang X, Ma L, Li J, Yang L, Yuan H, Pang D, Ouyang H. Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus–Host Interactions. Viruses. 2022; 14(11):2434. https://doi.org/10.3390/v14112434
Chicago/Turabian StyleZhang, Yuanzhu, Yiwu Chen, Jian Zhou, Xi Wang, Lerong Ma, Jianing Li, Lin Yang, Hongming Yuan, Daxin Pang, and Hongsheng Ouyang. 2022. "Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus–Host Interactions" Viruses 14, no. 11: 2434. https://doi.org/10.3390/v14112434
APA StyleZhang, Y., Chen, Y., Zhou, J., Wang, X., Ma, L., Li, J., Yang, L., Yuan, H., Pang, D., & Ouyang, H. (2022). Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus–Host Interactions. Viruses, 14(11), 2434. https://doi.org/10.3390/v14112434






