European Brown Hare Syndrome in Poland: Current Epidemiological Situation
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. The Characteristics of the Hunting Areas
2.3. Hares Body Weight, Sex and Age
2.4. Serological Methods
2.5. Virological and Molecular Tests EBHSV and RHDV2 Detection
3. Results
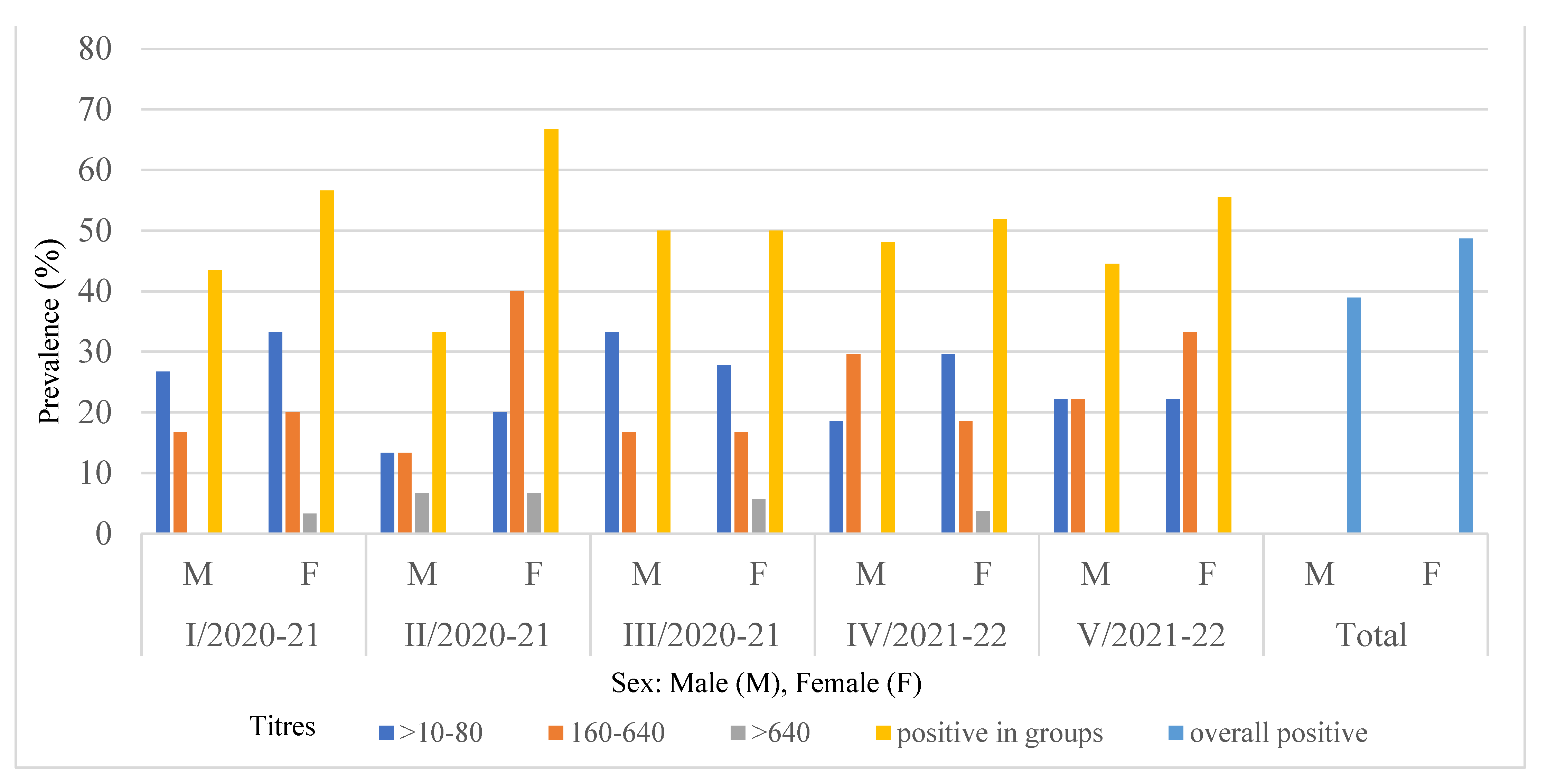
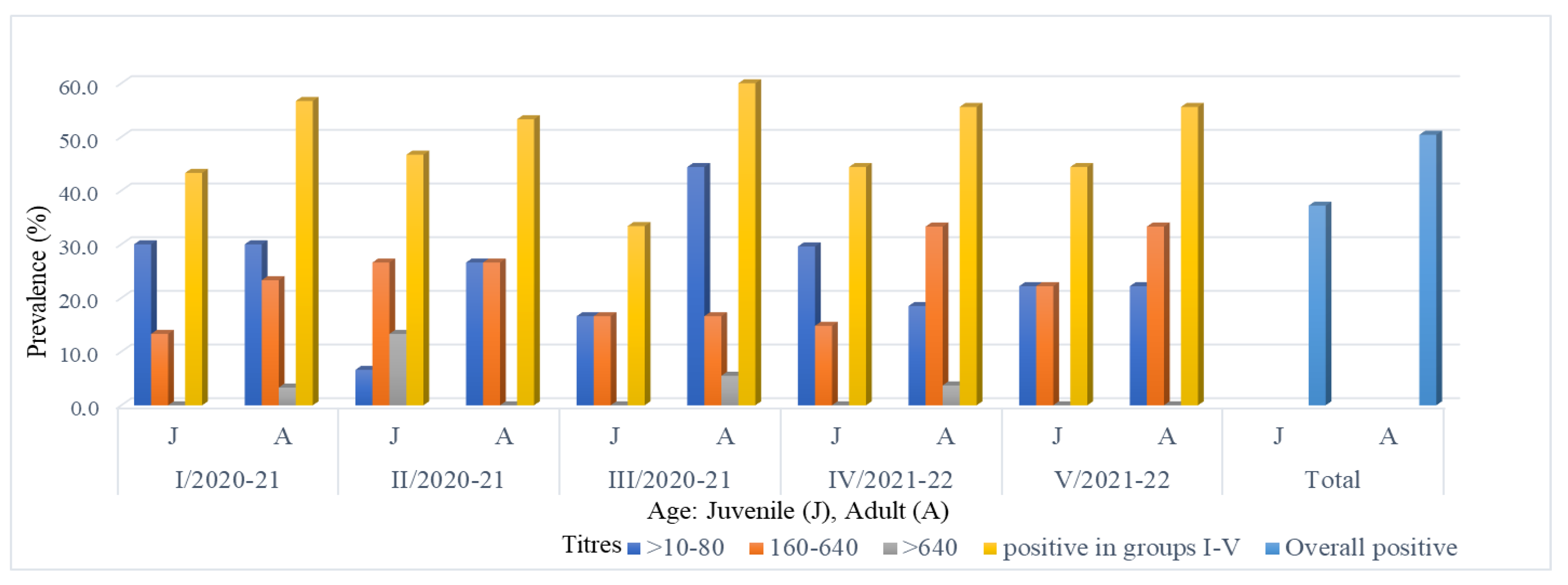
3.1. Seroprevalence
3.2. RHDV2 Serology
3.3. EBHSV and RHDV2 Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, P.J.; Fletcher, M.R.; Berny, P. Review of the factors affecting the decline of the European brown hare, Lepus europaeus (Pallas, 1778) and the use of wildlife incident data to evaluate the significance of paraquat. Agric. Ecosyst. Environ. 2000, 79, 95–103. [Google Scholar] [CrossRef]
- Gavier-Widén, D.; Mörner, T. Epidemiology and diagnosis of the European brown hare syndrome in Scandinavian countries: A review. Rev. Sci. Tech. 1991, 10, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.; Gavier, D.; Elling, F. Acute necrotising hepatitis in Danish farmed hares. Vet. Rec. 1989, 125, 486–487. [Google Scholar] [CrossRef]
- Löliger, H.C.; Eskens, U. Incidence, epizootiology and control of viral haemorrhagic disease of rabbits and the European brown hare syndrome in Germany. Rev. Sci. Tech. 1991, 10, 423–434. [Google Scholar] [CrossRef]
- Morisse, J.P.; Le Gall, G.; Boilletot, E. Hepatitis of viral origin in Leporidae: Introduction and aetiological hyptheses. Rev. Sci. Tech. 1991, 10, 283–295. [Google Scholar]
- Poli, A.; Nigro, M.; Gallazzi, D.; Sironi, G.; Lavazza, A.; Gelmetti, D. Acute hepatosis in the Eurpoean brown hare (Lepus europaeus) in Italy. J. Wildl. Dis. 1991, 27, 621–629. [Google Scholar] [CrossRef]
- Cancellotti, F.M.; Renzi, M. Epidemiology and current situation of viral haemorrhagic disease of rabbits and the European brown hare syndrome in Italy. Rev. Sci. Tech. 1991, 10, 409–422. [Google Scholar] [CrossRef]
- Chasey, D.; Duff, P. European brown hare syndrome and associated virus particles in the UK. Vet. Rec. 1990, 126, 623–624. [Google Scholar] [PubMed]
- Frölich, K.; Haerer, G.; Bacciarini, L.; Janovsky, M.; Rudolph, M.; Giacometti, M. European brown hare syndrome in free-ranging European brown and mountains hares from Switzerland. J. Wildl. Dis. 2001, 37, 803–807. [Google Scholar] [CrossRef]
- Frölich, K.; Wisser, J.; Schmüser, H.; Fehlberg, U.; Neubauer, H.; Grunow, R.; Nikolaou, K.; Priemer, J.; Thiede, S.; Streich, W.J.; et al. Epizootiologic and ecologic investigations of European brown hares (Lepus europaeus) in selected populations from Schleswig-Holstein, Germany. J. Wildl. Dis. 2003, 39, 751–761. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Zwingelstein, F.; Laurent, S.; Portejoie, Y.; Rasschaert, D. Molecular epidemiology of European brown hare syndrome virus in France between 1989 and 2003. Arch. Virol. 2006, 151, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Nauwynck, H.; Callebaut, P.; Peeters, J.; Ducatelle, R.; Uyttebroek, E. Susceptibility of hares and rabbits to a Belgian isolate of European brown hare syndrome virus. J. Wildl. Dis. 1993, 29, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, M.T.; Lavazza, A.; Capucci, L. European brown hare syndrome in northern Italy: Results of a virological and serological survey. Rev. Sci. Tech. 1994, 13, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Syrjälä, P.; Nylund, M.; Heinikainen, S. European brown hare syndrome in free-living mountain hares (Lepus timidus) and European brown hares (Lepus europaeus) in Finland 1990–2002. J. Wildl. Dis. 2005, 41, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.; Van der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef]
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.S.; Le Gall-Reculé, G.; Lopes, A.M.; Marchandeau, S.; Alda, F.; Almeida, T.; Celio, A.P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef]
- Chrobocińska, M.; Górski, J. Prevalence of infection with EBHS (European Brown Hare Syndrome) virus in hares in Poland. Bull. Vet. Inst. Pulawy 1995, 39, 17–21. [Google Scholar]
- Chrobocińska, M. Analysis of the fragment of capsid protein gene sequences of Polish strains of European brown hare syndrome virus. Bull. Vet. Inst. Pulawy 2002, 46, 213–222. [Google Scholar]
- Chrobocińska, M.; Górski, J. Prevalence of antibodies to EBHS (European brown hare syndrome) virus in sera of hares in Poland. Bull. Vet. Inst. Pulawy 1995, 39, 97–102. [Google Scholar]
- Frölich, K.; Meyer, H.; Pielowski, Z.; Ronsholt, L.; von Seck-Lanzendorf, S.; Stolte, M. European brown hare syndrome in free-ranging hares in Poland. J. Wildl. Dis. 1996, 32, 280–285. [Google Scholar] [CrossRef]
- Duarte, M.D.; Carvalho, C.L.; Barros, S.C.; Henriques, A.M.; Ramos, F.; Fagulha, T.; Luis, T.; Duarte, E.L.; Fevereiro, M. A real time Taqman RT-PCR for the detection of rabbit hemorrhagic disease virus 2 (RHDV2). J. Virol. Methods 2015, 219, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Reculé, G.; Lemaitre, E.; Bertagnoli, S.; Hubert, C.; Top, S.; Decors, A.; Marchandeau, S.; Guitton, J.S. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus). Vet. Res. 2017, 48, 70. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, F.; Melegari, I.; Sarchese, V.; Robetto, S.; Bermudez Sanchez, S.; Carella, E.; Orusa, R.; Cavadini, P.; Lavazza, A.; Marsilio, F.; et al. Potential role of wolf (Canis lupus) as passive carrier of European brown hare syndrome virus (EBHSV). Res. Vet. Sci. 2018, 117, 81–84. [Google Scholar] [CrossRef]
- Chiari, M.; Molinari, S.; Cavadini, P.; Bertasi, B.; Zanoni, M.; Capucci, L.; Lavazza, A. Red foxes (Vulpes vulpes) feeding brown hares (Lepus europaeus) infected by European brown hare syndrome virus (EBHSV) might be involved in the spread of the virus. Eur. J. Wildl. Res. 2016, 62, 761–765. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guérin, J.L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef]
- Abade dos Santos, F.A.; Pinto, A.; Burgoyne, T.; Dalton, K.P.; Carvalho, C.L.; Ramolo, D.W.; Carneiro, C.; Carvalho, T.; Peleteiro, M.C.; Parra, F.; et al. Spillover events of rabbit haemorrhagic disease virus 2 (recombinant GI.4P-GI.2) from Lagomorpha to Eurasian badger. Transbound. Emerg. Dis. 2022, 69, 1030–1045. [Google Scholar] [CrossRef]
- Calvete, C.; Mendoza, M.; Sarto, M.P.; de Bagüés, M.P.J.; Luján, L.; Molin, J.; Calvo, A.J.H. Detection of rabbit hemorrhagic disease virus GI.2/RHDV2/b in the Mediterranean Pine Vole (Microtus duodecimcostatus) and White-Toothed Shrew (Crocidura Russula). J. Wildl. Dis. 2019, 55, 467–472. [Google Scholar]
- Camarda, A.; Pugliese, N.; Cavadini, P.; Circella, E.; Capucci, L.; Caroli, A.; Legretto, M.; Malia, E.; Lavazza, A. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res. Vet. Sci. 2014, 97, 642–645. [Google Scholar] [CrossRef]
- Hall, R.N.; Peacock, D.E.; Kovaliski, J.; Mahar, J.E.; Mourant, R.; Piper, M.; Strive, T. Detection of RHDV2 in European brown hares (Lepus europaeus) in Australia. Vet. Rec. 2017, 180, 121. [Google Scholar] [CrossRef]
- Neimanis, A.; Ahola, H.; Larsson Petersson, U.; Lopes, A.M.; Abrantes, J.; Zohari, S.; Esteves, P.J.; Gavier-Widen, D. Overcoming species barriers: An outbreak of Lagovirus europaeus GI.2/RHDV2 in an isolated population of mountain hares (Lepus timidus). BMC Vet. Res. 2018, 14, 367. [Google Scholar] [CrossRef]
- Puggioni, G.; Cavadini, P.; Maestrale, C.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Reculé, G.; Lavazza, A.; Capucci, L. The new French 2010 variant of the rabbit of the hemorrhagic disease virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet. Res. 2013, 44, 96. [Google Scholar] [CrossRef] [PubMed]
- Velarde, R.; Cavadini, P.; Neimanis, A.; Cabezon, O.; Chiari, M.; Gaffuri, A.; Lavin, S.; Grilli, G.; Gavier-Widen, D.; Lavazza, A.; et al. Spillover events of infection of Brown hares (Lepus europaeus) with rabbit haemorrhagic disease type 2 virus (RHDV2) caused sporadic cases of an European Brown Hare Syndrome-like disease in Italy and Spain. Transbound. Emerg. Dis. 2017, 64, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, A.; Niedbalski, W. Detection of rabbit haemorrhagic disease virus 2 (GI.2) in Poland. Pol. J. Vet. Sci. 2018, 21, 451–458. [Google Scholar] [PubMed]
- Szillat, K.P.; Höper, D.; Beer, M.; König, P. Full-genome sequencing of German rabbit haemorrhagic disease virus uncovers recombination between RHDV (GI.2) and EBHSV (GII.1). Virus Evol. 2020, 6, veaa080. [Google Scholar] [CrossRef]
- Wirblich, C.; Meyers, G.; Ohlinger, V.F.; Capucci, L.; Eskens, U.; Haas, B.; Thiel, H.J. European brown hare syndrome virus: Relationship to Rabbit haemorrhagic disease virus and other caliciviruses. J. Virol. 1994, 68, 5164–5173. [Google Scholar] [CrossRef]
- Chiari, M.; Ferrari, N.; Giardiello, D.; Avisani, D.; Zanoni, M.; Alborali, G.L.; Lanfranchi, P.; Guberti, V.; Capucci, L.; Lavazza, A. Temporal dynamics of European brown hare syndrome infection in Northern Italian brown hares (Lepus europaeus). Eur. J. Wildl. Res. 2014, 60, 891–896. [Google Scholar] [CrossRef]
- Flis, M.; Rataj, B. Characteristics of population indicators of brown hare (Lepus europaeus Pall.) obtained during group hunting in the region with the highest density in western part of the Lublin region in Poland. Appl. Ecol. Environ. Res. 2019, 17, 13701–13711. [Google Scholar] [CrossRef]
- Pielowski, Z. Zając. In Monografia Przyrodniczo−Łowiecka; PWRiL: Warsaw, Poland, 1979. (In Polish) [Google Scholar]
- Stroh, G. Zwei sichere Altersmerkmale beim Hasen. Berl. Tierärztl. Wochenschr. 1931, 47, 180–181. [Google Scholar]
- Méres, J.; Ostrihoň, M.; Slamečka, M.; Kaštier, J. Population structure of brown hare (Lepus europaeus): A case study in selected areas of Nitra region. Acta Fac. For. Zvolen 2013, 1, 43–58. [Google Scholar]
- Caboń-Raczyńska, K.; Raczyński, J. Method of determination of age in the European hare. Acta Theriol. 1972, 17, 75–86. [Google Scholar] [CrossRef]
- Hruška, J.; Matoušková, J.; Ernst, M.; Slamečka, J. Populační dynamika zajíce polního v letech 2008–2010 na základe stanovení hmotnosti očných čoček. In Zajac Poľný pred Štvrťstoročím a Dnes; CVŽV Nitra: Nitra, Slovakia, 2011. (In Czech) [Google Scholar]
- Pintur, K.; Popović, N.; Alegro, A.; Severin, K.; Slavica, A.; Kolić, E. Selected indicators of brown hare (Lepus europaeus Pallas, 1778) population dynamics in northwestern Croatia. Vet. Arh. 2006, 76, 199–209. [Google Scholar]
- Fitzner, A.; Kęsy, A.; Bulenger, K.; Niedbalski, W. Evidence of independent introductions of RHDV2 strains in Poland based on the genome analysis of viral isolates from 2016–2018. Acta Biochim. Pol. 2021, 68, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gryz, J.; Krauze-Gryz, D. Why did Brown hare Lepus europaeus disappear from some areas in central Poland. Diversity 2022, 14, 465. [Google Scholar] [CrossRef]
- Hacklander, K.; Schai-Braun, S. Lepus europaeus. In The IUCN Red List of Threatened Species 2019; IUCN: Gland, Switzerland, 2019; p. eT41280A45187424. [Google Scholar]
- Misiorowska, M.; Ludwisiak, Ł.; Nasiadka, P. Population parameters of brown hare (Lepus europaeus L.) in regions of the species highest density in Poland. Sylwan 2014, 158, 901–910. [Google Scholar]
- Smith, R.K.; Jennings, N.V.; Harris, S. A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mammal Rev. 2005, 35, 1–24. [Google Scholar] [CrossRef]
- Garcia-Bocanegra, I.; Camach-Sillero, L.; Risalde, M.A.; Dalton, K.P.; Caballero-Gomez, J.; Agüero, M.; Zorilla, I.; Gomez-Guillamon, F. First outbreak of myxomatosisin Iberian hares (Lepus granatensis). Transbound. Emerg. Dis. 2019, 66, 2204–2208. [Google Scholar] [CrossRef]
- Panek, M. Current situation of hares and partridges and the management of their populations. In Animal Population Management; Polski Związek Łowiecki: Warsaw, Poland, 2016; pp. 99–109. Available online: www.czempin.pzlow.pl (accessed on 1 June 2022). (In Polish)
- Panek, M. Habitat factors associated with the decline in brown hare abundance in Poland in the beginning of the 21st century. Ecol. Indic. 2018, 85, 915–920. [Google Scholar] [CrossRef]
- Kamieniarz, R.; Panek, M. Game Animals in Poland at the Turn of the 20th and 21st Century; Stacja Badawcza Polskiego Związku Łowieckiego (Research Station of the Polish Hunting Association): Warsaw, Poland, 2008; pp. 78–83. Available online: www.czempin.pzlow.pl (accessed on 1 June 2022). (In Polish)
- Zalewski, D.; Okarma, H.; Panek, M. Monitoring Liczebności i Jakości Populacji Dzikich Zwierząt (Monitoring the Abundance and Quality of Wild Animal Populations); The Monograph; Uniwersytet Warmińsko-Mazurski w Olsztynie: Olsztyn, Poland, 2018; pp. 53–55. (In Polish) [Google Scholar]
- Misiorowska, M.; Wasilewski, M. Survival and causes of death among released brown hares (Lepus europaeus Pallas, 1778) in Central Poland. Acta Theriol. 2012, 54, 305–312. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of Forestry; Statistics Poland: Warsaw, Poland, 2021.
- Kwit, E.; Chrobocińska, M.; Grądzki, Z.; Jarosz, Ł.; Majer-Dziedzic, B.; Bigoraj, E. The genetic analysis of new Polish strains of European brown hare syndrome virus. Pol. J. Vet. Sci. 2014, 17, 353–355. [Google Scholar] [CrossRef]
- Górski, J.; Mizak, B.; Mizak, Z.; Komorowski, A. Clinical and anatomopathological features of rabbits peste (viral haemorrhagic disease of rabbits). Życie Weter. 1988, 63, 266–269. (In Polish) [Google Scholar]
- Capucci, L.; Fallacara, F.; Grazioli, S.; Lavazza, A.; Pacciarini, M.L.; Brocchi, E. A further step in the evolution of rabbit haemorrhagic disease virus: The appearance of the first consistent antigenic variant. Virus Res. 1998, 58, 115–126. [Google Scholar] [CrossRef]
- Chrobocińska, M.; Mizak, B. Phylogenetic analysis of partial capsid protein gene of rabbit haemorrhagic disease virus (RHDV) strains isolated between 1993 and 2005 in Poland. Bull. Vet. Inst. Pulawy 2007, 51, 189–197. [Google Scholar]
- Fitzner, A. Characterization and immunogenic properties of Polish strains of RHD virus. Bull. Vet. Inst. Pulawy 2009, 53, 575–582. [Google Scholar]
- Schirrmeyer, H.; Reimann, I.; Köllner, B.; Granzow, H. Pathogenic, antigenic and molecular properties of rabbit haemorrhagic disease virus (RHDV) isolated from vaccinated rabbits: Detection and characterization of antigenic variants. Arch. Virol. 1999, 144, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Steineck, T.; Novotny, N. European Brown Hare Syndrome (EBHS) in Austria—Epidemiologic investigations. Tierarztl. Umsch. 1993, 4, 225–229. [Google Scholar]
- Frölich, K.; Fickel, J.; Ludwig, A.; Lieckfeldt, D.; Streich, W.J.; Jurcik, R.; Slamecka, J.; Wibbelt, G. New variants of European brown hare syndrome virus strains in free-ranging European brown hares (Lepus europaeus) from Slovakia. J. Wildl. Dis. 2007, 43, 89–96. [Google Scholar] [CrossRef][Green Version]
- Paci, G.; Lavazza, A.; Ferretti, M.; Santilli, F.; Bagliacca, M. Relationship between anti-European Brown Hare syndrome serological titers and Brown Hare (Lepus europaeus Pallas) densities. Int. J. Zool. 2011, 2011, 436193. [Google Scholar] [CrossRef]
- Lavazza, A.; Guberti, V.; Ferri, M.; Zanni, M.L.; Poglayen, G.; Nardin, A.; Capucci, L. Epidemiology of Euroean brown hare syndrome (EBHS) in Modena Province (North Italy). In Proceedings of the 4th International Congress of the European Society for Veterinary Virology, Edinburgh, UK, 24–27 August 1997; pp. 34–37. [Google Scholar]
- Lavazza, A.; Cavadini, P.; Barbieri, I.; Tizzani, P.; Pinheiro, A.; Abrantes, J.; Esteves, P.J.; Grilli, G.; Gioia, E.; Zanoni, M.; et al. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrom (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet. Res. 2015, 46, 13. [Google Scholar] [CrossRef]
- Tsokana, C.N.; Sokos, C.; Giannakopoulos, A.; Birtsas, P.; Valiakos, G.; Spyrou, V.; Athanasiou, L.V.; Burriel, A.R.; Billinis, C. European Brown hare (Lepus europaeus) as a source of emerging and re-emerging pathogens of Public Health importance: A review. Vet. Med. Sci. 2020, 6, 550–564. [Google Scholar] [CrossRef]
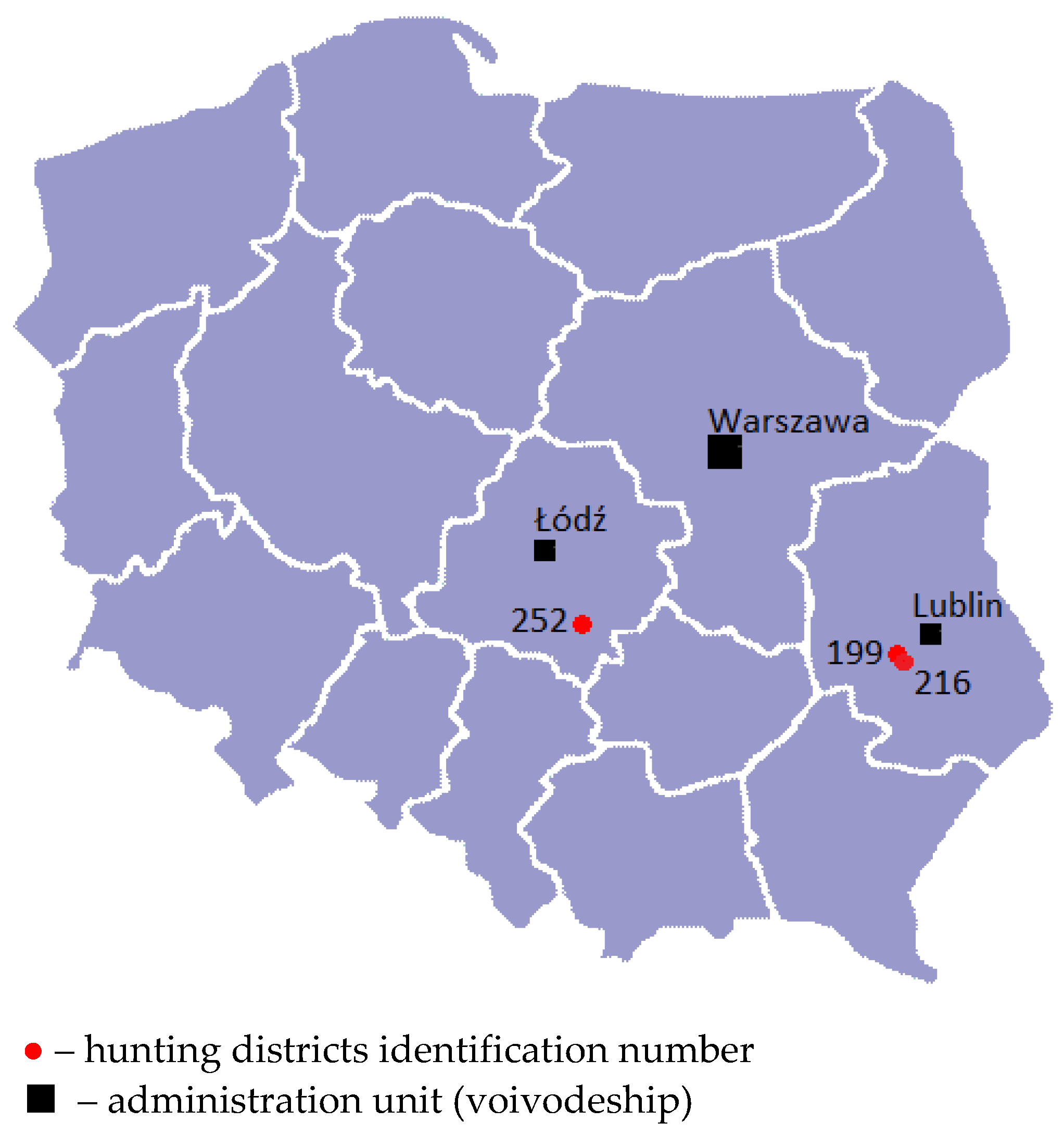
| Date of Collection/Additional Information | Geographical Region of Poland /Voivodeship | No. of Hares | Sex M/ F | Weight | Specimens Collected | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age J/A | (kg) | Blood | Liver | Duod. | Lungs | Kidneys | Spleen | Faeces (f)/Heart (h) | |||
| Hunting season 2021–2022 | Central/Łódź | 10 serol. study batch V | 2MJ, 2MA, 2FJ, 4FA | 3.3–4.8 | 10 | 10 | 7 | 0 | 0 | 0 | 0 |
| Hunting season 2021–2022 | Southeastern/ Lublin | 30 serol. study batch IV | 5MJ, 9MA, 8FJ, 8FA | 3.2–5.0 | 30 | 30 | 20 | 0 | 0 | 0 | 0 |
| Hunting season 2020–2021 | 20 serol. study batch III | 4MJ, 6MA, 4FJ, 6FA | 3.5–5.0 | 20 | 20 | 5 | 0 | 0 | 0 | 0 | |
| 20 serol. study batch II | 2MJ, 5MA, 7FJ, 6FA | 3.0–5.0 | 20 | 20 | 10 | 0 | 0 | 0 | 0 | ||
| 33 serol. study batch I | 7MJ, 8MA, 6FJ, 12FA | 3.6–5.0 | 33 | 32 | 32 | 0 | 0 | 0 | 0 | ||
| 2021/traffic incident | North-Central/ Kuyavian-Pomeranian (Bydgoszcz) | 1 | A | - | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 2021 /animals shelter | 3 | J (8 weeks) | - | 0 | 3 | 3 | 0 | 0 | 0 | 0 | |
| 2020 /animals shelter | 1 | J (4 weeks) | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2020/traffic incident | Southeastern/ Lublin | 1 | A | - | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| 2019/group hunting | 5 | A | - | 0 | 5 | 0 | 0 | 0 | 0 | 1 (h) | |
| 2019/animals shelter | North-Central/ Kuyavian-Pomeranian (Bydgoszcz) | 1 | J (4 months) | - | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| 2019/traffic incident | 3 | A | - | 0 | 3 | 0 | 3 | 3 | 0 | 3 (h) | |
| 2018/animals shelter | 3 | J (10 weeks) | - | 0 | 3 | 0 | 3 | 3 | 0 | 3 (f) | |
| 2016/traffic incident | 1 | J (11 weeks) | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | A | - | 0 | 2 | 0 | 2 | 2 | 0 | 0 | ||
| 2016/traffic incident | North/ Pomeranian (Gdańsk) | 1 | A | - | 0 | 1 | 0 | 1 | 0 | 1 | 1 (h) |
| 2016/found in the forest | 1 | A | - | 0 | 1 | 0 | 0 | 1 | 0 | 1 (h) | |
| 2015/traffic incident | Southwestern/ Lower Silesia (Wrocław) | 1 | A | - | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 2015/found in the forest | 1 | A | - | 0 | 1 | 0 | 1 | 1 | 0 | ||
| 2015/traffic incident | North-Central/ Kuyavian-Pomeranian (Bydgoszcz) | 2 | A | - | 0 | 2 | 0 | 1 | 0 | 2 | 0 |
| 8 | A | - | 0 | 8 | 0 | 0 | 0 | 2 | 0 | ||
| 2015/found in a rural environment | 2 | A | - | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 2015/group hunting | Central/Łódź | 12 | A | - | 0 | 12 | 0 | 0 | 0 | 0 | 0 |
| 2014/found in a rural environmnet | Central/Masovia (Warszawa) | 1 | A | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| 2014/traffic incident | Southwestern/Opole | 2 | A | - | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 2014/group hunting | Southeastern/ Lublin | 5 | A | - | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| Batch/ Hunting Season | No. of Hares Tested | Positive | Negative | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage 1 | Anti-EBHSV Antibody Titre Distribution 2 Titre (Pab ELISA/Mab ELISA) | No. | Percentage 1 % | |||||||
| >10–80 | 160–640 | >640 | <10 | ||||||||
| No. | % | No. | % | No. | % | No. | % | ||||
| I/2020–2021 | 33 | 30 | 90.9 | 18 | 60 | 11 | 36.66 | 1 | 3.33 | 3 | 9.1 |
| II/2020–2021 | 20 | 15 | 75 | 5 | 33.33 | 8 | 53.33 | 2 | 13.33 | 5 | 25 |
| III/2020–2021 | 20 | 18 | 90 | 11 | 61.1 | 6 | 33.33 | 1 | 5.6 | 2 | 10 |
| IV/2021–2022 | 30 | 27 | 90 | 13 | 48.1 | 13 | 48.1 | 1 | 3.7 | 3 | 10 |
| V/2021–2022 | 10 | 9 | 90 | 4 | 44.4 | 5 | 55.6 | 0 | 0 | 0 | 10 |
| Total | 113 | 99 | 87.6 | 51 | 51.5 | 43 | 43.4 | 5 | 5.1 | 14 | 12.4 |
| Males | 49 | 44 | 38.9 | 23 | 23.2 | 20 | 20.2 | 1 | 1 | 5 | 4.4 |
| J | 20 | 19 | 16.8 | 10 | 10.1 | 8 | 8.1 | 1 | 1 | 1 | 0.9 |
| A | 29 | 25 | 22.1 | 13 | 13.1 | 12 | 12.1 | 0 | 0 | 4 | 3.5 |
| Females | 64 | 55 | 48.7 | 28 | 28.3 | 23 | 23.2 | 4 | 4 | 9 | 8 |
| J | 28 | 23 | 20.4 | 13 | 13.1 | 9 | 9.1 | 1 | 1 | 5 | 4.44 |
| A | 36 | 32 | 28.3 | 15 | 15.2 | 14 | 14.1 | 3 | 3 | 4 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzner, A.; Niedbalski, W.; Kęsy, A.; Rataj, B.; Flis, M. European Brown Hare Syndrome in Poland: Current Epidemiological Situation. Viruses 2022, 14, 2423. https://doi.org/10.3390/v14112423
Fitzner A, Niedbalski W, Kęsy A, Rataj B, Flis M. European Brown Hare Syndrome in Poland: Current Epidemiological Situation. Viruses. 2022; 14(11):2423. https://doi.org/10.3390/v14112423
Chicago/Turabian StyleFitzner, Andrzej, Wiesław Niedbalski, Andrzej Kęsy, Bogusław Rataj, and Marian Flis. 2022. "European Brown Hare Syndrome in Poland: Current Epidemiological Situation" Viruses 14, no. 11: 2423. https://doi.org/10.3390/v14112423
APA StyleFitzner, A., Niedbalski, W., Kęsy, A., Rataj, B., & Flis, M. (2022). European Brown Hare Syndrome in Poland: Current Epidemiological Situation. Viruses, 14(11), 2423. https://doi.org/10.3390/v14112423




