Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Titration of Samples
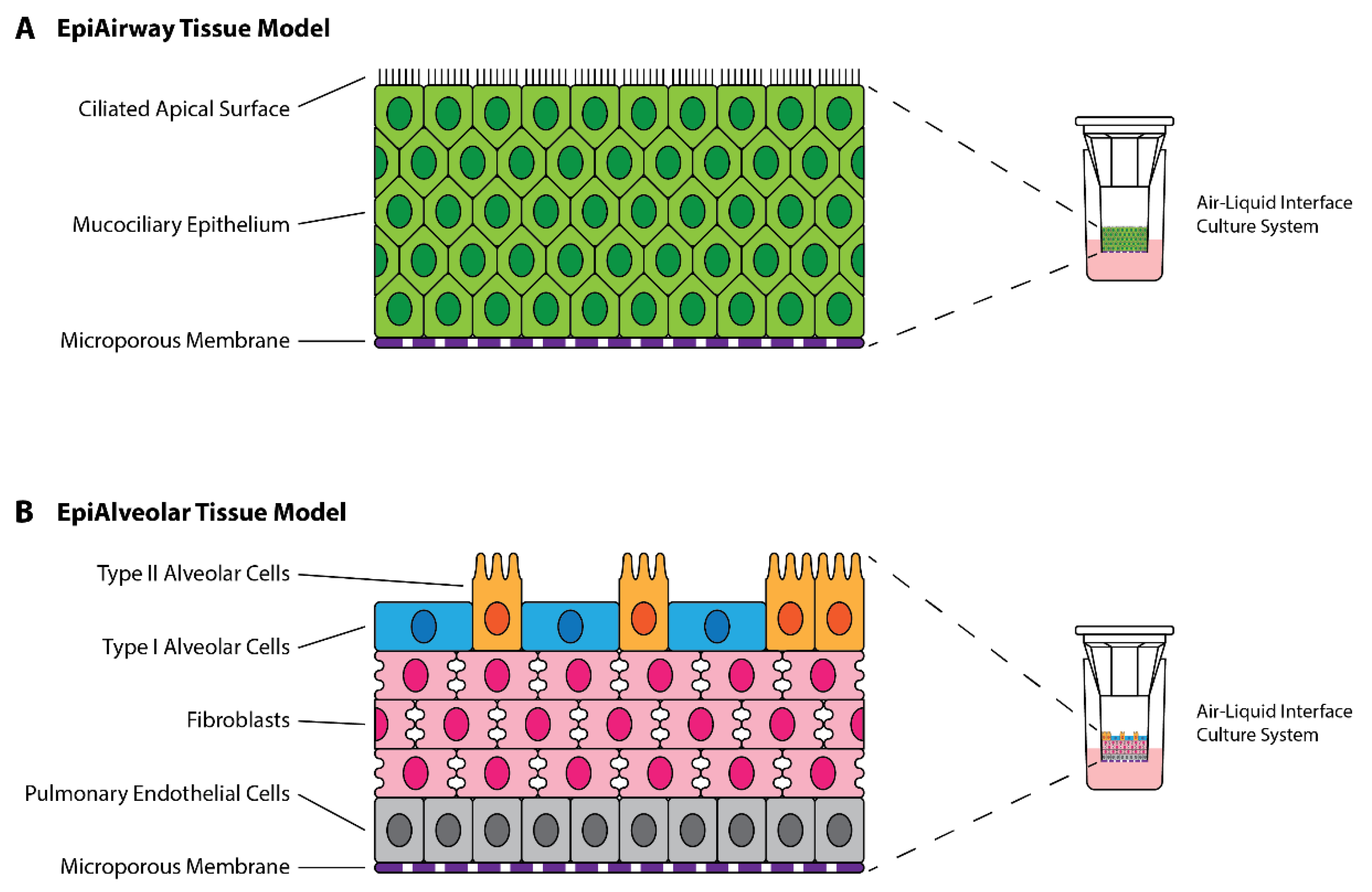
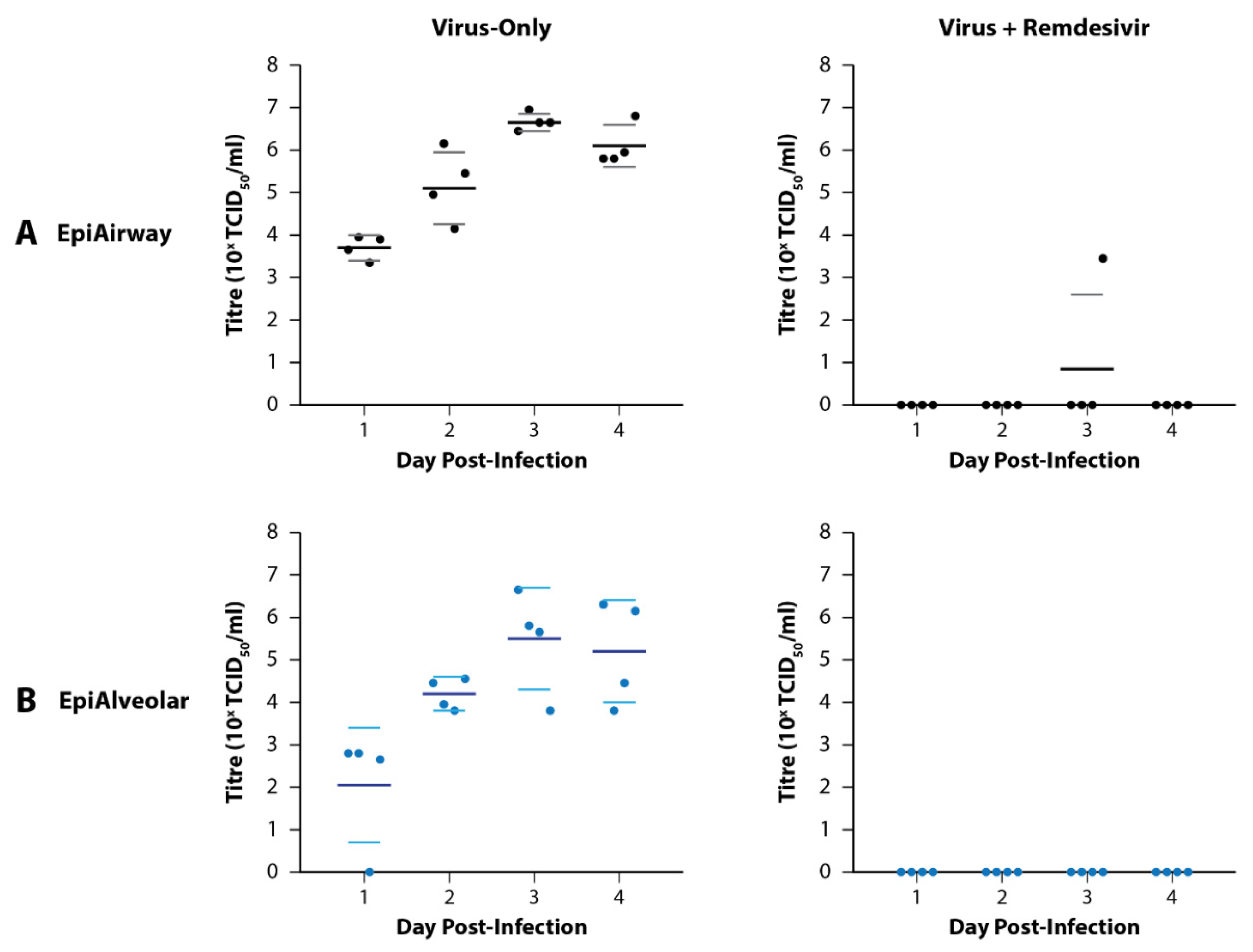
2.3. Preliminary Infections in Tissue Models
2.4. Drug Selection, Procurement, and Preparation
2.5. Drug Screening Using the EpiAirway Tissue Model
3. Results
3.1. Preliminary Infections of EpiAirway and EpiAlveolar Tissue Models
3.2. Rationale behind the Selection of Candidate Drugs
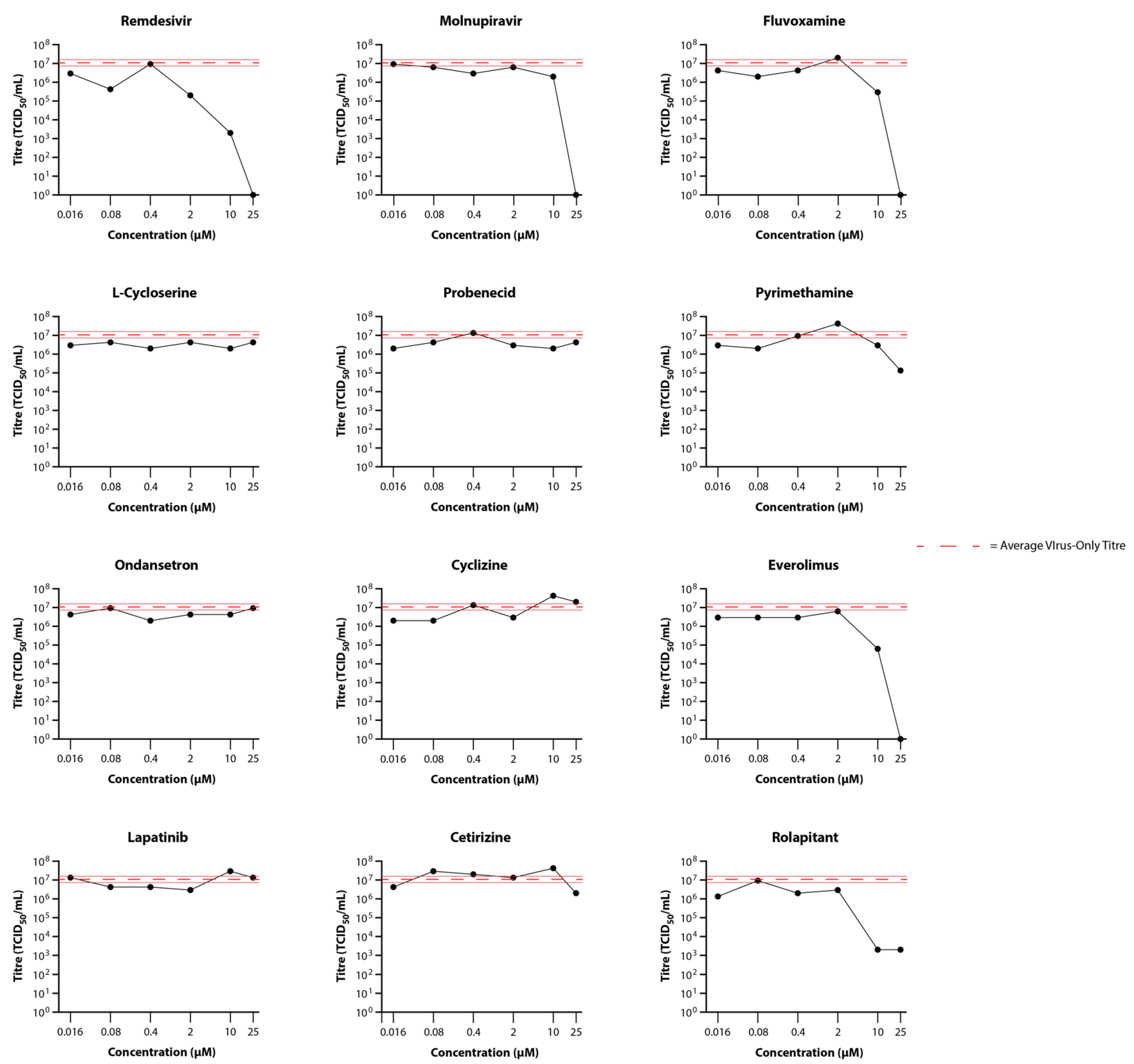
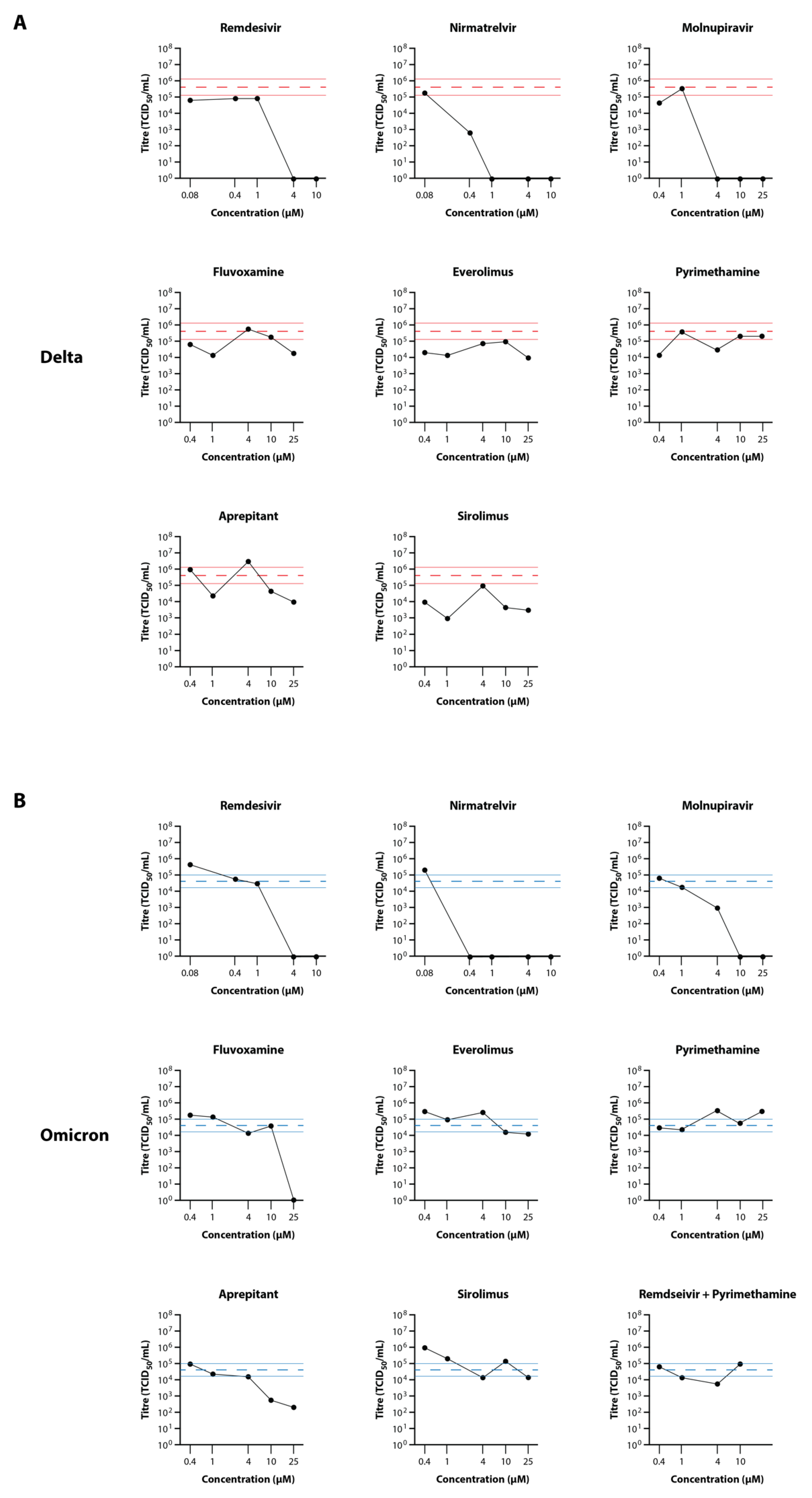
3.3. Primary Testing of Candidate Drugs in the EpiAirway Tissue Model
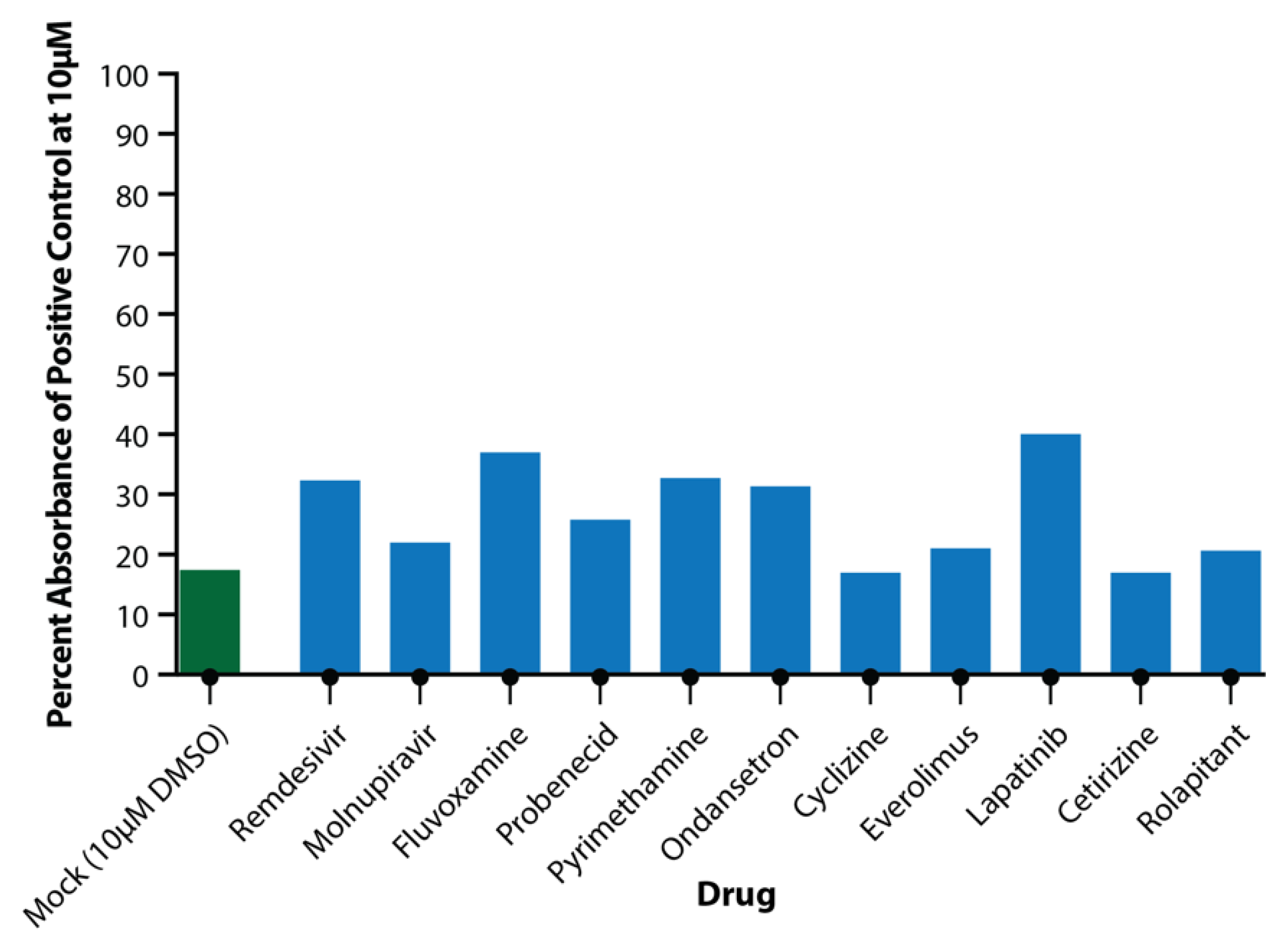
3.4. Secondary Testing of Candidate Drugs in the EpiAirway Tissue Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldometers.Info. COVID-19 Coronavirus Pandemic. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 18 September 2022).
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Abdool Karim, S.S.; Baden, L.R.; Farrar, J.J.; Hamel, M.B.; Longo, D.L.; Morrissey, S.; Rubin, E.J. Addressing Vaccine Inequity—COVID-19 Vaccines as a Global Public Good. N. Engl. J. Med. 2022, 386, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Researchers Fear Growing COVID Vaccine Hesitancy in Developing Nations. Nature 2022, 601, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Cavazzoni, P. Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant [Online]. 2022. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron (accessed on 18 September 2022).
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. COVID-19 Treatments [Online]. 2022. Available online: https://www.health.gov.au/health-alerts/covid-19/treatments (accessed on 18 September 2022).
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral COVID Antiviral Drugs. Clin. Infect. Dis. 2022, ciac180. [Google Scholar] [CrossRef]
- Kumar, M.; Fatma, A.; Bharti, N. Access to Medicines and Medical Equipment during COVID-19: Searching Compatibility between the WTO and the WHO. India Q. 2022, 78, 68–87. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- MacRaild, C.; Muzaffar-Ur-Rehman, M.; Faheem; Murugesan, S.; Styles, I.; Peterson, A.; Kirkpatrick, C.; Cooper, M.; Palombo, E.; Simpson, M.; et al. Systematic Down-Selection of Repurposed Drug Candidates for COVID-19. Int. J. Mol. Sci. 2022, 23, 11851. [Google Scholar] [CrossRef] [PubMed]
- North American 3Rs Collaborative. Microphysiological Systems Technology Hub [Online]. 2022. Available online: https://www.na3rsc.org/mps-tech-hub/ (accessed on 18 September 2022).
- Lawko, N.; Plaskasovitis, C.; Stokes, C.; Abelseth, L.; Fraser, I.; Sharma, R.; Kirsch, R.; Hasan, M.; Abelseth, E.; Willerth, S.M. 3D Tissue Models as an Effective Tool for Studying Viruses and Vaccine Development. Front. Mater. 2021, 8, 631373. [Google Scholar] [CrossRef]
- D’Agnillo, F.; Walters, K.-A.; Xiao, Y.; Sheng, Z.-M.; Scherler, K.; Park, J.; Gygli, S.; Rosas, L.A.; Sadtler, K.; Kalish, H.; et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021, 13, eabj7790. [Google Scholar] [CrossRef]
- Munker, D.; Osterman, A.; Stubbe, H.; Muenchhoff, M.; Veit, T.; Weinberger, T.; Barnikel, M.; Mumm, J.-N.; Milger, K.; Khatamzas, E.; et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur. Respir. J. 2021, 58, 2002724. [Google Scholar] [CrossRef]
- Boda, B.; Benaoudia, S.; Huang, S.; Bonfante, R.; Wiszniewski, L.; Tseligka, E.D.; Tapparel, C.; Constant, S. Antiviral drug screening by assessing epithelial functions and innate immune responses in human 3D airway epithelium model. Antivir. Res. 2018, 156, 72–79. [Google Scholar] [CrossRef]
- Ebisudani, T.; Sugimoto, S.; Haga, K.; Mitsuishi, A.; Takai-Todaka, R.; Fujii, M.; Toshimitsu, K.; Hamamoto, J.; Sugihara, K.; Hishida, T.; et al. Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening. Cell Rep. 2021, 35, 109218. [Google Scholar] [CrossRef]
- Gard, A.L.; Luu, R.J.; Miller, C.R.; Maloney, R.; Cain, B.P.; Marr, E.E.; Burns, D.M.; Gaibler, R.; Mulhern, T.J.; Wong, C.A.; et al. High-throughput human primary cell-based airway model for evaluating influenza, coronavirus, or other respiratory viruses in vitro. Sci. Rep. 2021, 11, 14961. [Google Scholar] [CrossRef]
- Mulay, A.; Konda, B.; Garcia, G.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K.; et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell Rep. 2021, 35, 109055. [Google Scholar] [CrossRef]
- Rijsbergen, L.C.; van Dijk, L.L.A.; Engel, M.F.M.; de Vries, R.D.; de Swart, R.L. In vitro Modelling of Respiratory Virus Infections in Human Airway Epithelial Cells—A Systematic Review. Front. Immunol. 2021, 12, 3301. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Jain, H.A.; Bansal, C.; Kumar, A.; Faheem; Muhammed, M.-U.-R.; Murugesan, S.; Simpson, M.M.; Karpe, A.V.; Chandra, R.; MacRaild, C.A.; et al. CoviRx: A user-friendly interface for systemic down-selection of repurposed drug candidates for COVID-19. Data, 2022; accepted. [Google Scholar]
- Pruijssers, A.J.; George, A.S.; Schäfer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H.; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Updates Its Treatment Guidelines to Include Molnupiravir [Online]. 2022. Available online: https://www.who.int/news/item/03-03-2022-molnupiravir (accessed on 18 September 2022).
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H.; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Drugbank. Aprepitant [Online]. 2022. Available online: https://go.drugbank.com/drugs/DB00673 (accessed on 18 September 2022).
- National Health Sservice. Cyclizine [Online]. 2022. Available online: https://www.nhs.uk/medicines/cyclizine/#:~:text=Cyclizine%20is%20an%20anti%2Dsickness,sickness%2C%20vertigo%20and%20travel%20sickness (accessed on 18 September 2022).
- NCATS. Cyclizine Hydrochloride [Online]. 2022. Available online: https://opendata.ncats.nih.gov/covid19/sample/summary/NCGC00016421 (accessed on 18 September 2022).
- Drugbank. Cetirizine [Online]. 2022. Available online: https://go.drugbank.com/drugs/DB00341 (accessed on 18 September 2022).
- NCI. Everolimus [Online]. 2022. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/everolimus (accessed on 18 September 2022).
- Mullen, P.J.; Garcia, G.; Purkayastha, A.; Matulionis, N.; Schmid, E.W.; Momcilovic, M.; Sen, C.; Langerman, J.; Ramaiah, A.; Shackelford, D.B.; et al. SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition. Nat. Commun. 2021, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- National Institues of Health. Fluvoxamine [Online]. 2022. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/fluvoxamine/ (accessed on 18 September 2022).
- Fred, S.M.; Kuivanen, S.; Ugurlu, H.; Casarotto, P.C.; Levanov, L.; Saksela, K.; Vapalahti, O.; Castrén, E. Antidepressant and Antipsychotic Drugs Reduce Viral Infection by SARS-CoV-2 and Fluoxetine Shows Antiviral Activity Against the Novel Variants in vitro. Front. Pharmacol. 2022, 12, 755600. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.; Kirkpatrick, P.; Kar, S.; Goss, P. Lapatinib. Nat. Rev. Drug Discov. 2007, 6, 431–432. [Google Scholar] [CrossRef]
- Saul, S.; Karim, M.; Huang, P.-T.; Ghita, L.; Chiu, W.; Kumar, S.; Bhalla, N.; Leyssen, P.; Cohen, C.A.; Huie, K.; et al. Discovery of pan-ErbB inhibitors protecting from SARS-CoV-2 replication, inflammation, and lung injury by a drug repurposing screen [Preprint]. bioRxiv 2021. [Google Scholar] [CrossRef]
- Amorim Franco, T.M.; Favrot, L.; Vergnolle, O.; Blanchard, J.S. Mechanism-Based Inhibition of the Mycobacterium tuberculosis Branched-Chain Aminotransferase by d- and l-Cycloserine. ACS Chem. Biol. 2017, 12, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Gilles, M.; Barral, K.; Nougairède, A.; van Helden, J.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020, 10, 13093. [Google Scholar] [CrossRef] [PubMed]
- Culy, C.R.; Bhana, N.; Plosker, G.L. Ondansetron. Paediatr. Drugs 2001, 3, 441–479. [Google Scholar] [CrossRef] [PubMed]
- Nougairède, A.; Touret, F. Antiviral Activity Against SARS-CoV-2: Ondansetron; Aix Marseille University: Marseille, France, 2020; Available online: https://www.european-virus-archive.com/sites/default/files/covid19-compounds-tests/Ondansetron.pdf (accessed on 18 September 2022).
- Pascale, L.R.; Dubin, A.; Hoffman, W.S. Therapeutic value of probenecid (Benemid®) in gout. J. Am. Med. Assoc. 1952, 149, 1188–1194. [Google Scholar] [CrossRef]
- Murray, J.; Hogan, R.J.; Martin, D.E.; Blahunka, K.; Sancilio, F.D.; Balyan, R.; Lovern, M.; Still, R.; Tripp, R.A. Probenecid inhibits SARS-CoV-2 replication in vivo and in vitro. Sci. Rep. 2021, 11, 18085. [Google Scholar] [CrossRef]
- Ellinger, B.; Bojkova, D.; Zaliani, A.; Cinatl, J.; Claussen, C.; Westhaus, S.; Keminer, O.; Reinshagen, J.; Kuzikov, M.; Wolf, M.; et al. A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection. Sci. Data 2021, 8, 70. [Google Scholar] [CrossRef]
- Zimmerman, J.; Selhub, J.; Rosenberg, I.H. Competitive inhibition of folate absorption by dihydrofolate reductase inhibitors, trimethoprim and pyrimethamine. Am. J. Clin. Nutr. 1987, 46, 518–522. [Google Scholar] [CrossRef]
- Syed, Y.Y. Rolapitant: First Global Approval. Drugs 2015, 75, 1941–1945. [Google Scholar] [CrossRef]
- Zaliani, A.; Vangeel, L.; Reinshagen, J.; Iaconis, D.; Kuzikov, M.; Keminer, O.; Wolf, M.; Ellinger, B.; Esposito, F.; Corona, A.; et al. Cytopathic SARS-CoV-2 Screening on VeroE6 Cells in a Large Repurposing Effort. Available online: https://www.ebi.ac.uk/chembl/document_report_card/CHEMBL4495565/ (accessed on 11 April 2022).
- Drugbank Sirolimus. Available online: https://go.drugbank.com/drugs/DB00877 (accessed on 19 April 2022).
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H.; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Approves First Treatment for COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 18 September 2022).
- Wang, A.Q.; Hagen, N.R.; Padilha, E.C.; Yang, M.; Shah, P.; Chen, C.Z.; Huang, W.; Terse, P.; Sanderson, P.; Zheng, W.; et al. Preclinical Pharmacokinetics and in vitro Properties of GS-441524, A Potential Oral Drug Candidate for COVID-19 Treatment. Front. Pharmacol. 2022, 13, 918083. [Google Scholar] [CrossRef]
- Lenze, E.J.; Mattar, C.; Zorumski, C.F.; Stevens, A.; Schweiger, J.; Nicol, G.E.; Miller, J.P.; Yang, L.; Yingling, M.; Avidan, M.S.; et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Merck’s COVID Pill Loses its Lustre: What That Means for the Pandemic. Nature 2021. Available online: https://www.nature.com/articles/d41586-021-03667-0 (accessed on 18 September 2022). [CrossRef] [PubMed]
- Abdelnabi, R.; Foo, C.S.; Jochmans, D.; Vangeel, L.; De Jonghe, S.; Augustijns, P.; Mols, R.; Weynand, B.; Wattanakul, T.; Hoglund, R.M.; et al. The Oral Protease Inhibitor (PF-07321332) Protects Syrian Hamsters Against Infection with SARS-CoV-2 Variants of Concern. Nat. Commun. 2022, 13, 719. [Google Scholar] [CrossRef] [PubMed]
- Seftel, D.; Boulware, D.R. Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19. Open Forum Infect. Dis. 2021, 8, ofab050. [Google Scholar] [CrossRef] [PubMed]
- Homolak, J.; Kodvanj, I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106044. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.; Dötsch, M.; Kick, H.; Vieira, A.; Möller, H.-J. Plasma concentrations of fluvoxamine and maprotiline in major depression: Implications on therapeutic efficacy and side effects. Eur. Neuropsychopharmacol. 1993, 3, 13–21. [Google Scholar] [CrossRef]
- Facente, S.N.; Reiersen, A.M.; Lenze, E.J.; Boulware, D.R.; Klausner, J.D. Fluvoxamine for the Early Treatment of SARS-CoV-2 Infection: A Review of Current Evidence. Drugs 2021, 81, 2081–2089. [Google Scholar] [CrossRef]
- Hosseini, M.; Chen, W.; Xiao, D.; Wang, C. Computational molecular docking and virtual screening revealed promising SARS-CoV-2 drugs. Precis. Clin. Med. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Heppler, L.N.; Attarha, S.; Persaud, R.; Brown, J.I.; Wang, P.; Petrova, B.; Tošić, I.; Burton, F.B.; Flamand, Y.; Walker, S.R.; et al. The antimicrobial drug pyrimethamine inhibits STAT3 transcriptional activity by targeting the enzyme dihydrofolate reductase. J. Biol. Chem. 2022, 298, 101531. [Google Scholar] [CrossRef]
- Appelberg, S.; Gupta, S.; Svensson Akusjärvi, S.; Ambikan, A.T.; Mikaeloff, F.; Saccon, E.; Végvári, Á.; Benfeitas, R.; Sperk, M.; Ståhlberg, M.; et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020, 9, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Sirolimus and COVID-19. 2022. Available online: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=sirolimus+&cntry=&state=&city=&dist= (accessed on 18 September 2022).
- Box, H.; Pennington, S.H.; Kijak, E.; Tatham, L.; Caygill, C.H.; Lopeman, R.C.; Jeffreys, L.N.; Herriott, J.; Sharp, J.; Neary, M.; et al. Lack of antiviral activity of probenecid in Vero E6 cells and Syrian golden hamsters: A need for better understanding of inter-lab differences in preclinical assays [Preprint]. bioRxiv 2022. [Google Scholar] [CrossRef]
- Schultz, D.C.; Johnson, R.M.; Ayyanathan, K.; Miller, J.; Whig, K.; Kamalia, B.; Dittmar, M.; Weston, S.; Hammond, H.L.; Dillen, C.; et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 2022, 604, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Why You Should Not Use Ivermectin to Treat or Prevent COVID-19. 2020. Available online: https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19 (accessed on 18 September 2022).
- Lim, S.C.L.; Hor, C.P.; Tay, K.H.; Mat Jelani, A.; Tan, W.H.; Ker, H.B.; Chow, T.S.; Zaid, M.; Cheah, W.K.; Lim, H.H.; et al. Efficacy of Ivermectin Treatment on Disease Progression Among Adults with Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 426–435. [Google Scholar] [CrossRef]
- Mehra, M.R.; Desai, S.S.; Ruschitzka, F.; Patel, A.N. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet, 2020; in press. [Google Scholar] [CrossRef]
- Saag, M.S. Misguided Use of Hydroxychloroquine for COVID-19: The Infusion of Politics into Science. JAMA 2020, 324, 2161–2162. [Google Scholar] [CrossRef]
- Martin, J.H.; Bowden, N.A. Drug repurposing in the era of COVID-19: A call for leadership and government investment. Med. J. Aust. 2020, 212, 450–452.e1. [Google Scholar] [CrossRef]
| Drugs | Original Indication | Catalogue Number | Purity | Solubility |
|---|---|---|---|---|
| Remdesivir | Antiviral | S8932 | 99.38% | DMSO, Ethanol |
| Molnupiravir | Antiviral | S0833 | 99.89% | DMSO, Water, Ethanol |
| Nirmatrelvir (PF-07321332) | Antiviral | S9866 | 99.82% | DMSO, Water, Ethanol |
| Aprepitant | Antiemetic | SML2215 | >98% | DMSO, Ethanol |
| Cyclizine | Antiemetic | S0897 | 98.17% | DMSO, Water, Ethanol |
| Cetirizine | Antihistamine | S1291 | 99.54% | DMSO, Water |
| Everolimus | Immunosuppressant, Anti-Cancer | S1120 | 99.69% | DMSO, Ethanol |
| Fluvoxamine | Antidepressant | S1336 | 99.80% | DMSO, Ethanol |
| Lapatinib | Anti-Cancer | S2111 | 99.86% | DMSO |
| L-Cycloserine | Antibiotic | S3945 | -- | DMSO, Water |
| Ondansetron | Antiemetic | S1996 | 99.35% | 0.5 M NaOH, 10 mM HCl |
| Probenecid | Anti-Gout | S4022 | 99.63% | DMSO, Ethanol |
| Pyrimethamine | Antiparasitic | S2006 | 100% | DMSO |
| Rolapitant | Antiemetic | S5476 | 99.67% | Ethanol |
| Sirolimus | Immunosuppressant | 37,094 | -- | DMSO, Ethanol |
| Drug | Class | Description | Activity Data (against SARS-CoV-2) | Cmax (µM) | Protein Binding (%) | Reference |
|---|---|---|---|---|---|---|
| Remdesivir | Antiviral | Nucleoside analogue used in the treatment of COVID-19 | EC50 = 0.01 μM in human airway epithelial cells | -- | 83–93.6 | [27] |
| Molnupiravir | Antiviral | Nucleoside analogue used in the treatment of COVID-19 | IC50 = 0.08 μM in Calu-3 cells | -- | -- | [28,29] |
| Nirmatrelvir (PF-07321332) | Antiviral | Inhibitor of SARS-CoV-2 main protease (Mpro) used in the treatment of COVID-19 | EC50 = 0.074 μM in Vero E6 cells | 4.42 | 69 | [30,31] |
| Aprepitant | Antiemetic | Neurokinin-1 receptor antagonist used in patients undergoing cancer chemotherapy | No activity data reported | 2.80 | >95 | [32] |
| Cyclizine | Antiemetic | Histamine H1 antagonist used to treat motion sickness | EC50 = 10 μM against SARS-CoV-1 pseudovirus entry in Vero E6 cells | 0.26 | 60–76 | [33,34] |
| Cetirizine | Antihistamine | Second-generation antihistamine used to treat allergic reactions such as rhinitis, urticaria, dermatitis, etc | No activity data reported | 0.80 | 93–96 | [35] |
| Everolimus | Immunosuppressant | mTOR inhibitor used as an immunosuppressant to prevent rejection of organ transplants | Reduced SARS-CoV-2 gene and protein expression in Vero cells at 1 μM | 0.19 | 74 | [36,37] |
| Fluvoxamine | Antidepressant | Selective serotonin re-uptake inhibitor (SSRI) used for the treatment of obsessive compulsive disorder (OCD) | IC50 = 10.54 μM in HEK293T-ACE2-TMPRSS2 cells | 0.28 | 77–80 | [38,39] |
| Lapatinib | Anti-Cancer | Receptor tyrosine kinase inhibitor used for the treatment of breast cancer | EC50 = 0.7 μM in Calu-3 cells | 4.18 | >99 | [40,41] |
| L-Cycloserine | Antibiotic | GABA transaminase inhibitor used to treat tuberculosis | Inhibition of replication in Vero E6 cells | 830 | None | [42,43] |
| Ondansetron | Antiemetic | 5HT3 receptor antagonist used as an antiemetic | EC50 = 2.47 μM in Vero E6 cells; no protection of hamsters | 0.43–0.66 | 73 | [44,45] |
| Probenecid | Anti-Gout | Uricosuric agent used to decrease uric acid in the body | IC50 = 0.0013 μM in Normal Human Bronchoepithelial cells | 521 | 75–95 | [46,47] |
| Pyrimethamine | Antiparasitic | DHFR inhibitor used as an antiparasitic for the treatment of cystoisosporiasis and toxoplasmosis | 58% inhibition of cytotoxicity in Caco-2 cells when administered at 10 μM | 0.94 | 87 | [48,49] |
| Rolapitant | Antiemetic | NK1 anatagonist used as an anti-emetic in patients undergoing chemotherapy | 20% Inhibition of cytotoxicity in Vero E6 | 1.90 | 99.8 | [50,51] |
| Sirolimus | Immunosuppressant | mTOR inhibitor used as an immunosuppressant to prevent rejection of organ transplants | Reduced SARS-CoV-2 gene and protein expression in Vero cells and airway cultures at 1μM | 0.016–0.098 | 92 | [37,52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAuley, A.J.; Jansen van Vuren, P.; Mohammed, M.-U.-R.; Faheem; Goldie, S.; Riddell, S.; Gödde, N.J.; Styles, I.K.; Bruce, M.P.; Chahal, S.; et al. Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection. Viruses 2022, 14, 2417. https://doi.org/10.3390/v14112417
McAuley AJ, Jansen van Vuren P, Mohammed M-U-R, Faheem, Goldie S, Riddell S, Gödde NJ, Styles IK, Bruce MP, Chahal S, et al. Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection. Viruses. 2022; 14(11):2417. https://doi.org/10.3390/v14112417
Chicago/Turabian StyleMcAuley, Alexander J., Petrus Jansen van Vuren, Muzaffar-Ur-Rehman Mohammed, Faheem, Sarah Goldie, Shane Riddell, Nathan J. Gödde, Ian K. Styles, Matthew P. Bruce, Simran Chahal, and et al. 2022. "Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection" Viruses 14, no. 11: 2417. https://doi.org/10.3390/v14112417
APA StyleMcAuley, A. J., Jansen van Vuren, P., Mohammed, M.-U.-R., Faheem, Goldie, S., Riddell, S., Gödde, N. J., Styles, I. K., Bruce, M. P., Chahal, S., Keating, S., Blasdell, K. R., Tachedjian, M., O’Brien, C. M., Singanallur, N. B., Viana, J. N., Vashi, A. V., Kirkpatrick, C. M., MacRaild, C. A., ... Vasan, S. S. (2022). Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection. Viruses, 14(11), 2417. https://doi.org/10.3390/v14112417









