In Vitro Infection Dynamics of Wuxiang Virus in Different Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Sequence Determination and Analysis
2.3. Viral Titer Determination
2.4. Virus Growth Kinetics
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Cell Cytopathic Changes and Cell Viability
2.7. Western Blotting
3. Results
3.1. Similarity Analysis of the Open Reading Frames of WUXV SXWX1813-2 and SXYQ1872-1
3.2. WUXV Induces Differential Cytopathic Changes and Cytotoxicity in Cell Lines
3.3. Effect of the Virus on Cell Viability
3.4. Cells Lines Support WUXV Replication and Produce Infectious Virus
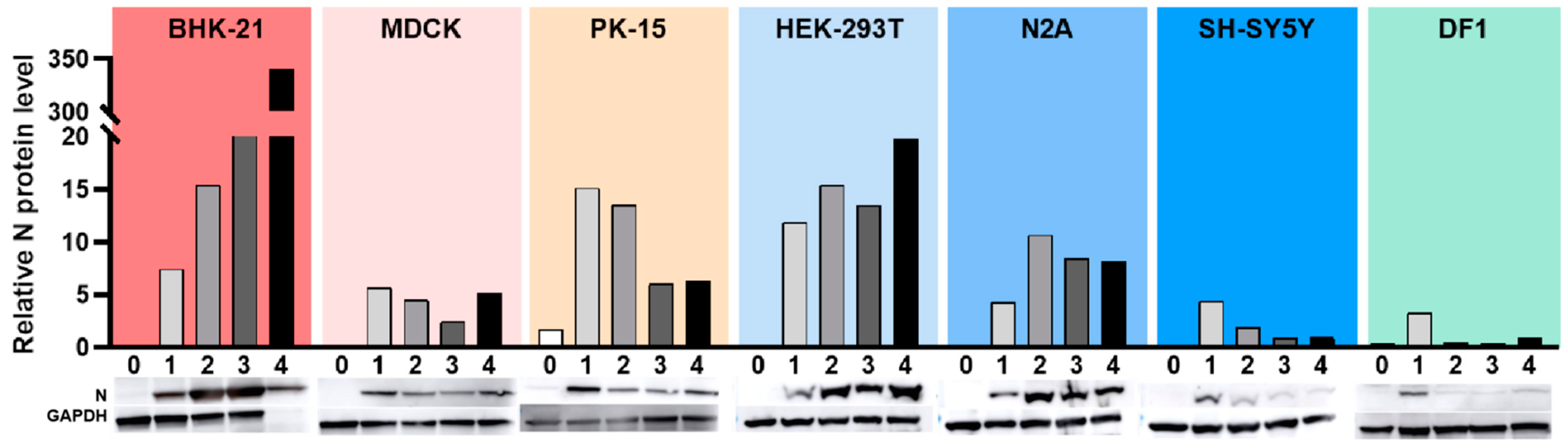
3.5. Western Blot Analysis of N Protein Expression in Different Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Fu, S.; Xu, Z.; Cheng, J.; Shi, M.; Fan, N.; Song, J.; Tian, X.; Cheng, J.; Ni, S.; et al. Emerging sand fly–borne phlebovirus in China. Emerg. Infect. Dis. 2020, 26, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Adkins, S.; Agwanda, B.R.; Al, K.R.; Alkhovsky, S.V.; Amarasinghe, G.K.; Avsic-Zupanc, T.; Ayllon, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. Correction to: 2021 taxonomic update of phylum negarnaviricota (riboviria:orthornavirae), including the large orders bunyavirales and mononegavirales. Arch. Virol. 2021, 166, 3567–3579. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, W.; Ye, C.; Smagghe, G.; Wang, J.J.; Niu, J. Discovery of a widespread presence bunyavirus that may have symbiont-like relationships with different species of aphids. Insect Sci. 2022, 29, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, S.; Conceicao-Neto, N.; Neyts, J.; Rocha-Pereira, J. Structural and functional similarities in bunyaviruses: Perspectives for pan-bunya antivirals. Rev. Med. Virol. 2019, 29, e2039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fu, S.; Cheng, J.; Xu, X.; Wang, J.; Wu, B.; Tian, X.; Li, Y.; He, Y.; Li, F.; et al. Re-isolation of wuxiang virus from wild sandflies collected from yangquan county, China. Virol. Sin. 2021, 36, 1177–1186. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Gao, G.F.; Tien, P.; Liu, W. Bunyavirales ribonucleoproteins: The viral replication and transcription machinery. Crit. Rev. Microbiol. 2018, 44, 522–540. [Google Scholar] [CrossRef] [PubMed]
- Hulswit, R.; Paesen, G.C.; Bowden, T.A.; Shi, X. Recent advances in bunyavirus glycoprotein research: Precursor processing, receptor binding and structure. Viruses 2021, 13, 353. [Google Scholar] [CrossRef]
- Ferron, F.; Weber, F.; de la Torre, J.C.; Reguera, J. Transcription and replication mechanisms of bunyaviridae and arenaviridae l proteins. Virus Res. 2017, 234, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fan, N.; Hou, X.; Wang, J.; Fu, S.; Song, J.; Shi, M.; Liang, G. Isolation and identification of a novel phlebovirus, hedi virus, from sandflies collected in China. Viruses 2021, 13, 772. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, N.; Hou, X.; Wang, J.; Fu, S.; Song, J.; Shi, M.; Liang, G. Reply to Charrel, R.N.; Depaquit, J. Comment on “Xu et al. Isolation and Identification of a Novel Phlebovirus, Hedi Virus, from Sandflies Collected in China. Viruses 2021, 13, 772”. Viruses 2021, 13, 2422. [Google Scholar] [CrossRef]
- Killick-Kendrick, R. The biology and control of phlebotomine sand flies. Clin. Dermatol. 1999, 17, 279–289. [Google Scholar] [CrossRef]
- Liang, G. Research progress of natural sandfly-borne viruses in China. Chin. J. Exp. Clin. Virol. 2022, 36, 469–474. [Google Scholar]
- Dong, X.; Soong, L. Emerging and re-emerging zoonoses are major and global challenges for public health. Zoonoses 2021, 1. [Google Scholar] [CrossRef]
- Wang, J.; Fan, N.; Fu, S.; Cheng, J.; Wu, B.; Xu, Z.; Song, J.; Tian, X.; Li, Y.; He, Y.; et al. Isolation and characterization of wuxiang virus from sandflies collected in yangquan county, shanxi province, china. Vector-Borne Zoonotic Dis. 2021, 21, 446–457. [Google Scholar] [CrossRef]
- Gandolfo, C.; Prathyumn, S.; Terrosi, C.; Anichini, G.; Gori, S.G.; Corti, D.; Bracci, L.; Lanzavecchia, A.; Roman-Sosa, G.; Cusi, M.G.; et al. Identification of a neutralizing epitope on tosv gn glycoprotein. Vaccines 2021, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Hum, N.R.; Bourguet, F.A.; Sebastian, A.; Lam, D.; Phillips, A.M.; Sanchez, K.R.; Rasley, A.; Loots, G.G.; Weilhammer, D.R. Mavs mediates a protective immune response in the brain to rift valley fever virus. PLoS Pathog. 2022, 18, e1010231. [Google Scholar] [CrossRef] [PubMed]
- Wuerth, J.D.; Habjan, M.; Kainulainen, M.; Berisha, B.; Bertheloot, D.; Superti-Furga, G.; Pichlmair, A.; Weber, F. Eif2b as a target for viral evasion of pkr-mediated translation inhibition. Mbio 2020, 11, e00976-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Jiang, R.R.; Ding, H.; Zhang, X.L.; Wang, N.; Zhang, Y.F.; Li, Y.; Chen, J.J.; Zhang, P.H.; Li, H.; et al. First detection of mukawa virus in ixodes persulcatus and haemaphysalis concinna in china. Front. Microbiol. 2022, 13, 791563. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.G.; Bini, L.; Gagliardi, A.; Anichini, G.; Gandolfo, C.; Prathyumnan, S.; Cusi, M.G. Ubiquitin and not only unfolded domains drives toscana virus non-structural nss protein degradation. Viruses 2020, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Habjan, M.; Penski, N.; Spiegel, M.; Weber, F. T7 rna polymerase-dependent and -independent systems for cdna-based rescue of rift valley fever virus. J. Gen. Virol. 2008, 89, 2157–2166. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Guan, Z.; Huang, W.; Zhang, Y.; Kortekaas, J.; Lozach, P.Y.; Peng, K. Nss filament formation is important but not sufficient for rvfv virulence in vivo. Viruses 2019, 11, 834. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, J.; Fu, S.; Wang, Q.; Wang, J.; Lu, X.; Tian, X.; Cheng, J.; Ni, S.; He, Y.; et al. Wuxiang virus is a virus circulated naturally in wuxiang county, china. Vector-Borne Zoonotic Dis. 2021, 21, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Cheng, M.; Ge, N.; Shu, J.; Xu, Z.; Su, X.; Kou, Z.; Tong, Y.; Qin, C.; et al. A single nonsynonymous mutation on zikv e protein-coding sequences leads to markedly increased neurovirulence in vivo. Virol. Sin. 2022, 37, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yao, X.; Fu, S.; Li, F.; Gu, T.; Chen, J.; He, Y.; Yin, J.; Xu, S.; Yin, Q.; et al. Establishment of TaqMan RT-PCR assay for Wuxiang virus. Chin. J. Exp. Clin. Virol. 2022, 36, 460–464. [Google Scholar]
- Smith, D.R.; Steele, K.E.; Shamblin, J.; Honko, A.; Johnson, J.; Reed, C.; Kennedy, M.; Chapman, J.L.; Hensley, L.E. The pathogenesis of rift valley fever virus in the mouse model. Virology 2010, 407, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Jones, D.; Trotter, R.; Donaldson, J.; White, J.; Stephen, E.; Slone, T.J. Experimental rift valley fever in rhesus macaques. Arch. Virol. 1988, 99, 31–44. [Google Scholar] [CrossRef]
- Quattrone, F.; Mazzetti, P.; Aquino, F.; Sani, S.; Carneglia, L.; Pistello, M.; Lopalco, P.L.; Tavoschi, L. Two clusters of toscana virus meningo-encephalitis in livorno province and elba island, July–September. Ann Ig 2020, 32, 674–681. [Google Scholar] [PubMed]
- Dersch, R.; Sophocleous, A.; Cadar, D.; Emmerich, P.; Schmidt-Chanasit, J.; Rauer, S. Toscana virus encephalitis in southwest germany: A retrospective study. BMC Neurol. 2021, 21, 495. [Google Scholar] [CrossRef]
- Clarke, L.L.; Mead, D.G.; Ruder, M.G.; Carter, D.L.; Bloodgood, J.; Howerth, E. Experimental infection of domestic piglets (sus scrofa) with rift valley fever virus. Am. J. Trop. Med. Hyg. 2021, 106, 182–186. [Google Scholar] [CrossRef]
- Mo, Q.; Xu, Z.; Deng, F.; Wang, H.; Ning, Y.J. Host restriction of emerging high-pathogenic bunyaviruses via mov10 by targeting viral nucleoprotein and blocking ribonucleoprotein assembly. PLoS Pathog. 2020, 16, e1009129. [Google Scholar] [CrossRef]
- Jin, C.; Liang, M.; Ning, J.; Gu, W.; Jiang, H.; Wu, W.; Zhang, F.; Li, C.; Zhang, Q.; Zhu, H.; et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in c57/bl6 mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 10053–10058. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Gaudreault, N.N.; Liu, Q.; Davis, A.S.; Shivanna, V.; Sunwoo, S.Y.; Lang, Y.; Morozov, I.; Ruder, M.; Drolet, B.; et al. Development of a sheep challenge model for rift valley fever. Virology 2016, 489, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Wilson, W.C.; Gaudreault, N.N.; Davis, A.S.; Shivanna, V.; Bawa, B.; Sunwoo, S.Y.; Ma, W.; Drolet, B.S.; Morozov, I.; et al. A recombinant rift valley fever virus glycoprotein subunit vaccine confers full protection against rift valley fever challenge in sheep. Sci. Rep. 2016, 6, 27719. [Google Scholar] [CrossRef]
- Odendaal, L.; Clift, S.J.; Fosgate, G.T.; Davis, A.S. Lesions and cellular tropism of natural rift valley fever virus infection in adult sheep. Vet. Pathol. 2019, 56, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, O.D.; Tomori, O.; Fajimi, J.L.; Schmitz, H. Experimental infection of three nigerian breeds of sheep with the zinga strain of the rift valley fever virus. Rev. Elev. Med. Vet. Pays Trop. 1996, 49, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ragan, I.K.; Schuck, K.N.; Upreti, D.; Odendaal, L.; Richt, J.A.; Trujillo, J.D.; Wilson, W.C.; Davis, A.S. Rift valley fever viral rna detection by in situ hybridization in formalin-fixed, paraffin-embedded tissues. Vector Borne Zoonotic Dis. 2019, 19, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.W.; TSlone, W.; Peters, C.J. The gerbil, meriones unguiculatus, a model for rift valley fever viral encephalitis. Arch. Virol. 1988, 102, 187–196. [Google Scholar] [CrossRef]


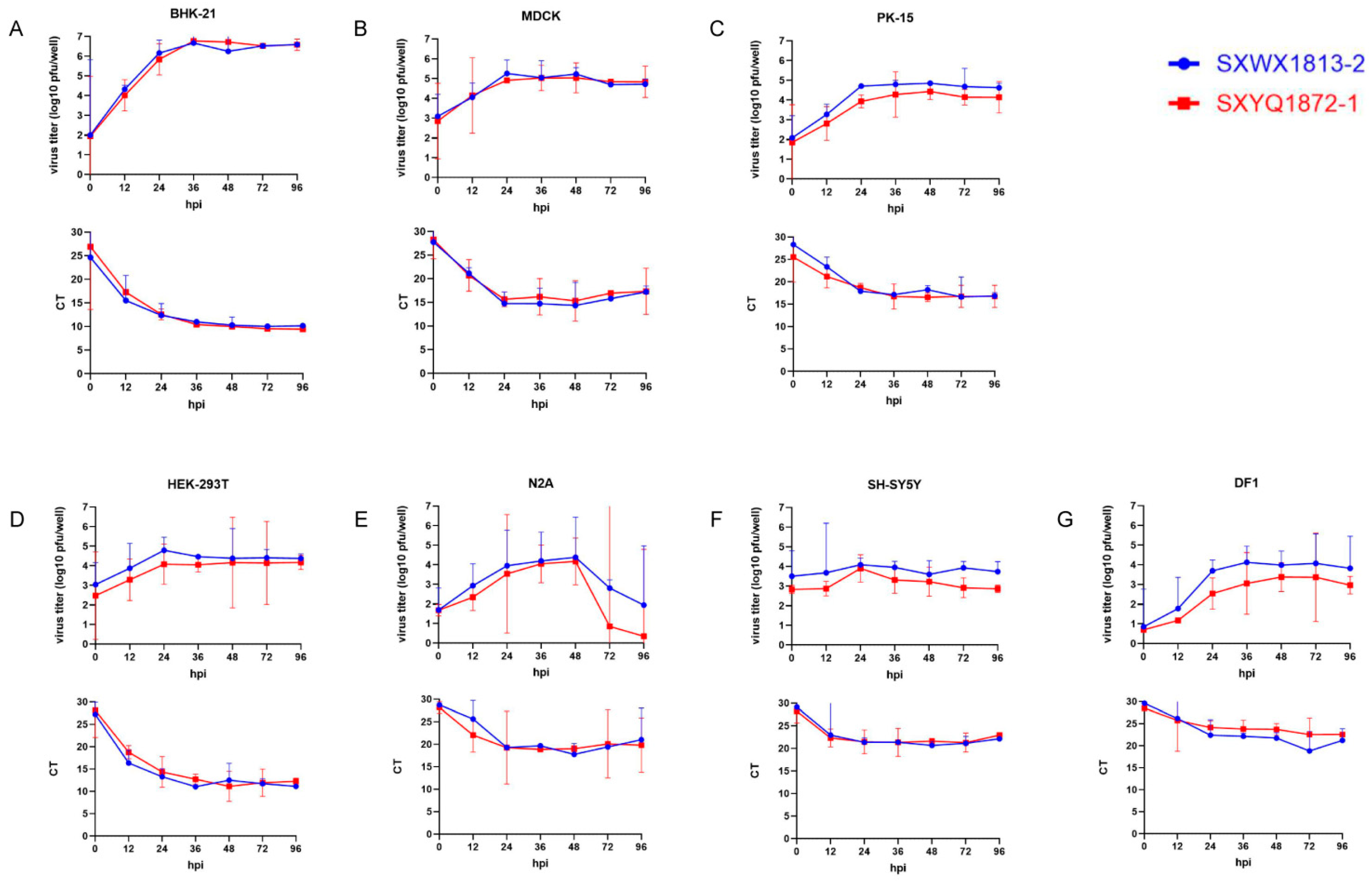
| Cell Line | Source | Tissue | Replication | CPE | Peak Titer (pfu/mL) | Time of Peak Titer (hpi) | ||
|---|---|---|---|---|---|---|---|---|
| SXWX1813-2 | SXYQ1872-1 | SXWX1813-2 | SXYQ1872-1 | |||||
| BHK-21 | hamster | kidney | + | + | 4.7 × 106 | 6.0 × 106 | 36 | 36 |
| MDCK | dog | kidney | + | + | 1.8 × 105 | 1.1 × 105 | 24 | 48 |
| PK-15 | pig | kidney | + | + | 7.1 × 104 | 2.7 × 104 | 48 | 48 |
| HEK-293T | human | embryonic kidney | + | − | 2.9 × 105 | 1.6 × 105 | 36 | 48 |
| N2A | mouse | neuroblastoma | + | − | 3.5 × 104 | 1.5 × 104 | 48 | 48 |
| SH-SY5Y | human | marrow | + | − | 1.2 × 104 | 2.8 × 103 | 24 | 24 |
| DF1 | chicken | embryo | + | − | 1.4 × 104 | 2.5 × 103 | 36 | 48 |
| Vero | monkey | kidney | − | − | / | / | / | / |
| S Segment | M Segment | L Segment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Virus Strains | NS | N | GP | RdRp | |||||||
| nt (%) | aa (%) | nt (%) | aa (%) | nt (%) | aa (%) | nt (%) | aa (%) | ||||
| SXWX1813-2 | 783 | 261 | 741 | 247 | 4089 | 1363 | 6273 | 2091 | |||
| SXYQ1872-1 | 783 (100) | 261 (100) | 741 (100) | 247 (100) | 4089 (96.7) | 1363 (97.4) | 6273 (97.7) | 2091 (99.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Yin, Q.; Hu, D.; Fu, S.; Zhang, W.; Nie, K.; Li, F.; Xu, S.; He, Y.; Liang, G.; et al. In Vitro Infection Dynamics of Wuxiang Virus in Different Cell Lines. Viruses 2022, 14, 2383. https://doi.org/10.3390/v14112383
Yao X, Yin Q, Hu D, Fu S, Zhang W, Nie K, Li F, Xu S, He Y, Liang G, et al. In Vitro Infection Dynamics of Wuxiang Virus in Different Cell Lines. Viruses. 2022; 14(11):2383. https://doi.org/10.3390/v14112383
Chicago/Turabian StyleYao, Xiaohui, Qikai Yin, Danhe Hu, Shihong Fu, Weijia Zhang, Kai Nie, Fan Li, Songtao Xu, Ying He, Guodong Liang, and et al. 2022. "In Vitro Infection Dynamics of Wuxiang Virus in Different Cell Lines" Viruses 14, no. 11: 2383. https://doi.org/10.3390/v14112383
APA StyleYao, X., Yin, Q., Hu, D., Fu, S., Zhang, W., Nie, K., Li, F., Xu, S., He, Y., Liang, G., Li, X., & Wang, H. (2022). In Vitro Infection Dynamics of Wuxiang Virus in Different Cell Lines. Viruses, 14(11), 2383. https://doi.org/10.3390/v14112383






