Abstract
Loss to follow-up (LTFU) from HIV pre-exposure prophylaxis (PrEP) care compromises the goal of HIV elimination. We investigated the proportion of LTFU and associated risk factors among men who have sex with men (MSM) enrolled in a PrEP demonstration project in Burkina Faso, Côte d’Ivoire, Mali, and Togo. CohMSM-PrEP, a prospective cohort study, was conducted between November 2017 and June 2021 in community-based clinics. MSM aged 18 years or older at substantial risk of HIV infection received a comprehensive prevention package, including PrEP and peer education. LTFU was defined as not returning to the clinic for six months. Associated risk factors were investigated using a time-varying Cox’s model. Of 647 participants followed up for a median time of 15 months, 372 were LTFU (57.5%). LTFU was associated with younger age (adjusted hazard ratio [95% Confidence Interval]; 1.50 [1.17–1.94]), unemployment (1.33 [1.03–1.71]), depression (1.63 [1.12–2.38]), and perceiving no HIV risk with stable male partners (1.61 [1.23–2.10]). Contacting peer educators outside of scheduled visits was protective (0.74 [0.56–0.97]). Our findings show that LTFU from PrEP care in West African MSM is a major challenge to achieving HIV elimination, but that the involvement of peer educators in PrEP delivery helps to limit LTFU by providing users with adequate support.
1. Introduction
In West Africa, the HIV epidemic is concentrated in key populations, who, together with their sexual partners, accounted for 72% of all new HIV infections in 2020 [1]. Therefore, prevention in these populations is crucial to reach the global goal of eliminating HIV infection as a public health threat by 2030 [2,3]. Pre-exposure prophylaxis (PrEP) is an effective prevention tool [4,5,6,7,8,9]. PrEP programs in West Africa started very recently and are mainly for men who have sex with men (MSM) and sex workers.
The effectiveness of PrEP programs is highly dependent on its use by those targeted. Besides low access to PrEP, high rates of disengagement (i.e., discontinuation or loss to follow-up [LTFU]) from PrEP care constitute another major issue. Unfortunately, data on PrEP disengagement in Sub-Saharan Africa (SSA) is sparse due to slow roll-out rates [4]. This is notably the case for MSM in West Africa, specifically. This situation is attributable to the complicated legal and sociocultural position for MSM there. Indeed, discriminatory policies and laws against same-sex behaviors perpetuate a culture of widespread stigma and discrimination [10,11,12]. At the institutional level, political stakeholders relegate less financial resources to programs dedicated to these populations [13], limiting the availability of adapted HIV prevention and care for them, including PrEP provision [14,15,16,17,18,19]. This heteronormativity causes MSM to be rejected by society, leading to economic and psychosocial marginalization [10,20]. The effect of this hostile environment on PrEP disengagement is unclear, but will most surely have an impact.
Worldwide, rates of PrEP disengagement in MSM range from 17% to 58% [7,21,22,23,24]. Most data comes from the United States, while in SSA the literature is limited to a handful of studies in Kenya, South Africa, and Zimbabwe [25,26,27,28,29] and one in West Africa [30]. In a recent meta-analysis, including studies from all regions except SSA, the rate of both LTFU and PrEP discontinuation in MSM and transgender women was 29.3% [31]. From the rare studies in SSA, LTFU ranged from 19.1% in a PrEP program for MSM and sex workers [25] to 42.5% in MSM only, both in Kenya [27]. In general, the barriers that drive LTFU and discontinuation are similar to those that affect other steps of the PrEP care cascade [32,33,34]—A framework designed to identify gaps in PrEP implementation, spanning from initiation, to engagement (i.e., uptake and adherence), to disengagement [23,35,36,37,38,39,40,41,42]. Based on findings from MSM in the United States, discontinuation of PrEP most often occurred within the first months of taking it [32,43] and tended to worsen over time [43,44], while in Kenya, spending less time in care was a risk factor for LTFU [26,27]. The large majority of discontinuations were related to logistical or financial barriers to accessing PrEP services in both the US [45,46,47,48,49], and in Kenya [26,27]. Another major reason for discontinuation in the US was related to decreased HIV risk perception [45,47,48,49,50,51,52,53], due to abstinence or entering into a monogamous relationship [54]. Younger age was associated with LTFU in the US only [45,47,53,55,56,57], while, in both SSA and in the US, substance use [26,27,46], PrEP side effects [28,29,45,47,50,52,58] and psychosocial problems [51,53], including stigma [25,28] were risk factors for disengagement.
One of the select few demonstration projects to provide PrEP to MSM in West Africa was CohMSM-PrEP [59]. From November 2017 until June 2021, MSM participants had access to PrEP and peer education as a part of a comprehensive sexual health prevention package in community-based clinics [30]. Initial findings showed that PrEP uptake helped prevent new HIV infections [30], and that certain community-based aspects of the cohort and accurate HIV-risk perception facilitated PrEP initiation and engagement [60,61]. Further findings showed that some factors related to the region’s hostile environment, like socioeconomic and psychosocial vulnerability were barriers to PrEP engagement and effective HIV protection [60,62]. Finally, preliminary data suggested that LTFU was high with 27% of participants being LTFU at the time of analysis.
In the present study, we aimed to estimate the proportion of participants LTFU at the cohort’s conclusion and to identify the risk factors for LTFU in CohMSM-PrEP. Our main hypothesis was that similar barriers and facilitators influencing the other steps of the PrEP care cascade would also play a role in study retention. To our knowledge—beyond the preliminary data from CohMSM-PrEP—There have been no other studies on retention of MSM in PrEP care in the West African context. Furthermore, this is the first study to explore risk factors for LTFU among MSM taking PrEP in West Africa and one of the very few in SSA. Informing on the state of the PrEP care cascade in West Africa and identifying a LTFU profile, will help guide PrEP delivery and rollout as more and more countries in the region add PrEP to their national AIDS programs.
2. Materials and Methods
2.1. Study Design and Participants
CohMSM-PrEP was a prospective cohort study initiated on 20 November 2017 and ended on 30 June 2021. It assessed the acceptability and feasibility of a comprehensive sexual health prevention package, including PrEP, for MSM in community-based clinics in West Africa. The four study sites were MSM-friendly clinics run by community-based organizations: Centre Oasis run by the Association African Solidarité (AAS) in Ouagadougou (Burkina Faso); Clinique de Confiance, run by the association Espace Confiance in Abidjan (Côte d’Ivoire); Clinique de Santé Sexuelle des Halles run by the Association pour la Résilience des Communautés pour l’Accès au Développement et à la Santé—ARCAD Santé PLUS (formerly ARCAD-SIDA) in Bamako (Mali); and Centre Lucia, run by Espoir Vie Togo (EVT) in Lomé (Togo). CohMSM-PrEP was designed as an addition to, and development of, a previous MSM cohort, CohMSM, which studied the implementation of HIV prevention and care services for MSM in the same study sites [63]. Except for PrEP provision, the comprehensive sexual health prevention package was the same in both cohort studies.
First, participants from CohMSM who wished to continue follow-up and take PrEP were recruited, thereby ending their participation in CohMSM. Then, “new” participants were identified by peer educators (PE) through a specific network of community-based organizations. Both ex-CohMSM participants and “new” participants had to meet the same eligibility criteria (a comparison of the two cohorts has been previously described [61].
Participants were eligible if they were 18 years or older, HIV-negative (status confirmed at study enrollment), MSM (defined as reporting at least one episode of anal intercourse [insertive or receptive] with another man in the six months preceding enrollment), and reported any of the following HIV at-risk criteria: (i) Non-virally suppressed seropositive sexual partner (male or female), (ii) condomless anal or vaginal sex with two or more partners in the previous six months, (iii) a history of sexually transmitted infection (STI) in the previous six months, (iv) post-exposure prophylaxis use in the previous six months, or (v) requesting PrEP. Exclusion criteria included signs or symptoms of acute HIV infection, probable exposure to HIV, a creatinine clearance of less than 60 mL/min (using the Cockroft-Gault equation); positive or indeterminate hepatitis B surface antigen test; and allergy or contraindication to PrEP drugs.
2.2. Procedures
Generic fixed-dose PrEP combinations of tenofovir disoproxil fumarate 300 mg and emtricitabine 200 mg were prescribed as follows: daily (one pill per day) or event-driven (2 + 1 + 1 dosing; i.e., 2 pills between 2–24 h before sex [1 if PrEP taken the previous day] followed by 1 pill 24 h and another 48 h after the first pill[s]) [64,65]. At each quarterly follow-up visit, in concertation with study doctors and/or PE, participants could decide to switch PrEP strategies or stop PrEP (temporarily or permanently) depending on their needs. The study procedures were the same regardless of the PrEP regimen.
During quarterly follow-up visits, participants received a refill of their PrEP prescription, clinical examinations by a physician, HIV testing, screening and treatment for other STIs, condoms and lubricants and peer-led psychosocial support and counseling. The latter included guidance on adherence, condom use, switching between PrEP strategies, abstaining from certain high-risk activities and study retention. Adherence-specific counseling was provided monthly beginning with first PrEP delivery and every three months thereafter. Furthermore, participants could come to their clinic for an unscheduled visit or contact PE by telephone at any time. If participants were 15 days late for their scheduled visit, PE contacted them by telephone (with previous consent). All services were offered free of charge and participants were compensated USD 5 for transport costs at every scheduled visit.
Participants were screened for HIV using national algorithms (Abbott Determine HIV 1/2 assay [Abbott Laboratories, Chiba, Japan] and, if the result was positive, SD Bioline HIV-1/2 3.0 [SD, Gyeonggi-do, South Korea] or First Response HIV-1/2 assay [Premier Medical Corporation, Mumbai, India]) and those diagnosed HIV positive during follow-up were invited to initiate antiretroviral treatment immediately.
Trained research assistants administered standardized face-to-face questionnaires at enrollment and every three months thereafter to collect socio-demographic and behavioral data on individual characteristics, cohort and PrEP-related factors, MSM-identity, psychosocial aspects, sexual behaviors, and substance use. Medical staff collected clinical data at each follow-up visit (scheduled or not), including LTFU, PrEP regimen and HIV and STI testing results.
2.3. Measures
2.3.1. Event of Interest (Outcome)
In the present study, the event of interest was the first episode of LTFU. A participant was defined as LTFU if they did not return to the clinic six months after their last visit, regardless if they re-engaged later in care. Participants refusing to continue in the cohort or those asking to withdraw their consent were also defined as LTFU. The outcome is time to LTFU. It was constructed as the time (in months) elapsed between the date of enrollment in the cohort (first prescription of PrEP) and the date of the last observed visit, before an absence of more than six months. Data for participants that completed follow-up were censored at the last quarterly visit before the end of the study (i.e., 30 June 2021). For HIV seroconverted participants, data was censored at the date of their positive HIV test; and for deceased participants, data was censored at the date of their death. A dichotomous variable indicated whether data for participants was censored (=1) or not (=0). Examples to illustrate the definition of LTFU in our study can be found in Supplemental Material Scheme S1.
2.3.2. Covariates
Covariates in the present analysis included:
Sociodemographic and socioeconomic characteristics. Age (grouped, 18–24 vs. >24 years old), country-fixed effects (Burkina Faso, Côte d’Ivoire, Mali, and Togo), employment status (employed vs. unemployed).
Cohort or PrEP-related characteristics. Recruitment type (ex-CohMSM participant vs. new participant), chosen PrEP regimen (event-driven or daily), self-reported PrEP adherence during most recent sexual intercourse (daily regimen, ‘optimal’ [7 pills taken in the week before most recent intercourse], vs. ‘suboptimal’ [4–6 pills], vs. ‘poor’ [1–3 pills], vs. ‘no PrEP’ [no pills]; event-driven regimen, ‘optimal’ [dosing schedule respected completely], vs. ‘suboptimal’ [taking at least one pill 2–24 h before sex act and one pill 24 h after sex act], vs. ‘poor’ [all other pill taking combinations], vs. ‘no PrEP’ [no tablets taken before or after sex act] [30]), contacted a PE outside of scheduled visits (‘yes’ vs. ‘no’).
MSM identity and psychosocial aspects. Self-defined sexual orientation (‘heterosexual’,‘homosexual/gay’ or ‘bisexual’), self-defined gender identity (‘man/boy’ vs. ‘both a man and a woman’, ‘more a woman’, and ‘neither a woman nor a man’), depression (based on the Patient Health Questionnaire-9, where a minimal (moderate to high) depression score was defined as ≤4 (>4) [66].
Sexual behaviors. Have a casual male/female partner (‘yes’ vs. ‘no’), number of male partners in the previous three months (≤2 vs. >2), HIV risk perception score with stable/casual partners ranged from 0–10 and was defined as no risk = 0, at risk = 1–10, and no stable/casual partner, combined prevention use during most recent anal intercourse (‘no PrEP & no condom use’ vs. ‘only PrEP use’ vs. ‘condom use only’ vs. ‘PrEP & condom use’).
2.4. Statistical Analysis
First, participants’ characteristics at baseline were described. In all longitudinal analyses, which ranged from baseline to M42 and used repeated measures, country and recruitment type were considered to be time-constant (i.e., measured at baseline); while the remaining covariates were time-dependent (i.e., varying over time). Next, bivariate analysis was conducted using the Kaplan–Meier technique to describe and explore significant associations between time-to-LTFU and covariates. Then, a time-varying Cox’s proportional hazards model was used to assess risk factors for LTFU. We identified potential covariates for the multivariate model using a threshold of 20% significance in univariate analysis. All univariate and multivariate models were adjusted for country-fixed effects. The forward selection technique was used to construct the final multivariate model and its goodness-of-fit was verified using the Akaike information criterion (AIC). All analyses were performed using STATA 16.1 statistical software.
2.5. Ethical Considerations
CohMSM-PrEP was registered with ClinicalTrials.gov, number NCT03459157. The study protocol was approved by the ethics committees of Mali (N°2017/113/CE/FMPOS), Burkina Faso (N°2017-7-105), Côte d’Ivoire (N°088/MSHP/CNER-kp) and Togo (N°338/2017/MSPS/CAB/SG/DGAS/DPML/CBRS), and Belgium (ethics committee of the Antwerp University). All participants provided written informed consent.
3. Results
3.1. Sample Characteristics
From 20 November 2020 until 30 June 2021, 647 participants enrolled in the CohMSM-PrEP study. Table 1 describes the characteristics of the study sample at baseline. Mean age was 25.3 years old (standard deviation, SD = 5.8) and 48.5% of participants were 18–24 years old. A majority of participants came from Mali (39.7%), 20.3% from both Côte d’Ivoire and Togo, and 19.7% from Burkina Faso. Almost half (46.2%) of participants were unemployed.

Table 1.
Characteristics of study participants at baseline, n = 647.
Half of the study sample (49.8%) were ex-CohMSM participants. A forth (25.0%) of participants contacted PE outside of their scheduled visits. Three-fourths (73.1%) of participants chose event-driven PrEP at baseline and after three months of pill intake, a third (40.9%) had optimal PrEP adherence, 16.1% had suboptimal adherence, 5.2% had poor adherence and 21.3% did not take PrEP during the week preceding their most recent intercourse.
At baseline, a majority of the study sample self-defined as bisexual (51.6%), 35.2% as homosexual or gay, and 2.3% as heterosexual. Over half (54.7%) of participants self-identified as a man or boy. Forty-five percent of the study sample (45.6%) had a moderate to high depression score (PHQ-9).
In the previous three months, 58.6% of the study sample had casual male partners, 20.3% had casual female partners and over a third (34.6%) of participants had more than two male sexual partners. With casual partners, 43.4% perceived themselves to be at risk of HIV infection, 14.8% at no risk, while the rest (40.2%) were not concerned because they had no casual partners. With stable partners, 41.0% perceived themselves to be at risk of HIV infection, 29.7% at no risk, while the rest (28.0%) were not concerned because they had no stable partner. After three months of pill intake, and in terms of combined prevention use during most recent intercourse, 33.0% used both PrEP and condoms, 9.6% used condoms only, 28.6% used PrEP only, and 26.7% used neither PrEP no condoms.
3.2. Loss to Follow-Up and Risk Factors
Overall, the median follow-up time was 15 months (IQR 6–30) and participants made up a total of 4831 scheduled follow-up visits from baseline to M42 (Figure 1). During follow-up, 372 participants were LTFU (57.5%). Of those 275 participants not LTFU, 25 participants seroconverted (3.9%), one participant died (0.1%), and 249 completed follow-up (38.5%). The median follow-up time for LTFU participants was 8 months (IQR 3–16). Of the 372 LTFU participants, 122 (33%) returned to care later, meaning 250 participants were LTFU definitively. None of those who returned to care seroconverted during the period they left care or after returning to care.
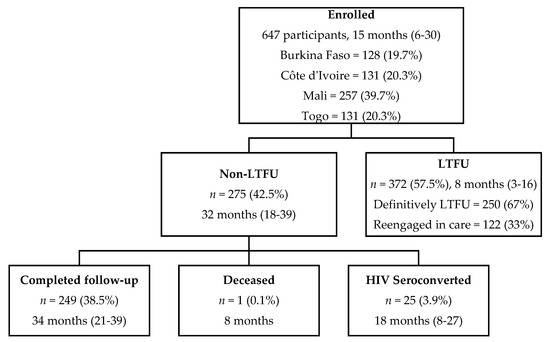
Figure 1.
CohMSM-PrEP study population flowchart with median follow-up time in months (IQR) (Follow-up from 20 November 2017–30 June 2021; 647 participants contributed to 4831 visits from baseline to M42).
Trends of LTFU according to patient’s characteristics can be found in Figure 2.


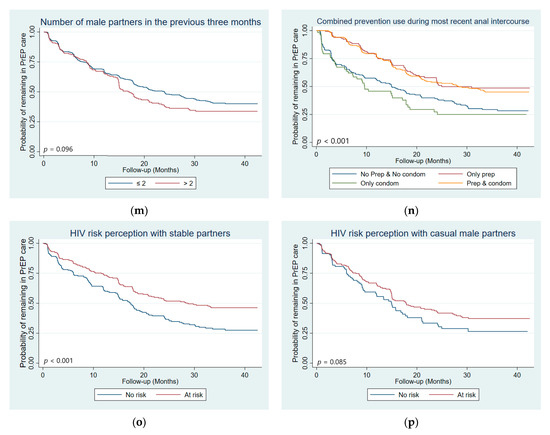
Figure 2.
Kaplan–Meier curves with log-rank test p-value comparing LTFU by: (a) age group, (b) country, (c) employment status, (d) recruitment type, (e) chosen PrEP regimen, (f) PrEP adherence, (g) contact with peer educators outside of scheduled visits, (h) self-defined sexual orientation, (i) self-defined gender identity, (j) depression score, (k) had a casual male sexual partner in the previous three months, (l) had a casual female sexual partner in the previous three months, (m) number of male sexual partners in the previous three months, (n) Combined prevention use during most recent anal intercourse, (o) HIV risk perception with stable male partner, and (p) HIV risk perception with casual male partners (n = 647 participants, 4831 visits).
In univariate Cox analysis, risk factors for LTFU included: younger age (18–24 years old, p < 0.001), being followed-up in Côte d’Ivoire (p = 0.017), being unemployed (p = 0.001), being non-adherent to PrEP (no PrEP, p < 0.001; poor adherence, p = 0.003; suboptimal adherence, p = 0.026), both a man and a woman/more a woman/neither a woman or a man gender identity (p = 0.023), having a moderate to severe depression score (p = 0.029), having a casual partner in the previous three months (male, p = 0.022; female, p = 0.030), perceiving no HIV risk with stable male partners (p = 0.007) and using neither PrEP nor condoms (p < 0.001) or condoms only (p < 0.001) during most recent anal intercourse (Table 2). Protective factors for LTFU included being followed-up in Togo (p < 0.001), contacting PE outside of scheduled visits (p = 0.014), and having no casual partners (p = 0.006).

Table 2.
Determinants of loss to follow-up from PrEP care (n = 647 participants, 4831 measures) 1.
In the final multivariate model, participants who were LTFU were more likely to be young (18–24, adjusted hazard ratio (AHR) [95% Confidence Interval, CI], p-value; 1.50 [1.17–1.94], 0.002) and unemployed (1.33 [1.03–1.71], 0.027), to have a moderate to high depression score (1.63 [1.12–2.38], 0.010), to have had a casual female partner in the previous three months (1.40 [1.00–1.95], 0.047), to have had more than two male sexual partners in the previous three months (1.37 [1.03–1.83], 0.031), and to perceive no HIV risk with their stable male partner (1.61 [1.23–2.10], <0.001) (Table 2). Furthermore, participants who used neither PrEP nor condoms (2.56 [1.92–3.42], <0.001), PrEP only (1.50 [1.05–2.13], 0.025), and condoms only (2.19 [1.47–3.25], <0.001) during their most recent anal intercourse were more likely to be LTFU compared to those who used both PrEP and condoms. Meanwhile, participants who were LTFU were less likely to have contacted PE outside of scheduled visits (0.74 [0.56–0.97], 0.029). Finally, a positive trend existed between being LTFU and self-identifying as heterosexual (1.83 [0.91–3.68], 0.088).
4. Discussion
This analysis showed that more than half of MSM taking PrEP were LTFU for six months or more at least once in the CohMSM-PrEP study in West Africa and of them a third later returned to care, while the rest were LTFU definitely. Compared to a preliminary analysis of the cohort, definitive LTFU increased from 27% to 39% (250/647) [30]. In both the United States and in SSA, the risk of PrEP disengagement tended to increase overtime [26,27,43,44] and could explain why we found higher rates of definitive LTFU at the end of the cohort compared to the preliminary analysis.
Compared to other studies in Kenya, rates of LTFU in the present study were higher [25,26,27]. However, comparison with other studies is difficult owing to the use of different definitions of LTFU and to different follow-up times. For example, in a one-year PrEP program in Kenya, 19.1% of MSM were LTFU or stopped taking PrEP after six months, but did not define the outcome any further [25]. In two other Kenyan studies defining LTFU as being more than ninety days late for an appointment (even if they later reengaged in care), LTFU ranged from 40.3% for a median follow-up time of 4.5 months [26] to 42.5% for a median follow-up time of 5.5 months [27]. It is to be noted that, on average, LTFU participants in our study stayed in care longer than those in the Kenyan studies did (8 months median follow-up time vs. 4.5–5.5 months). Indeed, the standardization of LTFU definitions across these programs would be helpful for their evaluation and comparison. Altogether, these studies and ours highlight that LTFU is a key issue to address in PrEP programs.
In this regard, one of the most important results from the present study was the positive influence peer education had on study retention, as participants who contacted PE were less likely to be LTFU. Indeed, in CohMSM-PrEP, one of PE’s main responsibilities was to contact LTFU participants in an effort to convince them to return to care. In large part due to their persistence, a third of these participants eventually returned to care. While the literature is limited on the direct effect of peer education on retention in PrEP care, it has been shown to be useful in encouraging PrEP initiation, uptake and adherence [41,60,67,68,69,70,71,72,73,74]. In general, MSM largely prefer community-based clinics for PrEP delivery [24,75,76,77,78,79,80,81,82,83], as they offer a more comprehensive and MSM-friendly approach to PrEP provision [75]. Our present finding reinforces previous findings from CohMSM-PrEP that showed peer-based outreach over time helped reach a new profile of MSM initiating PrEP [61], that the provision of PrEP in MSM-friendly community-based clinics promoted its use, and that peer education facilitated correct PrEP adherence [60].
Although peer education facilitated study retention in the present study, vulnerability was a barrier to it, mirroring findings from previous CohMSM-PrEP studies and the literature. Financial barriers can impede PrEP users from accessing care because of cost or not having time to attend appointments, ultimately leading to disengagement with PrEP care, as findings from the US suggest [45,46,47,48,49]. In the present study, unemployed participants were more likely to be LTFU. Although participants in CohMSM-PrEP received the prevention package free of charge and were reimbursed transport costs (USD 5), this still might not have been enough in light of the hostile environment towards MSM in the region. In terms of psychosocial vulnerability, studies in the US found higher rates of LTFU among patients with mental health problems [51,53], while in South Africa and Kenya, stigmatization was associated with PrEP discontinuation [25,28]. Our findings reflect the literature, as having a moderate to severe depression score predicted LTFU. In previous CohMSM-PrEP studies, financially insecure event-driven users were less likely to have correct adherence [60] and more likely to be ineffectively protected against HIV (i.e., incorrect PrEP adherence and no condom use) [62]. Furthermore, feeling alone was associated with incorrect PrEP adherence [60]. A final risk factor related to vulnerability in the present study was younger age—A common predictor of LTFU in other studies in the US [45,47,53,55,56,57]. In general, youth MSM are particularly vulnerable to HIV infection because of the compounding effect of power imbalances related to age on existing homonegativity [84]. Indeed, one can suppose that the accumulation of several of these vulnerabilities will produce an even stronger effect on LTFU.
A final result from the present study was that HIV risk perception played a substantial role in LTFU, reflecting findings from previous CohMSM-PrEP studies and the literature that showed HIV-risk perception also facilitated PrEP initiation and engagement [50,60,61,85,86,87,88,89,90,91,92]. It is important to note that PrEP is not a lifelong tool for everyone and it is not unusual to cycle in and out of PrEP use in accordance with “seasons of risk” [93]. Some instances of PrEP disengagement are voluntary due to decreasing perceived HIV risk [45,47,48,49,50,51,52,53], such as when users enter a period of abstinence or into a monogamous relationship with an HIV-negative or a virally suppressed HIV-positive partner [54]. This was evidenced in our study’s findings with participants who did not feel at risk of HIV infection with their stable partner being more likely to leave the cohort. Participants declaring casual female partners were also more likely to leave the cohort and a positive trend existed between LTFU and self-defining as heterosexual. These findings could reflect participants feeling less “at-risk” with female partners and having less need for PrEP.
However, this disengagement based on HIV risk perception requires accuracy. Indeed, multiple studies found recent high-risk behavior before LTFU despite reporting no longer being at-risk [49,50,55,56]. Indeed this period is critical and among MSM on PrEP in SSA, seroconversions occurred primarily among MSM who had stopped taking PrEP or those who had low or no detectable drug levels in their system [30,88]. In the present study, we also found relatively recent high-risk behavior was a risk factor for LTFU, i.e., having more than two male sexual partners in the previous three months. At the time, PrEP was only available through the cohort and these participants would have been at risk of HIV infection if they continued such practices without readopting appropriate risk reduction techniques after leaving the cohort. Nonetheless, sexual behavior is dynamic and multi-partnerships in the past do not necessarily continue in the future. LTFU participants did not return to care for over six months, during which time indication for PrEP could have changed.
In general, multiple findings from CohMSM-PrEP, including those from the present study, suggest certain behavioral, psychosocial and socioeconomic factors influence multiple levels of the PrEP care cascade simultaneously. They also highlight the value of community-based methods in such care. Specifically, in terms of improving LTFU, we recommend strengthening the role of PE in PrEP care as they can provide resources to users in matters related to HIV risk, and socioeconomic and psychosocial vulnerabilities. Indeed, PE could provide extra support for vulnerable users or provide risk reduction counseling and empowerment interventions to improve risk perception. Finally, we recommend providers deliver appropriate messaging about PrEP as a tool that can be stopped and started based on HIV risk, so users simply discontinue PrEP and do not leave care completely.
Our study has limitations. First, it was carried out in MSM-friendly community-based clinics located in the countries’ economic capitals, so participants might not be representative of the local MSM community and our findings might not be generalizable to other contexts. However, the differences found in an analysis comparing ex-participants from a previous MSM cohort, CohMSM, with newly recruited participants in CohMSM-PrEP, suggested that adding PrEP to the existing prevention package helped reach a new profile of MSM—less connected to these clinics [61]. Second, participants’ responses might have been affected by social desirability bias regarding sensitive topics, though; this bias was minimized by training research assistants to administer all the questionnaires and by having repeated and regular contact between participants and assistants. Furthermore, this limit only concerns covariates as LTFU was measured objectively, based on follow-up visit dates. Finally, we considered participants who reengaged in care after the 6-month cutoff to be LTFU, which led to a higher rate of LTFU. These participants represented 33% of the LTFU participants (122/372) and feedback from study staff and physicians suggested they missed appointments because they were highly mobile (travel, work, etc.) and/or had enough PrEP and did not feel the need to attend the clinic. Indeed, these participants would be ideal candidates for 6-month PrEP dispensing with HIV self-testing, which has been shown to be non-inferior to standard of care [94]. Furthermore, this definition is useful to determine interruptions in care, especially in contexts where PrEP is not available and for future long-acting injectable forms of PrEP. Indeed, up to one year or more after stopping injections trace amounts of these drugs remain present in the bloodstream, which are not enough to protect from HIV infection and could lead to HIV seroconversion and the development of drug resistant HIV [95]. Our definition is helpful for identifying optimal candidates for long-acting PrEP with no extended interruptions in care.
Despite these limitations, this is the first study to explore risk factors for LTFU among MSM taking PrEP in West Africa and one of the very few in Sub-Saharan Africa. Informing on the state of the PrEP care cascade in West Africa and identifying a LTFU profile, will help guide PrEP delivery and rollout as more and more countries in the region add PrEP to their national AIDS programs. Even though a high rate of LTFU was found in the present study, our results showed that for MSM participants who stayed in care, PrEP was a well-adopted and complementary HIV prevention tool. Indeed, stakeholders should not be discouraged by LTFU rates, but encouraged by the decrease in HIV incidence previously found in the cohort [30] and the fact that participants who used both PrEP and condoms during their most recent anal intercourse were more likely to be retained in care in the present study.
5. Conclusions
Our findings show that LTFU from PrEP care in West African MSM is a major challenge to achieving HIV elimination. However, they also show that the involvement of PEs in the management of PrEP users helps to limit LTFU by providing them with adequate support. There is an urgent need for the recognition and funding of these staff.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14112380/s1, Scheme S1: Definition of LTFU.
Author Contributions
A.E., B.S. and L.S.-T. designed the study. L.S.-T. supervised and B.C. conducted the data management and statistical analysis. A.E. and L.S.-T. co-drafted the article. A.E. drafted the first version of the manuscript. C.L. was a co-principal investigator of this study. B.D.K. was also a co-principal investigator and coordinated the study in Mali. T.T.E.D. coordinated the study in Burkina Faso. C.A. coordinated the study in Côte d’Ivoire. E.M. coordinated the study in Togo. I.Y. contributed to the coordination of the study and oversaw data collection. I.Y., L.S.-T. and B.C. accessed and verified the data. G.M., M.B., M.M. contributed to the study’s implementation and monitoring of the social sciences component. B.S. coordinated the social sciences component. L.R. and D.R.C. coordinated the community component. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the ANRS | Maladies infectieuses émergentes (ANRS 12369) and Expertise France (L’Initiative). August Eubanks and Ter Tiero Elias Dah were the recipients of doctoral fellowships from ANRS (ANRS 12369-B105) and Sidaction (2021-2-FJC-13012) for AE and from ANRS (ANRS 12324-B99) for TTED.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. CohMSM-PrEP was registered with ClinicalTrials.gov, number NCT03459157. The study protocol was approved by the ethics committees of Mali (N°2017/113/CE/FMPOS), Burkina Faso (N°2017-7-105), Côte d’Ivoire (N°088/MSHP/CNER-kp) and Togo (N°338/2017/MSPS/CAB/SG/DGAS/DPML/CBRS), and Belgium (ethics committee of the Antwerp University).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be requested by submitting a study proposal to the scientific board of the CohMSM-PrEP project (christian.laurent@ird.fr). Proposals will be evaluated for compatibility with the CohMSM-PrEP project and overlap with ongoing work. The codes generated during and/or analyzed during the current study are available from the corresponding author on request.
Acknowledgments
We sincerely thank the MSM participants and the staff of the community-based organizations (ARCAD Santé PLUS, Espace Confiance, Association African Solidarité, Espoir Vie Togo) who participated in the CohMSM-PrEP study. The CohMSM-PrEP Study Group: Christian Laurent, Issifou Yaya, Sayouba Ouedraogo, Bruno Granouillac, Benjamin Cuer, Laetitia Serrano, Martine Peeters, Clotilde Couderc (IRD, Inserm, Univ Montpellier, TransVIHMI, Montpellier, France); Bruno Spire, Luis Sagaon-Teyssier, Marion Mora, Gwenaëlle Maradan, Michel Bourrelly, Cyril Berenger, Sylvie Boyer (Inserm, IRD, Univ Aix-Marseille, SESSTIM, Marseille, France); Daniela Rojas Castro, Lucas Riegel, Paméla Palvadeau (Coalition PLUS, Pantin, France); Bea Vuylsteke, Irith De Baetselier, Thijs Reyniers, (Institut de Médecine Tropicale, Anvers, Belgique); Bintou Dembélé Keita, Fodié Diallo, Alou Coulibaly, Alassane Kader Maïga, Drissa Camara, Mahamadou Diarra, Aly Ouologuem, Naboh Sangaré, Abdoul Aziz Keita, Oumar Cissé, Fodé Traoré, Bréhima Abdrahamane Ouary, Ibrahima Kanta (ARCAD Santé PLUS, Bamako, Mali); Camille Anoma, Jean-Baptiste Malan, Rachelle Kotchi, Niamkey Thomas Aka, Kpassou Julien Lokrou, Noufo Hamed Coulibaly, Ekessi Jean Armel Koffi, Dibi Frédéric N’guessan, Stéphane-Alain Babo Yoro, Adama Cissé (Espace Confiance, Abidjan, Côte d’Ivoire); Ter Tiero Elias Dah, Issa Traoré, Camille Rajaonarivelo, Fayçal Rodrique Ouedraogo, Joseph Ouedraogo, Christian Coulibaly, Mamadou Ouedraogo, Ousseni Ilboudo, Abdoulazziz Traoré, Honoré Comsiambo (Association African Solidarité, Ouagadougou, Burkina Faso); Ephrem Mensah, Mawuényégan Kouamivi Agboyibor, Anani Attisso, Anouwarsadat Kokouba, Aléda Mawuli Badjassim, Kouakou Kokouvi Selom Agbomadji, Messan Attiogbe, Kossi Jeff Yaka, Agbégnigan Lorette Ekon, Julien Bimba (Espoir Vie Togo, Lomé, Togo); Claver Anoumou Dagnra, Kokou Dominique Tegueni (BIOLIM, Univ Lomé, Lomé, Togo).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- UNAIDS UNAIDS Data 2021. Available online: https://www.unaids.org/en/resources/documents/2021/2021_unaids_data (accessed on 11 May 2022).
- United Nations United Nations Political Declaration on Ending AIDS Sets World on the Fast-Track to End the Epidemic by 2030. Available online: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2016/june/20160608_PS_HLM_PoliticalDeclaration (accessed on 1 June 2022).
- United Nations United Nations Sustainable Development Goals (SDGs) 3: Ensure Healthy Lives and Promote Wellbeing for All at All Ages. Available online: https://www.un.org/sustainabledevelopment/health/ (accessed on 19 September 2022).
- Bavinton, B.R.; Grulich, A.E. HIV Pre-Exposure Prophylaxis: Scaling up for Impact Now and in the Future. Lancet Public Health 2021, 6, e528–e533. [Google Scholar] [CrossRef]
- Grulich, A.E.; Bavinton, B.R. Scaling up Preexposure Prophylaxis to Maximize HIV Prevention Impact. Curr. Opin. HIV AIDS 2022, 17, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Estcourt, C.; Yeung, A.; Nandwani, R.; Goldberg, D.; Cullen, B.; Steedman, N.; Wallace, L.; Hutchinson, S. Population-Level Effectiveness of a National HIV Preexposure Prophylaxis Programme in MSM. AIDS 2021, 35, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Grulich, A.E.; Guy, R.; Amin, J.; Jin, F.; Selvey, C.; Holden, J.; Schmidt, H.-M.A.; Zablotska, I.; Price, K.; Whittaker, B.; et al. Population-Level Effectiveness of Rapid, Targeted, High-Coverage Roll-out of HIV Pre-Exposure Prophylaxis in Men Who Have Sex with Men: The EPIC-NSW Prospective Cohort Study. Lancet HIV 2018, 5, e629–e637. [Google Scholar] [CrossRef]
- Public Health England towards Zero HIV Transmissions by 2030. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/965765/HIV_in_the_UK_2019_towards_zero_HIV_transmissions_by_2030.pdf (accessed on 1 July 2022).
- San Francisco Department of Public Health Population Health Division HIV Epidemiology Annual Report 2020. Available online: https://www.sfdph.org/dph/comupg/oprograms/hivepisec/hivepisecreports.asp (accessed on 3 July 2022).
- Beyrer, C.; Baral, S.D.; van Griensven, F.; Goodreau, S.M.; Chariyalertsak, S.; Wirtz, A.L.; Brookmeyer, R. Global Epidemiology of HIV Infection in Men Who Have Sex with Men. Lancet 2012, 380, 367–377. [Google Scholar] [CrossRef]
- Baral, S.; Scheibe, A.; Sullivan, P.; Trapence, G.; Lambert, A.; Bekker, L.-G.; Beyrer, C. Assessing Priorities for Combination HIV Prevention Research for Men Who Have Sex with Men (MSM) in Africa. AIDS Behav. 2013, 17 (Suppl. 1), S60–S69. [Google Scholar] [CrossRef]
- Beyrer, C.; Baral, S.D.; Collins, C.; Richardson, E.T.; Sullivan, P.S.; Sanchez, J.; Trapence, G.; Katabira, E.; Kazatchkine, M.; Ryan, O.; et al. The Global Response to HIV in Men Who Have Sex with Men. Lancet 2016, 388, 198–206. [Google Scholar] [CrossRef]
- Grosso, A.; Ryan, O.; Tram, K.H.; Baral, S. Financing the Response to HIV among Gay Men and Other Men Who Have Sex with Men: Case Studies from Eight Diverse Countries. Glob. Public Health 2015, 10, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Djomand, G.; Quaye, S.; Sullivan, P.S. HIV Epidemic among Key Populations in West Africa. Curr. Opin. HIV AIDS 2014, 9, 506–513. [Google Scholar] [CrossRef]
- Smith, A.D.; Tapsoba, P.; Peshu, N.; Sanders, E.J.; Jaffe, H.W. Men Who Have Sex with Men and HIV/AIDS in Sub-Saharan Africa. Lancet 2009, 374, 416–422. [Google Scholar] [CrossRef]
- Kushwaha, S.; Lalani, Y.; Maina, G.; Ogunbajo, A.; Wilton, L.; Agyarko-Poku, T.; Adu-Sarkodie, Y.; Boakye, F.; Zhang, N.; Nelson, L.E. “But the Moment They Find out That You Are MSM…”: A Qualitative Investigation of HIV Prevention Experiences among Men Who Have Sex with Men (MSM) in Ghana’s Health Care System. BMC Public Health 2017, 17, 770. [Google Scholar] [CrossRef] [PubMed]
- Ogunbajo, A.; Kershaw, T.; Kushwaha, S.; Boakye, F.; Wallace-Atiapah, N.-D.; Nelson, L.E. Barriers, Motivators, and Facilitators to Engagement in HIV Care Among HIV-Infected Ghanaian Men Who Have Sex with Men (MSM). AIDS Behav. 2018, 22, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Mendos, L.R. State Sponsored Homophobia; International Lesbian, Gay, Bisexual, Trans and Intersex Association (ILGA): Geneva, Switzerland, 2019. [Google Scholar]
- Poteat, T.; Diouf, D.; Drame, F.M.; Ndaw, M.; Traore, C.; Dhaliwal, M.; Beyrer, C.; Baral, S. HIV Risk among MSM in Senegal: A Qualitative Rapid Assessment of the Impact of Enforcing Laws That Criminalize Same Sex Practices. PLoS ONE 2011, 6, e28760. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Hakim, A.; Vuylsteke, B.; Semde, G.; Gbais, H.G.; Diarrassouba, M.; Thiam, M.; Laga, M. Exploring Risk Behaviors and Vulnerability for HIV among Men Who Have Sex with Men in Abidjan, Cote d’Ivoire: Poor Knowledge, Homophobia and Sexual Violence. PLoS ONE 2014, 9, e99591. [Google Scholar] [CrossRef]
- Grinsztejn, B.; Hoagland, B.; Moreira, R.I.; Kallas, E.G.; Madruga, J.V.; Goulart, S.; Leite, I.C.; Freitas, L.; Martins, L.M.S.; Torres, T.S.; et al. Retention, Engagement, and Adherence to Pre-Exposure Prophylaxis for Men Who Have Sex with Men and Transgender Women in PrEP Brasil: 48 Week Results of a Demonstration Study. Lancet HIV 2018, 5, e136–e145. [Google Scholar] [CrossRef]
- Shover, C.L.; Shoptaw, S.; Javanbakht, M.; Lee, S.-J.; Bolan, R.K.; Cunningham, N.J.; Beymer, M.R.; DeVost, M.A.; Gorbach, P.M. Mind the Gaps: Prescription Coverage and HIV Incidence among Patients Receiving Pre-Exposure Prophylaxis from a Large Federally Qualified Health Center in Los Angeles, California. AIDS Behav. 2019, 23, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.A.; Mena, L.; Patel, R.; Oldenburg, C.E.; Beauchamps, L.; Perez-Brumer, A.G.; Parker, S.; Mayer, K.H.; Mimiaga, M.J.; Nunn, A. Retention in Care Outcomes for HIV Pre-Exposure Prophylaxis Implementation Programmes among Men Who Have Sex with Men in Three US Cities. J. Int. AIDS Soc. 2016, 19, 20903. [Google Scholar] [CrossRef]
- Phanuphak, N.; Sungsing, T.; Jantarapakde, J.; Pengnonyang, S.; Trachunthong, D.; Mingkwanrungruang, P.; Sirisakyot, W.; Phiayura, P.; Seekaew, P.; Panpet, P.; et al. Princess PrEP Program: The First Key Population-Led Model to Deliver Pre-Exposure Prophylaxis to Key Populations by Key Populations in Thailand. Sex. Health 2018, 15, 542–555. [Google Scholar] [CrossRef]
- Kimani, M.; van der Elst, E.M.; Chirro, O.; Wahome, E.; Ibrahim, F.; Mukuria, N.; de Wit, T.F.R.; Graham, S.M.; Operario, D.; Sanders, E.J. “I Wish to Remain HIV Negative”: Pre-Exposure Prophylaxis Adherence and Persistence in Transgender Women and Men Who Have Sex with Men in Coastal Kenya. PLoS ONE 2021, 16, e0244226. [Google Scholar] [CrossRef]
- Wahome, E.W.; Graham, S.M.; Thiong’o, A.N.; Mohamed, K.; Oduor, T.; Gichuru, E.; Mwambi, J.; van der Elst, E.M.; Sanders, E.J. Risk Factors for Loss to Follow-up among at-Risk HIV Negative Men Who Have Sex with Men Participating in a Research Cohort with Access to Pre-Exposure Prophylaxis in Coastal Kenya. J. Int. AIDS Soc. 2020, 23, e25593. [Google Scholar] [CrossRef]
- Wahome, E.; Boyd, A.; Thiong’o, A.N.; Mohamed, K.; Oduor, T.; Gichuru, E.; Mwambi, J.; van der Elst, E.; Graham, S.M.; Prins, M.; et al. Stopping and Restarting PrEP and Loss to Follow-up among PrEP-Taking Men Who Have Sex with Men and Transgender Women at Risk of HIV-1 Participating in a Prospective Cohort Study in Kenya. HIV Med. 2022, 23, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Pillay, D.; Stankevitz, K.; Lanham, M.; Ridgeway, K.; Murire, M.; Briedenhann, E.; Jenkins, S.; Subedar, H.; Hoke, T.; Mullick, S. Factors Influencing Uptake, Continuation, and Discontinuation of Oral PrEP among Clients at Sex Worker and MSM Facilities in South Africa. PLoS ONE 2020, 15, e0228620. [Google Scholar] [CrossRef] [PubMed]
- Parmley, L.E.; Harris, T.G.; Chingombe, I.; Mapingure, M.; Mugurungi, O.; Rogers, J.H.; Gozhora, P.; Wu, Y.; Samba, C.; Musuka, G.; et al. Engagement in the Pre-Exposure Prophylaxis (PrEP) Cascade among a Respondent-Driven Sample of Sexually Active Men Who Have Sex with Men and Transgender Women during Early PrEP Implementation in Zimbabwe. J. Int. AIDS Soc. 2022, 25, e25873. [Google Scholar] [CrossRef]
- Laurent, C.; Keita, B.D.; Yaya, I.; Guicher, G.L.; Sagaon-Teyssier, L.; Agboyibor, M.K.; Coulibaly, A.; Traoré, I.; Malan, J.-B.; Baetselier, I.D.; et al. HIV Pre-Exposure Prophylaxis for Men Who Have Sex with Men in West Africa: A Multicountry Demonstration Study. Lancet HIV 2021, 8, e420–e428. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Xu, J.; Hu, Z.; Rutstein, S.E.; Tucker, J.D.; Ong, J.J.; Jiang, Y.; Geng, W.; Wright, S.T.; et al. Discontinuation, Suboptimal Adherence, and Reinitiation of Oral HIV Pre-Exposure Prophylaxis: A Global Systematic Review and Meta-Analysis. Lancet HIV 2022, 9, e254–e268. [Google Scholar] [CrossRef]
- Rutstein, S.E.; Smith, D.K.; Dalal, S.; Baggaley, R.C.; Cohen, M.S. Initiation, Discontinuation, and Restarting HIV Pre-Exposure Prophylaxis: Ongoing Implementation Strategies. Lancet HIV 2020, 7, e721–e730. [Google Scholar] [CrossRef]
- Garrison, L.E.; Haberer, J.E. Pre-Exposure Prophylaxis Uptake, Adherence, and Persistence: A Narrative Review of Interventions in the U.S. Am. J. Prev. Med. 2021, 61, S73–S86. [Google Scholar] [CrossRef]
- Golub, S.A.; Enemchukwu, C.U. The Critical Importance of Retention in HIV Prevention. Lancet HIV 2018, 5, e475–e476. [Google Scholar] [CrossRef]
- Nunn, A.S.; Brinkley-Rubinstein, L.; Oldenburg, C.E.; Mayer, K.H.; Mimiaga, M.; Patel, R.; Chan, P.A. Defining the HIV Pre-Exposure Prophylaxis Care Continuum. AIDS 2017, 31, 731–734. [Google Scholar] [CrossRef]
- Parsons, J.T.; Rendina, H.J.; Lassiter, J.M.; Whitfield, T.H.F.; Starks, T.J.; Grov, C. Uptake of HIV Pre-Exposure Prophylaxis (PrEP) in a National Cohort of Gay and Bisexual Men in the United States: The Motivational PrEP Cascade. J. Acquir. Immune Defic. Syndr. 2017, 74, 285–292. [Google Scholar] [CrossRef]
- Liu, A.; Cohen, S.; Follansbee, S.; Cohan, D.; Weber, S.; Sachdev, D.; Buchbinder, S. Early Experiences Implementing Pre-Exposure Prophylaxis (PrEP) for HIV Prevention in San Francisco. PLoS Med. 2014, 11, e1001613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Newman, P.A.; Guta, A.; Lacombe-Duncan, A.; Tepjan, S. Clinical Exigencies, Psychosocial Realities: Negotiating HIV Pre-Exposure Prophylaxis beyond the Cascade among Gay, Bisexual and Other Men Who Have Sex with Men in Canada. J. Int. AIDS Soc. 2018, 21, e25211. [Google Scholar] [CrossRef] [PubMed]
- Hillis, A.; Germain, J.; Hope, V.; McVeigh, J.; Van Hout, M.C. Pre-Exposure Prophylaxis (PrEP) for HIV Prevention Among Men Who Have Sex with Men (MSM): A Scoping Review on PrEP Service Delivery and Programming. AIDS Behav. 2020, 24, 3056–3070. [Google Scholar] [CrossRef] [PubMed]
- Beach, L.B.; Greene, G.J.; Lindeman, P.; Johnson, A.K.; Adames, C.N.; Thomann, M.; Washington, P.C.T.; Phillips, G. Barriers and Facilitators to Seeking HIV Services in Chicago Among Young Men Who Have Sex with Men: Perspectives of HIV Service Providers. AIDS Patient Care STDS 2018, 32, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Galindo, G.R.; Garrett-Walker, J.J.; Hazelton, P.; Lane, T.; Steward, W.T.; Morin, S.F.; Arnold, E.A. Community Member Perspectives from Transgender Women and Men Who Have Sex with Men on Pre-Exposure Prophylaxis as an HIV Prevention Strategy: Implications for Implementation. Implement. Sci. 2012, 7, 116. [Google Scholar] [CrossRef]
- Kelley, C.F.; Kahle, E.; Siegler, A.; Sanchez, T.; del Rio, C.; Sullivan, P.S.; Rosenberg, E.S. Applying a PrEP Continuum of Care for Men Who Have Sex With Men in Atlanta, Georgia. Clin. Infect. Dis. 2015, 61, 1590–1597. [Google Scholar] [CrossRef]
- Hosek, S.; Rudy, B.; Landovitz, R.; Kapogiannis, B.; Siberry, G.; Rutledge, B.; Liu, N.; Brothers, J.; Mulligan, K.; Zimet, G.; et al. An HIV Pre-Exposure Prophylaxis (PrEP) Demonstration Project and Safety Study for Young MSM. J. Acquir. Immune Defic. Syndr. 2017, 74, 21–29. [Google Scholar] [CrossRef]
- Stankevitz, K.; Grant, H.; Lloyd, J.; Gomez, G.B.; Kripke, K.; Torjesen, K.; Ong, J.J.; Terris-Prestholt, F. Oral Preexposure Prophylaxis Continuation, Measurement and Reporting. AIDS 2020, 34, 1801–1811. [Google Scholar] [CrossRef]
- Whitfield, T.H.F.; John, S.A.; Rendina, H.J.; Grov, C.; Parsons, J.T. Why I Quit Pre-Exposure Prophylaxis (PrEP)? A Mixed-Method Study Exploring Reasons for PrEP Discontinuation and Potential Re-Initiation among Gay and Bisexual Men. AIDS Behav. 2018, 22, 3566–3575. [Google Scholar] [CrossRef]
- Marcus, J.L.; Hurley, L.B.; Hare, C.B.; Nguyen, D.P.; Phengrasamy, T.; Silverberg, M.J.; Stoltey, J.E.; Volk, J.E. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J. Acquir. Immune Defic. Syndr. 2016, 73, 540–546. [Google Scholar] [CrossRef]
- Spinelli, M.A.; Scott, H.M.; Vittinghoff, E.; Liu, A.Y.; Gonzalez, R.; Morehead-Gee, A.; Gandhi, M.; Buchbinder, S.P. Missed Visits Associated with Future Preexposure Prophylaxis (PrEP) Discontinuation among PrEP Users in a Municipal Primary Care Health Network. Open Forum Infect. Dis. 2019, 6, ofz101. [Google Scholar] [CrossRef] [PubMed]
- Hojilla, J.C.; Vlahov, D.; Crouch, P.-C.; Dawson-Rose, C.; Freeborn, K.; Carrico, A. HIV Pre-Exposure Prophylaxis (PrEP) Uptake and Retention among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav. 2018, 22, 1096–1099. [Google Scholar] [CrossRef]
- Morgan, E.; Ryan, D.T.; Newcomb, M.E.; Mustanski, B. High Rate of Discontinuation May Diminish PrEP Coverage Among Young Men Who Have Sex with Men. AIDS Behav. 2018, 22, 3645–3648. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Cohen, S.E.; Vittinghoff, E.; Anderson, P.L.; Doblecki-Lewis, S.; Bacon, O.; Chege, W.; Postle, B.S.; Matheson, T.; Amico, K.R.; et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern. Med. 2016, 176, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Krakower, D.; Maloney, K.M.; Powell, V.E.; Levine, K.; Grasso, C.; Melbourne, K.; Marcus, J.L.; Mayer, K.H. Patterns and Clinical Consequences of Discontinuing HIV Preexposure Prophylaxis during Primary Care. J. Int. AIDS Soc. 2019, 22, e25250. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, J.C.; Golden, M.R.; Barbee, L.A.; Khosropour, C.M. Patient Disengagement from an HIV Pre-Exposure Prophylaxis Program in a Sexually Transmitted Disease Clinic. Sex Transm. Dis. 2018, 45, e62–e64. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, E.; Knapper, C. Audit of Patient Retention in Pre-Exposure Prophylaxis (PrEP) Services in an Integrated Sexual Reproductive Health Service Setting. Int. J. STD AIDS 2019, 30, 1432–1435. [Google Scholar] [CrossRef]
- Haberer, J.E.; Bangsberg, D.R.; Baeten, J.M.; Curran, K.; Koechlin, F.; Amico, K.R.; Anderson, P.; Mugo, N.; Venter, F.; Goicochea, P.; et al. Defining Success with HIV Pre-Exposure Prophylaxis: A Prevention-Effective Adherence Paradigm. AIDS 2015, 29, 1277–1285. [Google Scholar] [CrossRef]
- Spinelli, M.A.; Glidden, D.V.; Anderson, P.L.; Gandhi, M.; Cohen, S.; Vittinghoff, E.; Coleman, M.E.; Scott, H.; Bacon, O.; Elion, R.; et al. Brief Report: Short-Term Adherence Marker to PrEP Predicts Future Nonretention in a Large PrEP Demo Project: Implications for Point-of-Care Adherence Testing. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 81, 158–162. [Google Scholar] [CrossRef]
- Doblecki-Lewis, S.; Liu, A.Y.; Feaster, D.J.; Cohen, S.E.; Elion, R.; Bacon, O.; Coleman, M.; Cardenas, G.; Kolber, M.A. Patterns and Correlates of Participant Retention in a Multi-City Pre-Exposure Prophylaxis Demonstration Project. J. Acquir. Immune Defic. Syndr. 2018, 79, 62–69. [Google Scholar] [CrossRef]
- Huang, Y.-L.A.; Tao, G.; Smith, D.K.; Hoover, K.W. Persistence With Human Immunodeficiency Virus Pre-Exposure Prophylaxis in the United States, 2012–2017. Clin. Infect. Dis. 2021, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Holloway, I.; Dougherty, R.; Gildner, J.; Beougher, S.C.; Pulsipher, C.; Montoya, J.A.; Plant, A.; Leibowitz, A. PrEP Uptake, Adherence, and Discontinuation among California YMSM Using Geosocial Networking Applications. J. Acquir. Immune Defic. Syndr. 2017, 74, 15–20. [Google Scholar] [CrossRef] [PubMed]
- AVAC PrEPWatch. Available online: https://www.prepwatch.org/ (accessed on 12 May 2022).
- Eubanks, A.; Coulibaly, B.; Dembélé Keita, B.; Anoma, C.; Dah, T.T.E.; Mensah, E.; Kaba, S.; Lokrou, K.J.; Ouedraogo, F.R.; Badjassim, A.M.F.; et al. Socio-Behavioral Correlates of Pre-Exposure Prophylaxis Use and Correct Adherence in Men Who Have Sex with Men in West Africa. BMC Public Health 2022, 22, 1832. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, A.; Dembélé Keita, B.; Anoma, C.; Dah, T.T.E.; Mensah, E.; Maradan, G.; Bourrelly, M.; Mora, M.; Riegel, L.; Rojas Castro, D.; et al. Reaching a Different Population of MSM in West Africa With the Integration of PrEP Into a Comprehensive Prevention Package (CohMSM-PrEP ANRS 12369-Expertise France). J. Acquir. Immune Defic. Syndr. 2020, 85, 292–301. [Google Scholar] [CrossRef]
- Eubanks, A.; Coulibaly, B.; Dembélé Keita, B.; Anoma, C.; DAH, T.T.E.; Mensah, E.; Maradan, G.; Bourrelly, M.; Mora, M.; Riegel, L.; et al. Rate and Predictors of Ineffective HIV Protection in African Men Who Have Sex with Men Taking Pre-Exposure Prophylaxis. AIDS Behav. 2022, 26, 3524–3537. [Google Scholar] [CrossRef]
- Dah, T.T.E.; Yaya, I.; Sagaon-Teyssier, L.; Coulibaly, A.; Kouamé, M.J.-B.; Agboyibor, M.K.; Maiga, K.; Traoré, I.; Mora, M.; Palvadeau, P.; et al. Adherence to Quarterly HIV Prevention Services and Its Impact on HIV Incidence in Men Who Have Sex with Men in West Africa (CohMSM ANRS 12324—Expertise France). BMC Public Health 2021, 21, 972. [Google Scholar] [CrossRef]
- World Health Organization. What’s the 2 + 1 + 1? Event-Driven Oral Pre-Exposure Prophylaxis to Prevent HIV for Men Who Have Sex with Men: Update to Who’s Recommendation on Oral Prep. Available online: http://www.who.int (accessed on 3 June 2021).
- Molina, J.-M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.-M.; Cua, E.; Pasquet, A.; et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Kelly, J.A.; Amirkhanian, Y.A.; Walsh, J.L.; Brown, K.D.; Quinn, K.G.; Petroll, A.E.; Pearson, B.M.; Rosado, A.N.; Ertl, T. AIDSImpact Special Issue Social Network Intervention to Increase Pre-Exposure Prophylaxis (PrEP) Awareness, Interest, and Use Among African American Men Who Have Sex with Men. AIDS Care 2020, 32, 40–46. [Google Scholar] [CrossRef]
- Schneider, J.A.; Young, L.; Ramachandran, A.; Michaels, S.; Cohen, H.; Robinson, I.; Alon, L.; Hill, B.; Nakasone, S.; Balenciaga, M.; et al. A Pragmatic Randomized Controlled Trial to Increase PrEP Uptake for HIV Prevention: 55-Week Results From PrEPChicago. J. Acquir. Immune Defic. Syndr. 2021, 86, 31–37. [Google Scholar] [CrossRef]
- McMahan, V.M.; Martin, A.; Garske, L.; Violette, L.R.; Andrasik, M.P.; Baeten, J.M.; Banta-Green, C.; Stekler, J.D.; McMahan, V.M.; Martin, A.; et al. Development of a Targeted Educational Intervention to Increase Pre-Exposure Prophylaxis Uptake among Cisgender Men and Transgender Individuals Who Have Sex with Men and Use Methamphetamine in Seattle (WA, USA). Sex. Health 2019, 16, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Daughtridge, G.W.; Conyngham, S.C.; Ramirez, N.; Koenig, H.C. I Am Men’s Health: Generating Adherence to HIV Pre-Exposure Prophylaxis (PrEP) in Young Men of Color Who Have Sex with Men. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2015, 14, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Klassen, B.J.; Lachowsky, N.J.; Lin, S.Y.; Edward, J.B.; Chown, S.A.; Hogg, R.S.; Moore, D.M.; Roth, E.A. Gay Men’s Understanding and Education of New HIV Prevention Technologies in Vancouver, Canada. Qual. Health Res. 2017, 27, 1775–1791. [Google Scholar] [CrossRef] [PubMed]
- Raifman, J.R.G.; Flynn, C.; German, D. Healthcare Provider Contact and Pre-Exposure Prophylaxis in Baltimore Men Who Have Sex With Men. Am. J. Prev. Med. 2017, 52, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.; Griffin, M.; Singer, S.N.; Greene, R.E.; Acosta, I.L.Z.; Kaudeyr, S.K.; Kapadia, F.; Halkitis, P.N. Structural Barriers to Pre-Exposure Prophylaxis Use Among Young Sexual Minority Men: The P18 Cohort Study. Curr. HIV Res. 2018, 16, 237–249. [Google Scholar] [CrossRef]
- John, S.A.; Rendina, H.J.; Grov, C.; Parsons, J.T. Home-Based Pre-Exposure Prophylaxis (PrEP) Services for Gay and Bisexual Men: An Opportunity to Address Barriers to PrEP Uptake and Persistence. PLoS ONE 2017, 12, e0189794. [Google Scholar] [CrossRef]
- Anand, T.; Nitpolprasert, C.; Trachunthong, D.; Kerr, S.J.; Janyam, S.; Linjongrat, D.; Hightow-Weidman, L.B.; Phanuphak, P.; Ananworanich, J.; Phanuphak, N.; et al. A Novel Online-to-Offline (O2O) Model for Pre-Exposure Prophylaxis and HIV Testing Scale Up. J. Int. AIDS Soc. 2017, 20, 21326. [Google Scholar] [CrossRef]
- Aloysius, I.; Savage, A.; Zdravkov, J.; Korologou-Linden, R.; Hill, A.; Smith, R.; Houghton-Price, V.; Boffito, M.; Nwokolo, N. InterPrEP. Internet-Based Pre-Exposure Prophylaxis with Generic Tenofovir DF/Emtricitabine in London: An Analysis of Outcomes in 641 Patients. J. Virus Erad. 2017, 3, 218–222. [Google Scholar] [CrossRef]
- Levy, M.E.; Watson, C.C.; Glick, S.N.; Kuo, I.; Wilton, L.; Brewer, R.A.; Fields, S.D.; Criss, V.; Magnus, M. Receipt of HIV Prevention Interventions Is More Common in Community-Based Clinics than in Primary Care or Acute Care Settings for Black Men Who Have Sex with Men in the District of Columbia. AIDS Care 2016, 28, 660–664. [Google Scholar] [CrossRef]
- Eaton, L.A.; Matthews, D.D.; Bukowski, L.A.; Friedman, M.R.; Chandler, C.J.; Whitfield, D.L.; Sang, J.M.; Stall, R.D. Elevated HIV Prevalence and Correlates of PrEP Use among a Community Sample of Black Men Who Have Sex with Men. J. Acquir. Immune Defic. Syndr. 2018, 79, 339–346. [Google Scholar] [CrossRef]
- Doblecki-Lewis, S.; Liu, A.; Feaster, D.; Cohen, S.E.; Cardenas, G.; Bacon, O.; Andrew, E.; Kolber, M.A. Healthcare Access and PrEP Continuation in San Francisco and Miami After the US PrEP Demo Project. J. Acquir. Immune Defic. Syndr. 2017, 74, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.E.; Okeke, N.L.; Munn, T.; Hunter, M.; Alexis, K.; Corneli, A.; Seña, A.C.; McGee, K.; McKellar, M.S. Partnerships Between a University-Affiliated Clinic and Community-Based Organizations to Reach Black Men Who Have Sex with Men for PrEP Care. J. Acquir. Immune Defic. Syndr. 2018, 77, e25–e27. [Google Scholar] [CrossRef] [PubMed]
- Arnold, E.A.; Hazelton, P.; Lane, T.; Christopoulos, K.A.; Galindo, G.R.; Steward, W.T.; Morin, S.F. A Qualitative Study of Provider Thoughts on Implementing Pre-Exposure Prophylaxis (PrEP) in Clinical Settings to Prevent HIV Infection. PLoS ONE 2012, 7, e40603. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.J.; Anderson, K.M.; Bangsberg, D.; Toevs, K.; Morrison, D.; Wells, C.; Clark, P.; Nicolaidis, C. Access to HIV Pre-Exposure Prophylaxis in Practice Settings: A Qualitative Study of Sexual and Gender Minority Adults’ Perspectives. J. Gen. Intern. Med. 2019, 34, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.; Guidry, J.A.; Collier, K.L.; Mantell, J.E.; Boccher-Lattimore, D.; Kaighobadi, F.; Sandfort, T.G.M. A Clinical Home for Preexposure Prophylaxis: Diverse Health Care Providers’ Perspectives on the “Purview Paradox”. J. Int. Assoc. Provid. AIDS Care 2016, 15, 59–65. [Google Scholar] [CrossRef]
- World Health Organization. Briefs on Young Key Populations; World Health Organization: Geneva, Switzerland, 2016.
- Stephenson, R.; Darbes, L.A.; Chavanduka, T.; Essack, Z.; van Rooyen, H. HIV Testing, Knowledge and Willingness to Use PrEP Among Partnered Men Who Have Sex with Men in South Africa and Namibia. AIDS Behav. 2021, 25, 1993–2004. [Google Scholar] [CrossRef]
- Coulaud, P.; Sagaon-Teyssier, L.; M’madi Mrenda, B.; Maradan, G.; Mora, M.; Bourrelly, M.; Dembélé Keita, B.; Keita, A.A.; Anoma, C.; Babo Yoro, S.-A.; et al. Interest in HIV Pre-Exposure Prophylaxis in Men Who Have Sex with Men in West Africa (CohMSM ANRS 12324—Expertise France). Trop. Med. Int. Health 2018, 23, 1084–1091. [Google Scholar] [CrossRef]
- Grant, R.M.; Anderson, P.L.; McMahan, V.; Liu, A.; Amico, K.R.; Mehrotra, M.; Hosek, S.; Mosquera, C.; Casapia, M.; Montoya, O.; et al. Uptake of Pre-Exposure Prophylaxis, Sexual Practices, and HIV Incidence in Men and Transgender Women Who Have Sex with Men: A Cohort Study. Lancet Infect. Dis. 2014, 14, 820–829. [Google Scholar] [CrossRef]
- Wahome, E.W.; Graham, S.M.; Thiong’o, A.N.; Mohamed, K.; Oduor, T.; Gichuru, E.; Mwambi, J.; Prins, M.; van der Elst, E.; Sanders, E.J. PrEP Uptake and Adherence in Relation to HIV-1 Incidence among Kenyan Men Who Have Sex with Men. EClinicalMedicine 2020, 26, 100541. [Google Scholar] [CrossRef]
- Hanum, N.; Cambiano, V.; Sewell, J.; Phillips, A.N.; Rodger, A.J.; Speakman, A.; Nwokolo, N.; Asboe, D.; Gilson, R.; Clarke, A.; et al. Use of HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in England: Data from the AURAH2 Prospective Study. Lancet Public Health 2020, 5, e501–e511. [Google Scholar] [CrossRef]
- Hammoud, M.A.; Vaccher, S.; Jin, F.; Bourne, A.; Maher, L.; Holt, M.; Bavinton, B.R.; Haire, B.; Degenhardt, L.; Grulich, A.; et al. HIV Pre-Exposure Prophylaxis (PrEP) Uptake among Gay and Bisexual Men in Australia and Factors Associated with the Nonuse of PrEP among Eligible Men: Results from a Prospective Cohort Study. J. Acquir. Immune Defic. Syndr. 2019, 81, e73–e84. [Google Scholar] [CrossRef] [PubMed]
- Gafos, M.; Horne, R.; Nutland, W.; Bell, G.; Rae, C.; Wayal, S.; Rayment, M.; Clarke, A.; Schembri, G.; Gilson, R.; et al. The Context of Sexual Risk Behaviour among Men Who Have Sex with Men Seeking PrEP, and the Impact of PrEP on Sexual Behaviour. AIDS Behav. 2019, 23, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wahome, E.; Graham, S.; Thiong’o, A.; Chirro, O.; Mohamed, K.; Gichuru, E.; Mwambi, J.; Price, M.; Sanders, E.J. Assessment of PrEP Eligibility and Uptake among At-Risk MSM Participating in a HIV-1 Vaccine Feasibility Cohort in Coastal Kenya. Wellcome Open Res. 2019, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Elsesser, S.A.; Oldenburg, C.E.; Biello, K.B.; Mimiaga, M.J.; Safren, S.A.; Egan, J.E.; Novak, D.S.; Krakower, D.S.; Stall, R.; Mayer, K.H. Seasons of Risk: Anticipated Behavior on Vacation and Interest in Episodic Antiretroviral Pre-Exposure Prophylaxis (PrEP) among a Large National Sample of U.S. Men Who Have Sex with Men (MSM). AIDS Behav. 2016, 20, 1400–1407. [Google Scholar] [CrossRef]
- Ngure, K.; Ortblad, K.F.; Mogere, P.; Bardon, A.R.; Thomas, K.K.; Mangale, D.; Kiptinness, C.; Gakuo, S.; Mbaire, S.; Nyokabi, J.; et al. Efficiency of 6-Month PrEP Dispensing with HIV Self-Testing in Kenya: An Open-Label, Randomised, Non-Inferiority, Implementation Trial. Lancet HIV 2022, 9, e464–e473. [Google Scholar] [CrossRef]
- AVAC about Cabotegravir (CAB-LA). Available online: https://www.prepwatch.org (accessed on 3 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).