Isolation and Characterization of Lytic Pseudomonas aeruginosa Bacteriophages Isolated from Sewage Samples from Tunisia
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Isolation and Propagation of Bacteriophages
2.3. Extraction, Sequencing, Annotation, and Taxonomic Assessment of the Phages and Bacterial Genomes
2.4. Host Range Analysis
2.5. Thermal Stability and pH Sensitivity
2.6. Phage Adsorption Assay
2.7. One-Step Growth Curve
2.8. Accession Numbers
3. Results and Discussion
3.1. Isolation and Selection of the Phages
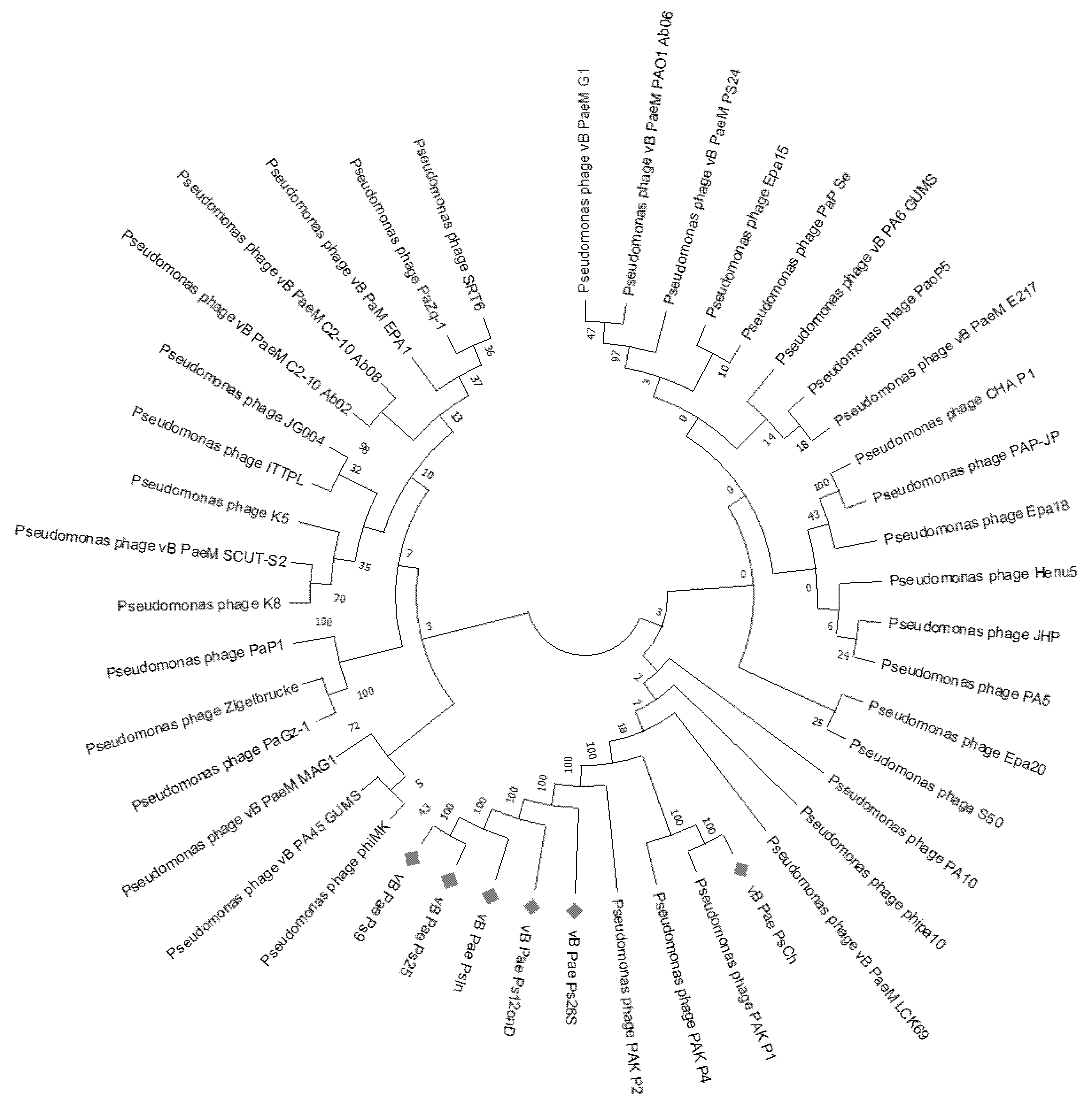
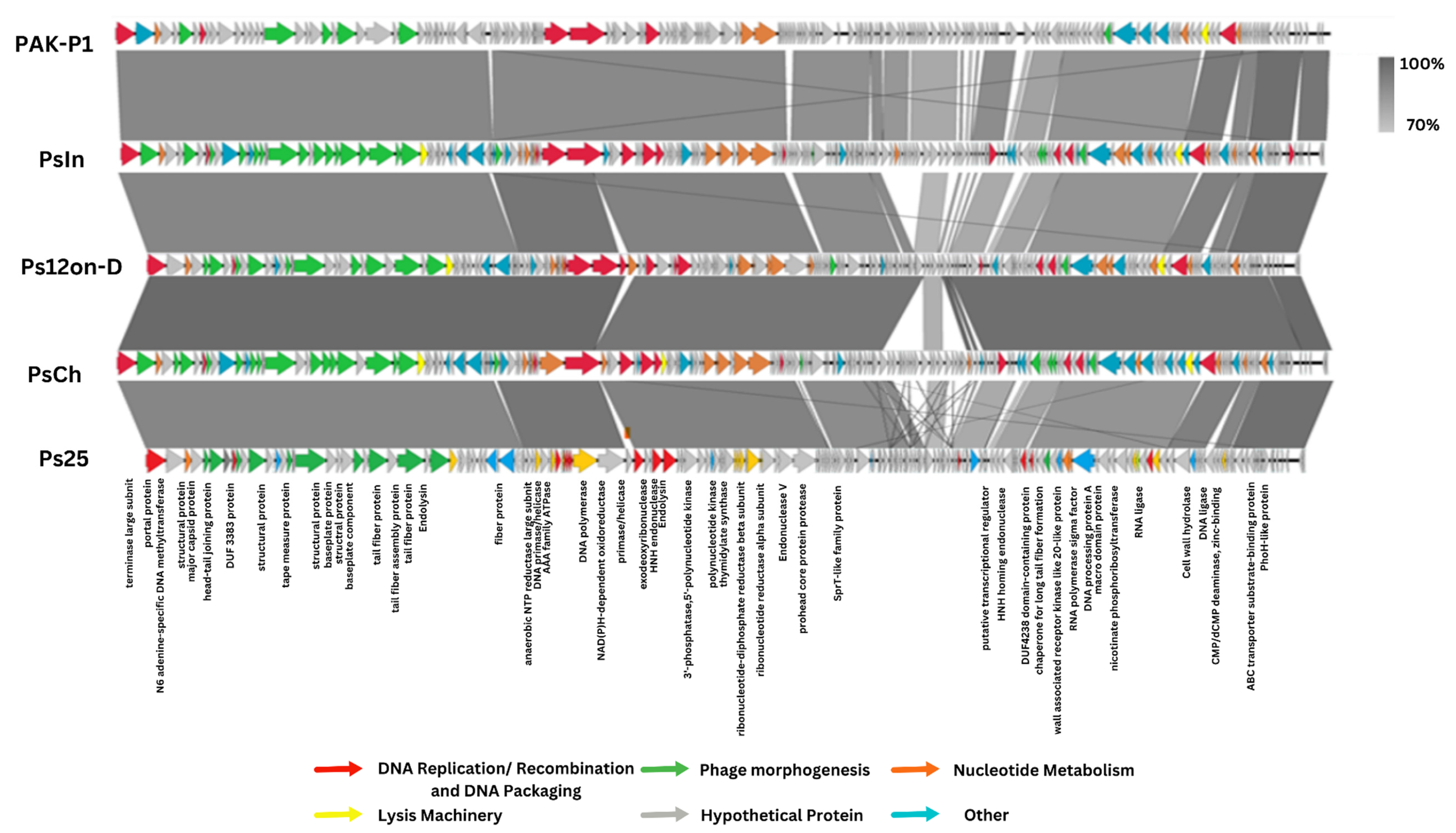
3.2. Genomic Identification of the PsIn, PsCh, Ps12on-D, and Ps25 Phages
3.3. Host Range Analysis
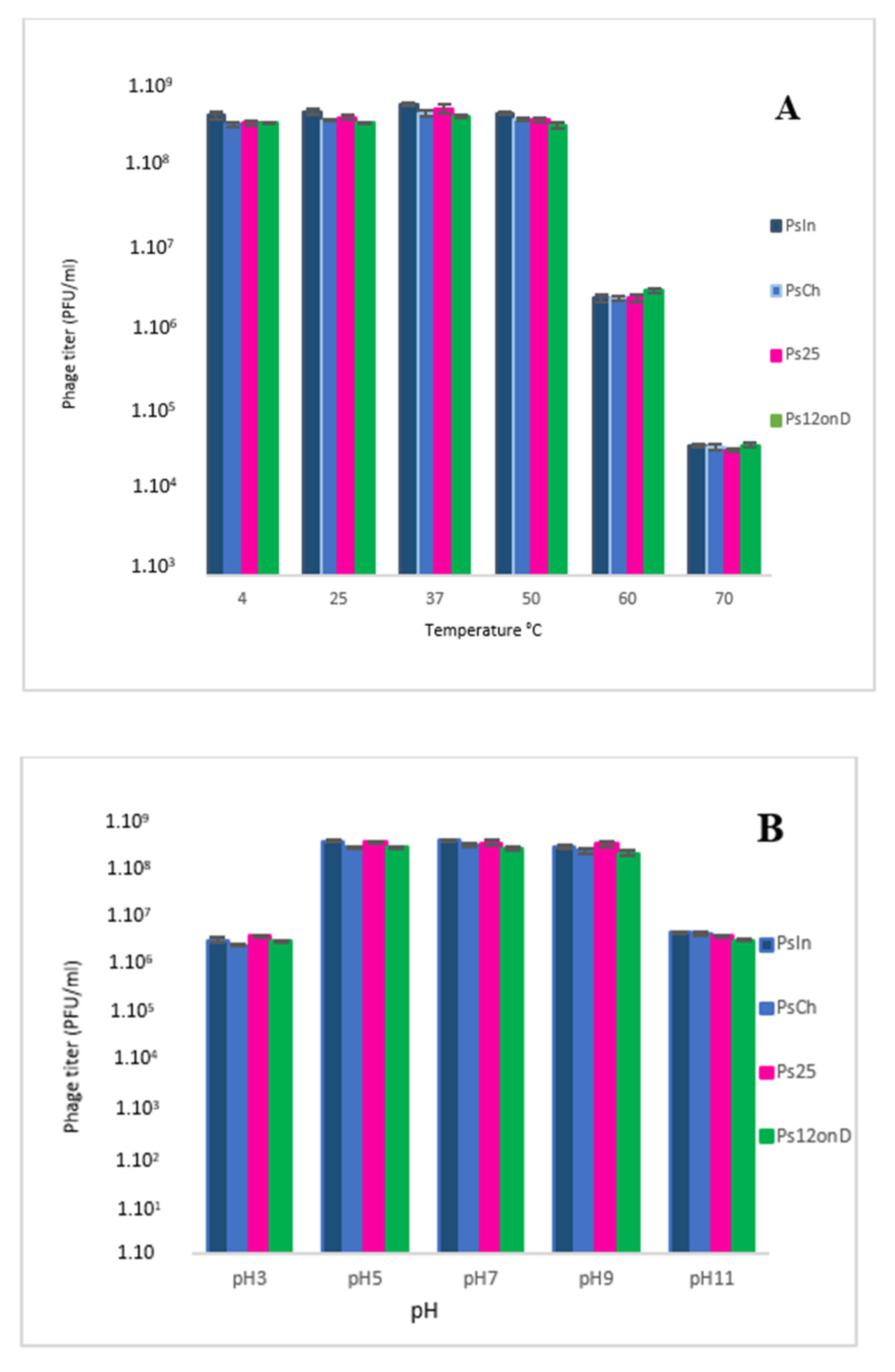
3.4. Thermal and pH Stability
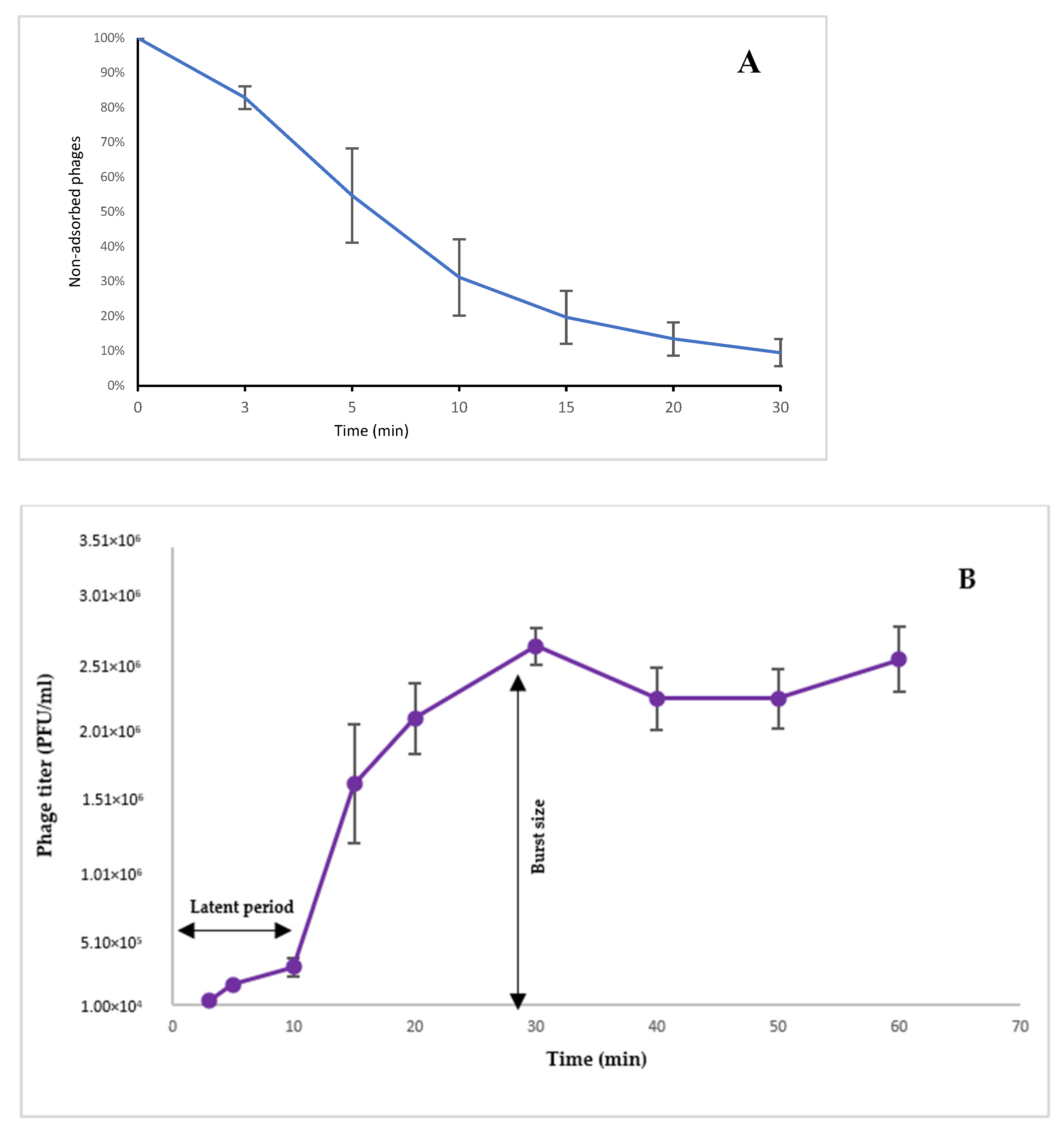
3.5. Adsorption Assay
3.6. One-Step Growth Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wise, R. Antimicrobial Resistance: Priorities for Action. J. Antimicrob. Chemother. 2002, 49, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Monnet, D.L. Consommation d’antibiotiques et Résistance Bactérienne. In Proceedings of the Annales Françaises D’anesthésie et de Réanimation; Elsevier: Amsterdam, The Netherlands, 2000; Volume 19, pp. 409–417. [Google Scholar]
- Moore, L.S.P.; Freeman, R.; Gilchrist, M.J.; Gharbi, M.; Brannigan, E.T.; Donaldson, H.; Livermore, D.M.; Holmes, A.H. Homogeneity of Antimicrobial Policy, yet Heterogeneity of Antimicrobial Resistance: Antimicrobial Non-Susceptibility among 108717 Clinical Isolates from Primary, Secondary and Tertiary Care Patients in London. J. Antimicrob. Chemother. 2014, 69, 3409–3422. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and Economic Impact of Antibiotic Resistance in Developing Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed]
- Lekunberri, I.; Villagrasa, M.; Balcázar, J.L.; Borrego, C.M. Contribution of Bacteriophage and Plasmid DNA to the Mobilization of Antibiotic Resistance Genes in a River Receiving Treated Wastewater Discharges. Sci. Total Environ. 2017, 601–602, 206–209. [Google Scholar] [CrossRef]
- Harnisz, M.; Kiedrzyńska, E.; Kiedrzyński, M.; Korzeniewska, E.; Czatzkowska, M.; Koniuszewska, I.; Jóźwik, A.; Szklarek, S.; Niestępski, S.; Zalewski, M. The Impact of WWTP Size and Sampling Season on the Prevalence of Antibiotic Resistance Genes in Wastewater and the River System. Sci. Total Environ. 2020, 741, 140466. [Google Scholar] [CrossRef]
- De Bentzmann, S.; Plesiat, P. Pseudomonas Aeruginosa: Une Virulence Complexe. Rev. Francoph. Des Lab. 2011, 2011, 73–81. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of Antibiotic Resistance Pseudomonas Aeruginosa in Intensive Care Unit; A Critical Review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Ben Haj Khalifa, A.; Moissenet, D.; Vu Thien, H.; Khedher, M. Virulence factors in Pseudomonas aeruginosa: Mechanisms and modes of regulation. Ann. Biol. Clin. 2011, 69, 393–403. [Google Scholar] [CrossRef]
- Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Hancock, R.E.W. Pseudomonas Aeruginosa: All Roads Lead to Resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Chairat, S.; Ben Yahia, H.; Rojo-Bezares, B.; Sáenz, Y.; Torres, C.; Ben Slama, K. High Prevalence of Imipenem-Resistant and Metallo-Beta-Lactamase-Producing Pseudomonas Aeruginosa in the Burns Hospital in Tunisia: Detection of a Novel Class 1 Integron. J. Chemother. 2019, 31, 120–126. [Google Scholar] [CrossRef]
- Hedfi, M.; Khouni, H.; Massoudi, Y.; Abdelhedi, C.; Sassi, K.; Chouchen, A. Epidemiology of Nosocomial Infections: About 70 Cases. Tunis Med. 2016, 94, 401–406. [Google Scholar] [PubMed]
- Krir, A.; Dhraief, S.; Messadi, A.A.; Thabet, L. Profil Bactériologique et Résistance Aux Antibiotiques Des Bactéries Isolées Dans Un Service de Réanimation Des Brûlés Durant Sept Ans. Ann Burn. Fire Disasters 2019, 32, 197–202. [Google Scholar]
- Lucena, A.; Dalla Costa, L.M.; Nogueira, K.S.; Matos, A.P.; Gales, A.C.; Paganini, M.C.; Castro, M.E.S.; Raboni, S.M. Nosocomial Infections with Metallo-Beta-Lactamase-Producing Pseudomonas Aeruginosa: Molecular Epidemiology, Risk Factors, Clinical Features and Outcomes. J. Hosp. Infect. 2014, 87, 234–240. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Silva, K.d.C.F.; Calomino, M.A.; Deutsch, G.; de Castilho, S.R.; de Paula, G.R.; Esper, L.M.R.; Teixeira, L.A. Molecular Characterization of Multidrug-Resistant (MDR) Pseudomonas Aeruginosa Isolated in a Burn Center. Burns 2017, 43, 137–143. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and Efficacy of Phage Therapy in Difficult-to-Treat Infections: A Systematic Review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Kellenberger, G.; Kellenberger, E. Electron Microscopical Studies of Phage Multiplication: III. Observation of Single Cell Bursts. Virology 1957, 3, 275–285. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Bim, F.L.; Monteiro, R.M.; Macedo, A.P.; Santos, E.S.; Silva-Lovato, C.H.; Paranhos, H.F.O.; Melo, L.D.R.; Santos, S.B.; Watanabe, E. Identification and Characterization of New Bacteriophages to Control Multidrug-Resistant Pseudomonas Aeruginosa Biofilm on Endotracheal Tubes. Front. Microbiol. 2020, 11, 580779. [Google Scholar] [CrossRef]
- Sharma, S.; Datta, S.; Chatterjee, S.; Dutta, M.; Samanta, J.; Vairale, M.G.; Gupta, R.; Veer, V.; Dwivedi, S.K. Isolation and Characterization of a Lytic Bacteriophage against Pseudomonas Aeruginosa. Sci. Rep. 2021, 11, 19393. [Google Scholar] [CrossRef]
- Myelnikov, D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922–1955. J. Hist Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage Therapy of Venous Leg Ulcers in Humans: Results of a Phase I Safety Trial. J. Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Parys, L.V.; et al. Quality-Controlled Small-Scale Production of a Well-Defined Bacteriophage Cocktail for Use in Human Clinical Trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef] [PubMed]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and Tolerability of a Cocktail of Bacteriophages to Treat Burn Wounds Infected by Pseudomonas Aeruginosa (PhagoBurn): A Randomised, Controlled, Double-Blind Phase 1/2 Trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Änggård, E.E.; Harper, D.R. A Controlled Clinical Trial of a Therapeutic Bacteriophage Preparation in Chronic Otitis Due to Antibiotic-Resistant Pseudomonas Aeruginosa; A Preliminary Report of Efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Gabard, J.; Jault, P. How to Achieve a Good Phage Therapy Clinical Trial? In Phage Therapy: A Practical Approach; Górski, A., Międzybrodzki, R., Borysowski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 147–168. [Google Scholar]
- Zaldastanishvili, E.; Leshkasheli, L.; Dadiani, M.; Nadareishvili, L.; Askilashvili, L.; Kvatadze, N.; Goderdzishvili, M.; Kutateladze, M.; Balarjishvili, N. Phage Therapy Experience at the Eliava Phage Therapy Center: Three Cases of Bacterial Persistence. Viruses 2021, 13, 1901. [Google Scholar] [CrossRef]
- Antoine, C.; Laforêt, F.; Blasdel, B.; Glonti, T.; Kutter, E.; Pirnay, J.P.; Mainil, J.; Delcenserie, V.; Thiry, D. Efficacy Assessment of PEV2 Phage on Galleria Mellonella Larvae Infected with a Pseudomonas Aeruginosa Dog Otitis Isolate. Res. Vet. Sci. 2021, 136, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cheng, X.; Li, J.; Yuan, X.; Huang, X.; Lian, M.; Li, W.; Huang, T.; Xie, Y.; Liu, J.; et al. Novel Lytic Phages Protect Cells and Mice against Pseudomonas Aeruginosa Infection. J. Virol. 2021, 95, e01832-20. [Google Scholar] [CrossRef]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Bilocq, F.; Pot, B.; Cornelis, P.; Zizi, M.; Eldere, J.V.; Deschaght, P.; Vaneechoutte, M.; Jennes, S.; Pitt, T.; et al. Pseudomonas Aeruginosa Population Structure Revisited. PLoS ONE 2009, 4, e7740. [Google Scholar] [CrossRef]
- Adams, M.H. Methods of study of bacterial viruses. Bacteriophages 1959, 592, 450–451. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive Visualization of de Novo Genome Assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the All-Bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC—A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Cui, X.; You, J.; Sun, L.; Yang, X.; Zhang, T.; Huang, K.; Pan, X.; Zhang, F.; He, Y.; Yang, H. Characterization of Pseudomonas Aeruginosa Phage C11 and Identification of Host Genes Required for Virion Maturation. Sci. Rep. 2016, 6, 39130. [Google Scholar] [CrossRef]
- Kleinheinz, K.A.; Joensen, K.G.; Larsen, M.V. Applying the ResFinder and VirulenceFinder Web-Services for Easy Identification of Acquired Antibiotic Resistance and E. Coli Virulence Genes in Bacteriophage and Prophage Nucleotide Sequences. Bacteriophage 2014, 4, e27943. [Google Scholar] [CrossRef]
- Cosentino, S.; Voldby Larsen, M.; Møller Aarestrup, F.; Lund, O. PathogenFinder—Distinguishing Friend from Foe Using Bacterial Whole Genome Sequence Data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Khan Mirzaei, M.; Nilsson, A.S. Isolation of Phages for Phage Therapy: A Comparison of Spot Tests and Efficiency of Plating Analyses for Determination of Host Range and Efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef]
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; Van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P.; et al. Characterization of Novel Bacteriophages for Biocontrol of Bacterial Blight in Leek Caused by Pseudomonas Syringae Pv. Porri. Front. Microbiol. 2016, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Vandenheuvel, D.; Kropinski, A.M.; Mast, J.; De Vos, D.; Verbeken, G.; Noben, J.-P.; Lavigne, R.; Vaneechoutte, M.; Pirnay, J.-P. Characterization of Newly Isolated Lytic Bacteriophages Active against Acinetobacter Baumannii. PLoS ONE 2014, 9, e104853. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, W.; Zhong, Q.; Chen, Q.; He, X.; Baker, J.L.; Xiong, K.; Jin, X.; Wang, J.; Hu, F.; et al. Development of a Bacteriophage Cocktail to Constrain the Emergence of Phage-Resistant Pseudomonas Aeruginosa. Front. Microbiol. 2020, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Bobay, L.-M.; Chevallereau, A.; Saussereau, E.; Ceyssens, P.-J.; Debarbieux, L. The Search for Therapeutic Bacteriophages Uncovers One New Subfamily and Two New Genera of Pseudomonas-Infecting Myoviridae. PLoS ONE 2015, 10, e0117163. [Google Scholar] [CrossRef]
- Shen, M.; Le, S.; Jin, X.; Li, G.; Tan, Y.; Li, M.; Zhao, X.; Shen, W.; Yang, Y.; Wang, J.; et al. Characterization and Comparative Genomic Analyses of Pseudomonas Aeruginosa Phage PaoP5: New Members Assigned to PAK_P1-like Viruses. Sci. Rep. 2016, 6, 34067. [Google Scholar] [CrossRef]
- Debarbieux, L.; Leduc, D.; Maura, D.; Morello, E.; Criscuolo, A.; Grossi, O.; Balloy, V.; Touqui, L. Bacteriophages Can Treat and Prevent Pseudomonas Aeruginosa Lung Infections. J. Infect. Dis. 2010, 201, 1096–1104. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, S.; Shen, W.; Zhao, X.; Shen, M.; Tan, Y.; Li, G.; Li, M.; Wang, J.; Hu, F.; et al. Characterization of the First Double-Stranded RNA Bacteriophage Infecting Pseudomonas Aeruginosa. Sci. Rep. 2016, 6, 38795. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P.; Rodríguez, A. The Perfect Bacteriophage for Therapeutic Applications—A Quick Guide. Antibiotics 2019, 8, 126. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Liu, J.-Q.; Zhao, C.-Y.; Yu, S.; Sun, Y.-B.; Shi, H.-Y.; Huang, H.-L. Isolation and Genome Sequencing of a Novel Pseudomonas Aeruginosa Phage PA-YS35. Curr. Microbiol. 2020, 77, 123–128. [Google Scholar] [CrossRef]
- Kwiatek, M.; Parasion, S.; Rutyna, P.; Mizak, L.; Gryko, R.; Niemcewicz, M.; Olender, A.; Łobocka, M. Isolation of Bacteriophages and Their Application to Control Pseudomonas Aeruginosa in Planktonic and Biofilm Models. Res. Microbiol. 2017, 168, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Bechet, M.; Vergassola, M.; Rocha, E. Causes for the Intriguing Presence of TRNAs in Phages. Genome Res. 2007, 17, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Le, S.; Tan, Y.; Zhu, J.; Li, M.; Rao, X.; Zou, L.; Li, S.; Wang, J.; Jin, X.; et al. Genomic and Proteomic Analyses of the Terminally Redundant Genome of the Pseudomonas Aeruginosa Phage PaP1: Establishment of Genus PaP1-like Phages. PLoS ONE 2013, 8, e62933. [Google Scholar] [CrossRef]
- Henry, M.; Lavigne, R.; Debarbieux, L. Predicting In Vivo Efficacy of Therapeutic Bacteriophages Used To Treat Pulmonary Infections. Antimicrob. Agents Chemother. 2013, 57, 5961–5968. [Google Scholar] [CrossRef] [PubMed]
- Garbe, J.; Bunk, B.; Rohde, M.; Schobert, M. Sequencing and Characterization of Pseudomonas Aeruginosa Phage JG004. BMC Microbiol. 2011, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Saussereau, E.; Maura, D.; Huerre, M.; Touqui, L.; Debarbieux, L. Pulmonary Bacteriophage Therapy on Pseudomonas Aeruginosa Cystic Fibrosis Strains: First Steps Towards Treatment and Prevention. PLoS ONE 2011, 6, e16963. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Lysis from Without. Bacteriophage 2011, 1, 46–49. [Google Scholar] [CrossRef]
- Essoh, C.; Latino, L.; Midoux, C.; Blouin, Y.; Loukou, G.; Nguetta, S.-P.A.; Lathro, S.; Cablanmian, A.; Kouassi, A.K.; Vergnaud, G.; et al. Investigation of a Large Collection of Pseudomonas Aeruginosa Bacteriophages Collected from a Single Environmental Source in Abidjan, Côte d’Ivoire. PLoS ONE 2015, 10, e0130548. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Vogele, K.; Malik, D.J. Production of Phage Therapeutics and Formulations: Innovative Approaches. In Phage Therapy: A Practical Approach; Górski, A., Międzybrodzki, R., Borysowski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–41. [Google Scholar]
- Capra, M.L.; Quiberoni, A.; Reinheimer, J. Phages of Lactobacillus Casei/Paracasei: Response to Environmental Factors and Interaction with Collection and Commercial Strains. J. Appl. Microbiol. 2006, 100, 334–342. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Ceyssens, P.-J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef]

| Feature | Genome PsIn | Genome PsCh | Genome Ps12onD | Genome Ps25 |
|---|---|---|---|---|
| Genome size | 92,515 bp | 92,710 bp | 88,705 bp | 87,887 bp |
| G+C content (G+C content host) | 49.28 % (66.17%) | 49.30 % (66.17%) | 49.14 % (66.17%) | 49.13 % (66.17%) |
| No. of predicted CDSs | 174 | 167 | 166 | 164 |
| Predicted tRNAs | tRNAGln; tRNATrp; tRNAArg; tRNALeu; tRNAIIe; tRNAAsp; tRNACys; tRNAAsn; tRNAPro; tRNAGly; tRNAPhe; tRNAGlu | tRNAGln; tRNATrp; tRNAArg; tRNALys; tRNALeu; tRNAIIe; tRNAAsp; tRNACys; tRNAAsn; tRNAPro; tRNAGly; tRNAPhe; tRNAGlu | tRNAGln; tRNATrp; tRNAArg; tRNALeu; tRNAIIe; tRNAAsp; tRNACys; tRNAAsn; tRNAPro; tRNAGly; tRNAPhe; tRNAGlu | tRNAGln; tRNATrp; tRNAArg; tRNALeu; tRNAIIe; tRNAAsp; tRNACys; tRNAAsn; tRNAPro; tRNAGly; tRNAPhe; tRNAGlu |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akremi, I.; Merabishvili, M.; Jlidi, M.; Haj Brahim, A.; Ben Ali, M.; Karoui, A.; Lavigne, R.; Wagemans, J.; Pirnay, J.-P.; Ben Ali, M. Isolation and Characterization of Lytic Pseudomonas aeruginosa Bacteriophages Isolated from Sewage Samples from Tunisia. Viruses 2022, 14, 2339. https://doi.org/10.3390/v14112339
Akremi I, Merabishvili M, Jlidi M, Haj Brahim A, Ben Ali M, Karoui A, Lavigne R, Wagemans J, Pirnay J-P, Ben Ali M. Isolation and Characterization of Lytic Pseudomonas aeruginosa Bacteriophages Isolated from Sewage Samples from Tunisia. Viruses. 2022; 14(11):2339. https://doi.org/10.3390/v14112339
Chicago/Turabian StyleAkremi, Ismahen, Maya Merabishvili, Mouna Jlidi, Adel Haj Brahim, Manel Ben Ali, Anis Karoui, Rob Lavigne, Jeroen Wagemans, Jean-Paul Pirnay, and Mamdouh Ben Ali. 2022. "Isolation and Characterization of Lytic Pseudomonas aeruginosa Bacteriophages Isolated from Sewage Samples from Tunisia" Viruses 14, no. 11: 2339. https://doi.org/10.3390/v14112339
APA StyleAkremi, I., Merabishvili, M., Jlidi, M., Haj Brahim, A., Ben Ali, M., Karoui, A., Lavigne, R., Wagemans, J., Pirnay, J.-P., & Ben Ali, M. (2022). Isolation and Characterization of Lytic Pseudomonas aeruginosa Bacteriophages Isolated from Sewage Samples from Tunisia. Viruses, 14(11), 2339. https://doi.org/10.3390/v14112339









