Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Product (IP) VG161
2.2. Animal Models and Administration of mVG161
2.3. Patient-Derived Xenograft (PDX) Humanized Mouse Models
2.4. ELISPOT Assay to Verify Specific Anticancer Immunity
2.5. Immune Profiling of the Tumor Microenvironment
2.6. Data Management and Statistical Analysis
3. Results
3.1. Anti-HSV-1 Antibodies Were Successfully Induced after Subcutaneous Inoculation of VG161
3.2. Enhanced Anticancer Efficacy in Anti-HSV-1 Antibody-Positive Animals
3.3. Enhanced Anticancer Efficacy in Humanized PDX Mouse Model Treated with VG161
3.4. Systemic Specific Anticancer Immunity in Anti-HSV-1 Antibody-Positive Mice
3.5. Tumor Microenvironment Changes Due to Immunization; NanoString nCounter Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, C.; Liu, H.; Robins, E.; Song, W.; Liu, D.; Li, Z.; Zheng, L. Next-generation immuno-oncology agents: Current momentum shifts in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Russell, S.J. History of Oncolytic Viruses: Genesis to Genetic Engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.E.; Speranza, M.-C.; Cho, C.-F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Coffin, R.S.; Davis, C.J.; Graham, N.J.; Groves, N.; Guest, P.J.; Harrington, K.J.; James, N.D.; Love, C.A.; McNeish, I.; et al. A Phase I Study of OncoVEXGM-CSF, a Second-Generation Oncolytic Herpes Simplex Virus Expressing Granulocyte Macrophage Colony-Stimulating Factor. Clin. Cancer Res. 2006, 12, 6737–6747. [Google Scholar] [CrossRef]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 2016, 4, 53. [Google Scholar] [CrossRef]
- Otani, Y.; Yoo, J.Y.; Shimizu, T.; Kurozumi, K.; Date, I.; Kaur, B. Implications of immune cells in oncolytic herpes simplex virotherapy for glioma. Brain Tumor Pathol. 2022, 39, 57–64. [Google Scholar] [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.-Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Chesney, J.; Awasthi, S.; Curti, B.; Hutchins, L.; Linette, G.; Triozzi, P.; Tan, M.C.B.; Brown, R.E.; Nemunaitis, J.; Whitman, E.; et al. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB–IVM1c melanoma. Melanoma Res. 2018, 28, 44–51. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Öhrling, K.; Kaufman, H.L. Final analyses of OPTiM: A randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef]
- Chouljenko, D.; Ding, J.; Lee, I.-F.; Murad, Y.; Bu, X.; Liu, G.; Delwar, Z.; Sun, Y.; Yu, S.; Samudio, I.; et al. Induction of Durable Antitumor Response by a Novel Oncolytic Herpesvirus Expressing Multiple Immunomodulatory Transgenes. Biomedicines 2020, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Cosman, R.; Aggarwal, N.; O’Grady, A.; Hsu, E.; Ardolino, L.; Xia, H.; Chi, Y.; Qui, S.; Murad, Y.; et al. Initial results from a first in human trial incorporating accelerated dose titration of a novel immune stimulating oncolytic virus—VG161. J. Clin. Oncol. 2021, 39, e14574. [Google Scholar] [CrossRef]
- Wald, A.; Corey, L. HSV: Persistence in the population: Epidemiology, Transmission; Cambridge University Press: Cambridge, UK, 2007; pp. 656–672. [Google Scholar] [CrossRef]
- Smith, J.S.; Robinson, N.J. Age-Specific Prevalence of Infection with Herpes Simplex Virus Types 2 and 1: A Global Review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef]
- Rosario, M.; Liu, B.; Kong, L.; Collins, L.I.; Schneider, S.E.; Chen, X.; Han, K.; Jeng, E.K.; Rhode, P.R.; Leong, J.W.; et al. The IL-15-Based ALT-803 Complex Enhances FcγRIIIa-Triggered NK Cell Responses and In Vivo Clearance of B Cell Lymphomas. Clin. Cancer Res. 2016, 22, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kong, L.; Han, K.; Hong, H.; Marcus, W.D.; Chen, X.; Jeng, E.K.; Alter, S.; Zhu, X.; Rubinstein, M.P.; et al. A Novel Fusion of ALT-803 (Interleukin (IL)-15 Superagonist) with an Antibody Demonstrates Antigen-specific Antitumor Responses. J. Biol. Chem. 2016, 291, 23869. [Google Scholar] [CrossRef]
- Kim, P.S.; Kwilas, A.R.; Xu, W.; Alter, S.; Jeng, E.K.; Wong, H.C.; Schlom, J.; Hodge, J.W. IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016, 7, 16130–16145. [Google Scholar] [CrossRef]
- Gomes-Giacoia, E.; Miyake, M.; Goodison, S.; Sriharan, A.; Zhang, G.; You, L.; Egan, J.O.; Rhode, P.R.; Parker, A.S.; Chai, K.X.; et al. Intravesical ALT-803 and BCG Treatment Reduces Tumor Burden in a Carcinogen Induced Bladder Cancer Rat Model; a Role for Cytokine Production and NK Cell Expansion. PLoS ONE 2014, 9, e96705. [Google Scholar] [CrossRef]
- Clinical Study of VG161 in Subjects With Advanced Primary Liver Cancer—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04806464 (accessed on 30 September 2022).
- Russell, S.J. For the Success of Oncolytic Viruses: Single Cycle Cures or Repeat Treatments? (One Cycle Should Be Enough). Mol. Ther. 2018, 26, 1876–1880. [Google Scholar] [CrossRef]
- Chahlavi, A.; Rabkin, S.D.; Todo, T.; Sundaresan, P.; Martuza, R.L. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther. 1999, 6, 1751–1758. [Google Scholar] [CrossRef]
- Johnson, D.C.; Huber, M.T. Directed Egress of Animal Viruses Promotes Cell-to-Cell Spread. J. Virol. 2002, 76, 1–8. [Google Scholar] [CrossRef]
- Johnson, D.C.; Baines, J.D. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Genet. 2011, 9, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2020; Volume 108, pp. 85–125. [Google Scholar] [CrossRef]
- Melcher, A. Oncolytic Virotherapy: Single Cycle Cures or Repeat Treatments? (Repeat Dosing Is Crucial!). Mol. Ther. 2018, 26, 1875–1876. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.K.; Kmieciak, M.; Knutson, K.L.; Bear, H.D.; Manjili, M.H. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res. Treat. 2010, 123, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sprague, L.; Braidwood, L.; Conner, J.; Cassady, K.A.; Benencia, F.; Cripe, T.P. Please stand by: How oncolytic viruses impact bystander cells. Futur. Virol. 2018, 13, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Tough, D.F.; Borrow, P.; Sprent, J. Induction of Bystander T Cell Proliferation by Viruses and Type I Interferon in Vivo. Science 1996, 272, 1947–1950. [Google Scholar] [CrossRef]
- Tietze, J.K.; Wilkins, D.E.C.; Sckisel, G.D.; Bouchlaka, M.N.; Alderson, K.L.; Weiss, J.M.; Ames, E.; Bruhn, K.W.; Craft, N.; Wiltrout, R.H.; et al. Delineation of antigen-specific and antigen-nonspecific CD8+ memory T-cell responses after cytokine-based cancer immunotherapy. Blood 2012, 119, 3073–3083. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Shin, D.H.; Nguyen, T.; Ozpolat, B.; Lang, F.; Alonso, M.; Gomez-Manzano, C.; Fueyo, J. Current strategies to circumvent the antiviral immunity to optimize cancer virotherapy. J. Immunother. Cancer 2021, 9, 2086. [Google Scholar] [CrossRef]
- Tähtinen, S.; Feola, S.; Capasso, C.; Laustio, N.; Groeneveldt, C.; Ylösmäki, E.O.; Ylösmäki, L.; Martins, B.; Fusciello, M.; Medeot, M.; et al. Exploiting Preexisting Immunity to Enhance Oncolytic Cancer Immunotherapy. Cancer Res. 2020, 80, 2575–2585. [Google Scholar] [CrossRef]
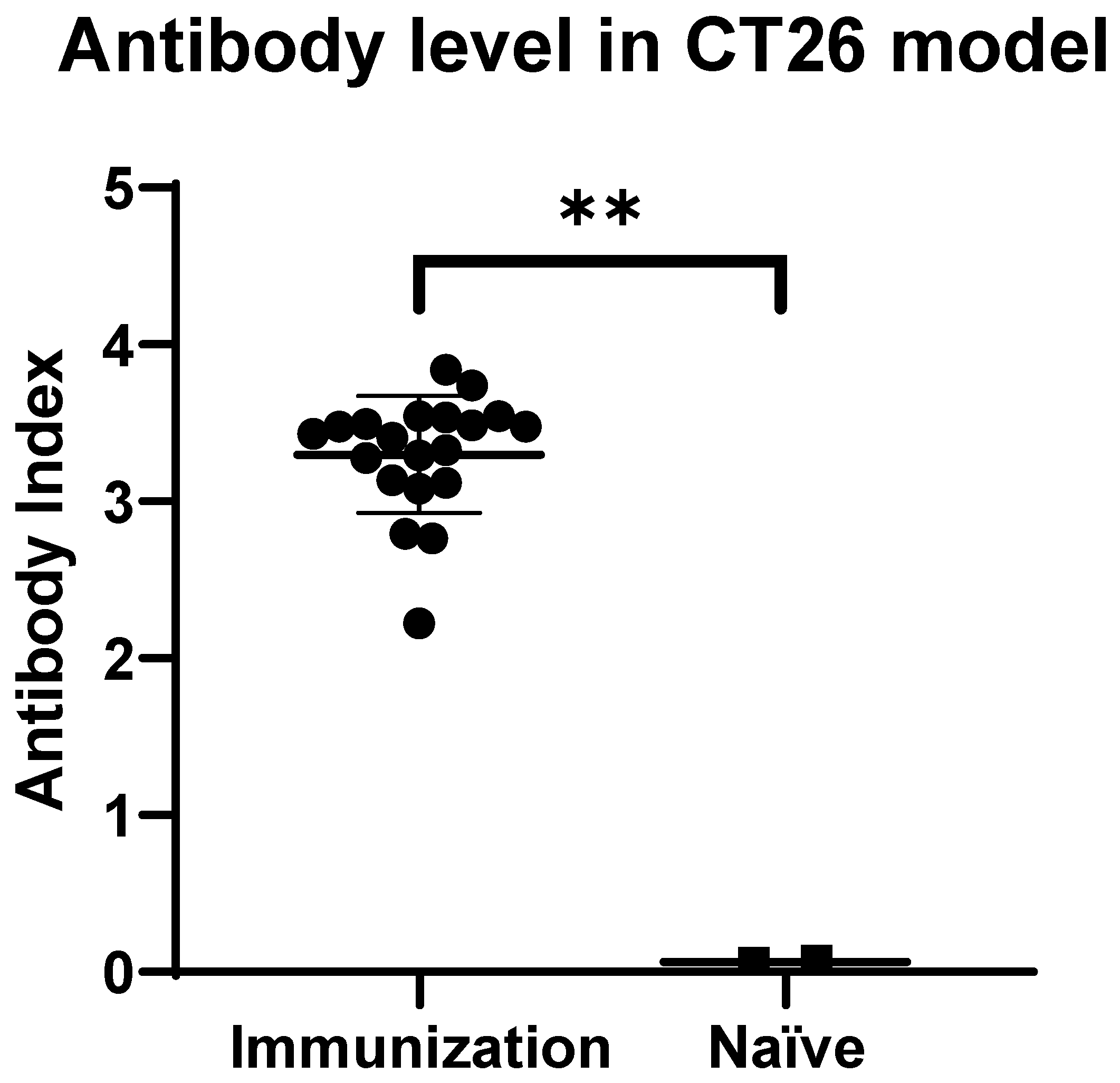




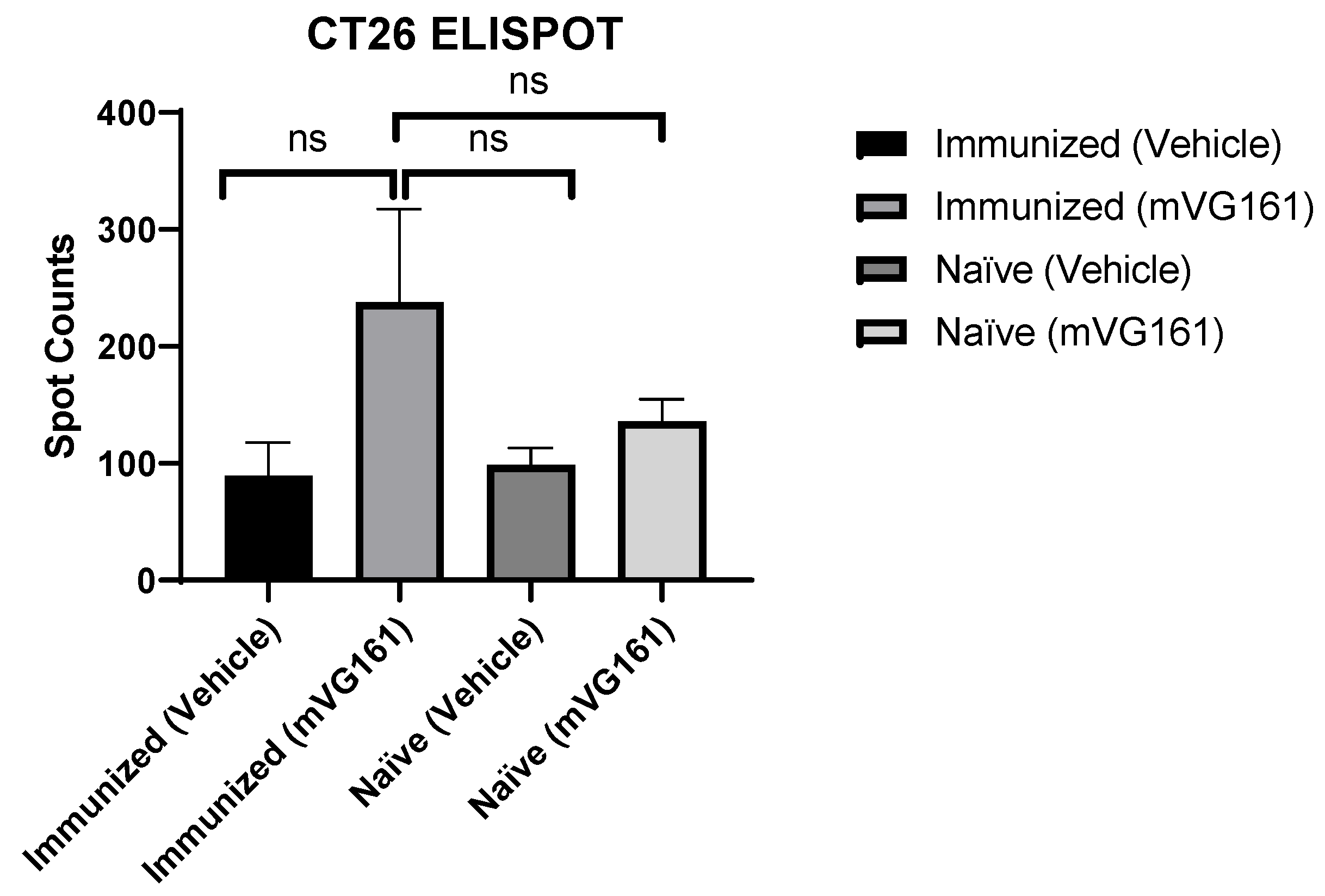
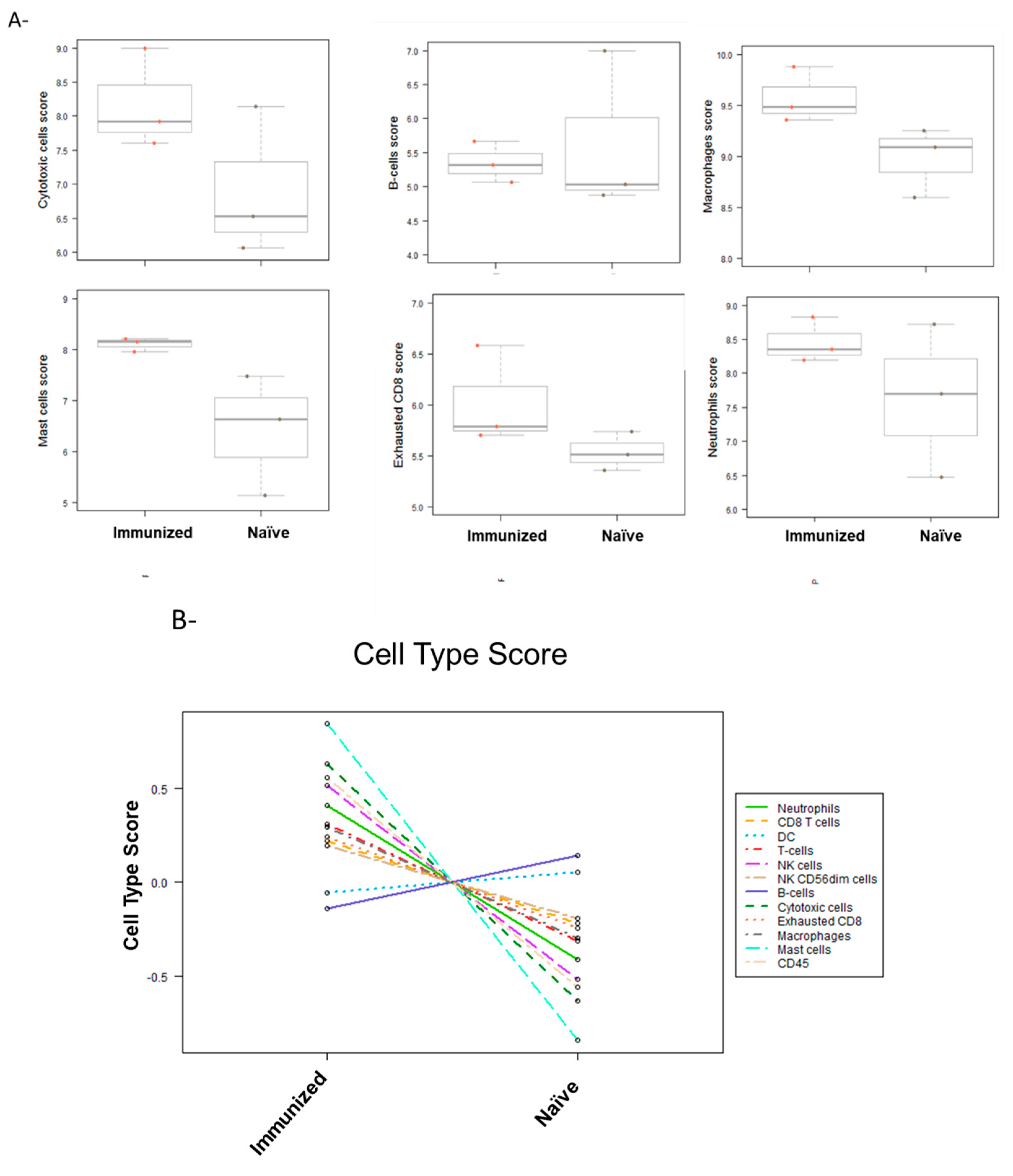
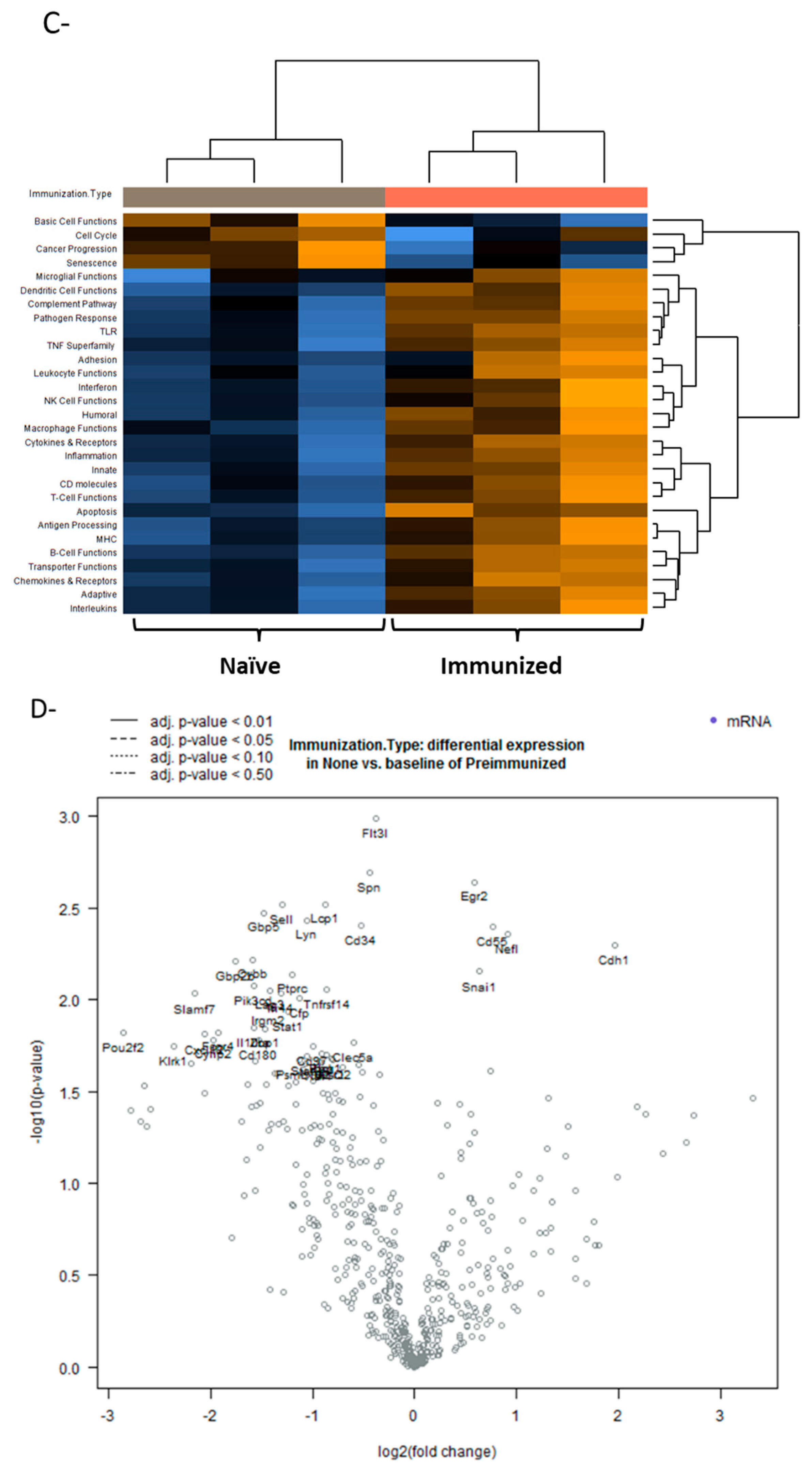
| Group | D1 | D3 | D5 | D7 | D10 | D12 | D14 | D17 | D19 |
|---|---|---|---|---|---|---|---|---|---|
| Immunized (mVG161) | 100.00% | 100.59% | 87.30% | 59.33% | 41.88% | 32.52% | 19.72% | 14.79% | 10.83% |
| Naïve (mVG161) | 100.00% | 94.53% | 83.44% | 89.42% | 69.04% | 55.02% | 33.41% | 22.89% | 20.01% |
| Day | D1 | D3 | D5 | D8 | D10 | D12 | D15 | D17 | D19 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean T/C% 1 | ||||||||||
| Injected Side | immunized (mVG161) | 100.0% | 72.1% | 52.2% | 37.0% | 27.9% | 25.2% | 22.9% | 28.7% | 28.3% |
| naïve (mVG161) | 100.0% | 98.8% | 73.2% | 50.8% | 39.1% | 32.5% | 32.1% | 48.2% | 58.3% | |
| Non-injected Side | immunized (mVG161) | 100.0% | 112.0% | 112.4% | 81.1% | 54.9% | 39.6% | 36.2% | 45.2% | 44.2% |
| naïve (mVG161) | 100.0% | 96.0% | 103.1% | 78.7% | 65.4% | 58.7% | 62.4% | 81.2% | 84.1% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Murad, Y.M.; Sun, Y.; Lee, I.-F.; Samudio, I.; Liu, X.; Jia, W.W.-G.; Zhao, R. Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus. Viruses 2022, 14, 2327. https://doi.org/10.3390/v14112327
Ding J, Murad YM, Sun Y, Lee I-F, Samudio I, Liu X, Jia WW-G, Zhao R. Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus. Viruses. 2022; 14(11):2327. https://doi.org/10.3390/v14112327
Chicago/Turabian StyleDing, Jun, Yanal M. Murad, Yi Sun, I-Fang Lee, Ismael Samudio, Xiaohu Liu, William Wei-Guo Jia, and Ronghua Zhao. 2022. "Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus" Viruses 14, no. 11: 2327. https://doi.org/10.3390/v14112327
APA StyleDing, J., Murad, Y. M., Sun, Y., Lee, I.-F., Samudio, I., Liu, X., Jia, W. W.-G., & Zhao, R. (2022). Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus. Viruses, 14(11), 2327. https://doi.org/10.3390/v14112327






