Immunogenicity, Safety, and Anti-Viral Efficacy of a Subunit SARS-CoV-2 Vaccine Candidate in Captive Black-Footed Ferrets (Mustela nigripes) and Their Susceptibility to Viral Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunization of Black-Footed Ferrets
2.2. Serology
2.3. In Vivo Passive Serum Transfer Studies
2.4. Histopathology of Mice from Serum Transfer Study
2.5. Challenge of Vaccinated and Unvaccinated BFF with SARS-CoV-2 Virus
2.6. Analyses
3. Results
3.1. Ferret Immunization
3.2. Serology
3.3. Passive Serum Transfer Studies in Mice
3.4. Viral Loads by Treatment and by ELISA Antibody Titers in Mice
3.5. Histologic Examination of Tissues from Mice
3.6. SARS-CoV-2 Challenge in Black-Footed Ferrets
3.7. Histopathology of Challenged Black-Footed Ferrets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Munnink, B.B.O.; Der Honing, R.W.H.-V.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef] [PubMed]
- APHIS One Health—SARS-CoV-2 in Animals. Available online: https://www.aphis.usda.gov/aphis/ourfocus/onehealth/one-health-sarscov2-in-animals (accessed on 17 May 2022).
- Oude Munnink, B.B.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2020, 371, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Mediannikov, O.; Maurin, M.; Devaux, C.; Colson, P.; Levasseur, A.; Fournier, P.E.; Raoult, D. Mink, SARS-CoV-2, and the Human-Animal Interface. Front. Microbiol. 2021, 12, 663815. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-K.; Ali, G.D.; Jia, F.; Li, Q.; Kelvin, D.; Couch, R.C.; Harrod, K.S.; Hutt, J.A.; Cameron, C.; Weiss, S.R.; et al. The SARS-CoV ferret model in an infection–challenge study. Virology 2008, 374, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Kim, S.-G.; Kim, S.-M.; Kim, E.-H.; Park, S.-J.; Yu, K.-M.; Chang, J.-H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Kok, A.; De Meulder, D.; Bestebroer, T.M.; Lamers, M.M.; Okba, N.M.A.; Van Vlissingen, M.F.; Rockx, B.; Haagmans, B.L.; Koopmans, M.; et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat. Commun. 2020, 11, 3496. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.L.; Diel, D.G.; Wang, L.; Zec, S.; Laverack, M.; Martins, M.; Caserta, L.C.; Killian, M.L.; Terio, K.; Olmstead, C.; et al. Sars-COV-2 Infection and Longitudinal Fecal Screening in Malayan Tigers (Panthera Tigris Jacksoni), Amur Tigers (Panthera Tigris Altaica), and African Lions (Panthera Leo Krugeri) at the Bronx Zoo, New York, USA. J. Zoo Wildl. Med. 2021, 51, 733–744. [Google Scholar] [CrossRef] [PubMed]
- USDA Confirmation of COVID-19 in Gorillas at a California Zoo. Available online: https://content.govdelivery.com/accounts/USDAAPHIS/bulletins/2b5837f/ (accessed on 15 May 2022).
- WOAH COVID-19: Events in Animals. Available online: https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/events-in-animals/ (accessed on 17 May 2022).
- Santymire, R.; Branvold-Faber, H.; Marinari, P.E. Recovery of the black-footed ferret. In Biology and Diseases of the Ferret, 3rd ed.; Fox, J.G., Marini, R.P., Eds.; John Wiley & Sons: New York, NY, USA, 2014; pp. 219–231. [Google Scholar]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.W.; Kien, F.; Roberts, A.; Cheung, Y.C.; Lamirande, E.W.; Vogel, L.; Chu, S.L.; Tse, J.; Guarner, J.; Zaki, S.R.; et al. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcγRII-dependent entry into B cells in vitro. Vaccine 2007, 25, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; Queen, K.; Tao, Y.; Paden, C.R.; Zhang, J.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States. Emerg. Infect. Dis. 2020, 26, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Park, J.-G.; Pino, P.A.; Gonzalez, O.; Akhter, A.; Allué-Guardia, A.; Olmo-Fontánez, A.; Gautam, S.; Garcia-Vilanova, A.; Ye, C.; et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat. Commun. 2020, 11, 6122. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Bosco-Lauth, A.M.; Root, J.J.; Porter, S.M.; Walker, A.E.; Guilbert, L.; Hawvermale, D.; Pepper, A.; Maison, R.M.; Hartwig, A.E.; Gordy, P.; et al. Peridomestic Mammal Susceptibility to Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Emerg. Infect. Dis. 2021, 27, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R Core Team. R A Lang. Environ. Stat. Comput. R Found. Stat. Comput. Vienna, Austria. 2021. Available online: https://www.r-project.org (accessed on 15 August 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Package ‘Lme4’ Linear Mixed-Effects Models Using “Eigen” and S4. 2017. Available online: https://github.com/lme4/lme4/ (accessed on 2 February 2022).
- Fox, J.; Weisberg, S. Package ‘Car’: An R Companion to Applied Regression. 2022. Available online: https://www.rdocumentation.org/packages/car/versions/3.1-0 (accessed on 2 February 2022).
- Rocke, T.E.; Tripp, D.; Lorenzsonn, F.; Falendysz, E.; Smith, S.; Williamson, J.; Abbott, R. Age at Vaccination May Influence Response to Sylvatic Plague Vaccine (SPV) in Gunnison’s Prairie Dogs (Cynomys gunnisoni). EcoHealth 2015, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wong, L.-Y.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Musiu, C.; Caligola, S.; Fiore, A.; Lamolinara, A.; Frusteri, C.; Del Pizzo, F.D.; De Sanctis, F.; Canè, S.; Adamo, A.; Hofer, F.; et al. Fatal cytokine release syndrome by an aberrant FLIP/STAT3 axis. Cell Death Differ. 2021, 29, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.-L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.-M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Najjar-Debbiny, R.; Hanna, A.; Jabbour, A.; Abu Ahmad, Y.; Saffuri, A.; Abu-Sinni, M.; Shkeiri, R.; Elemy, A.; Hakim, F. COVID-19 vaccine—Long term immune decline and breakthrough infections. Vaccine 2021, 39, 6984–6989. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Tiwari, R.; Saied, A.R.A.; Dhama, K. SARS-CoV-2 Vaccine for Domestic and Captive Animals: An Effort to Counter COVID-19 Pandemic at the Human-Animal Interface. Vaccine 2021, 39, 7119–7122. [Google Scholar] [CrossRef] [PubMed]

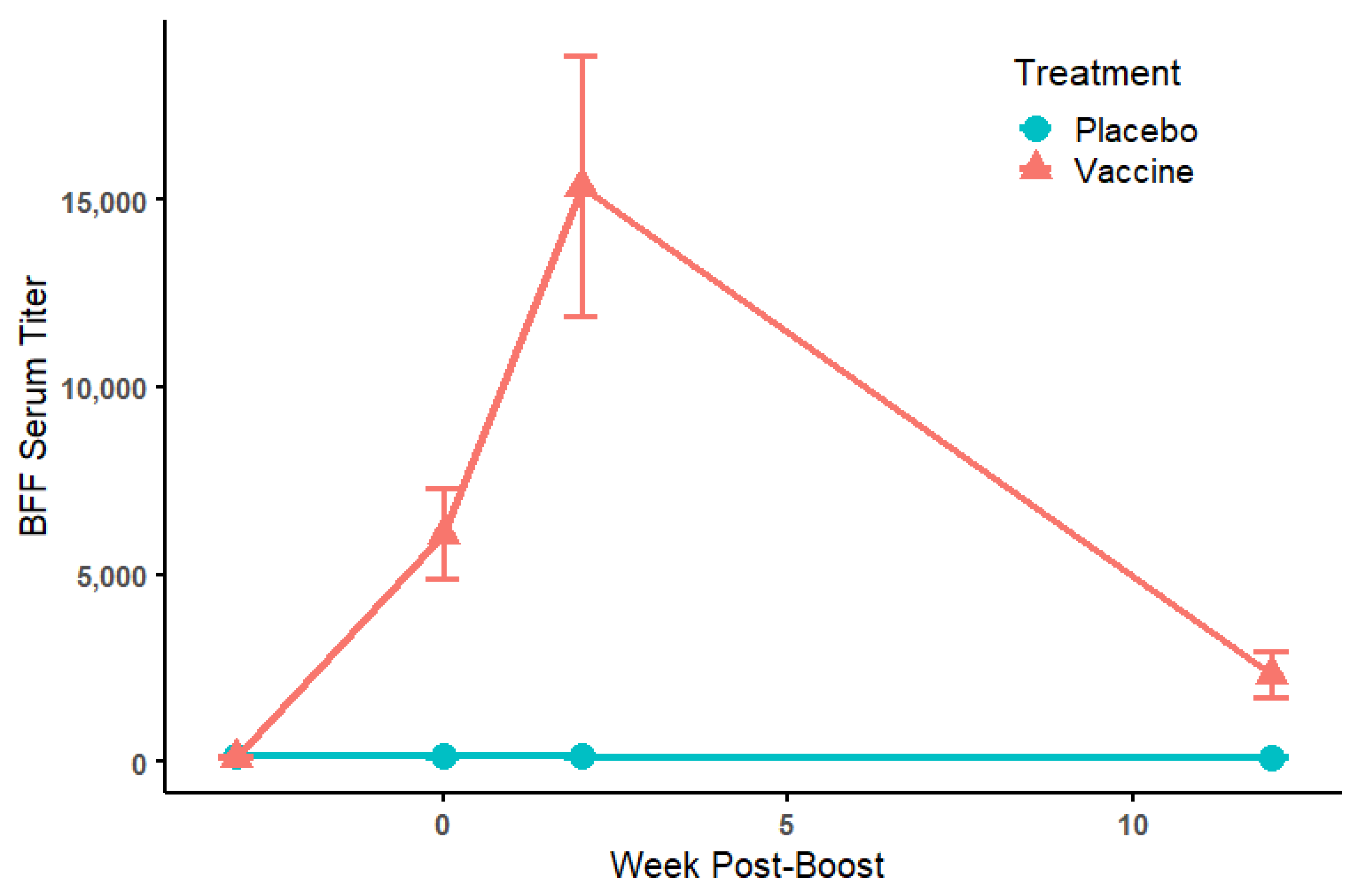
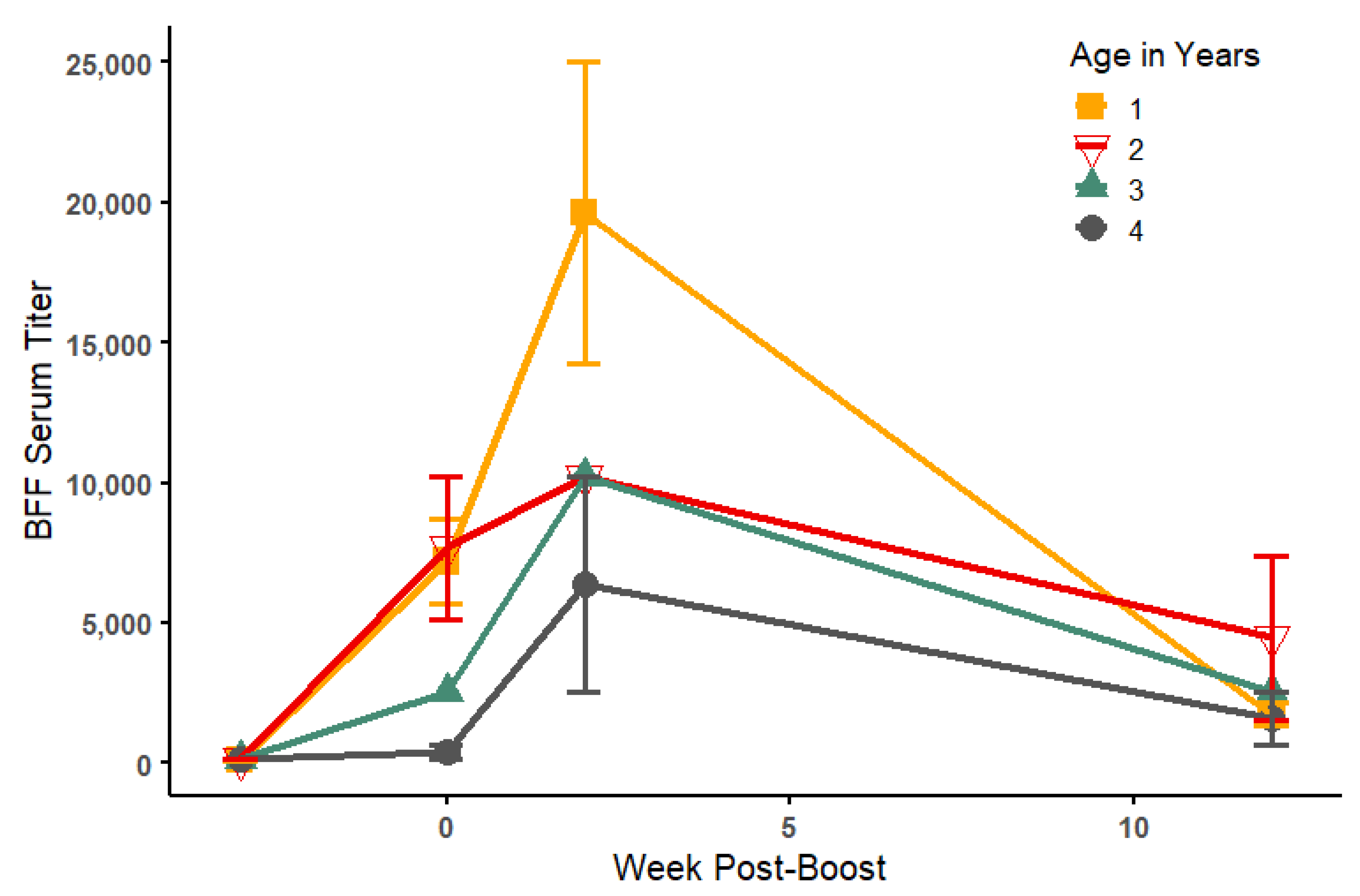
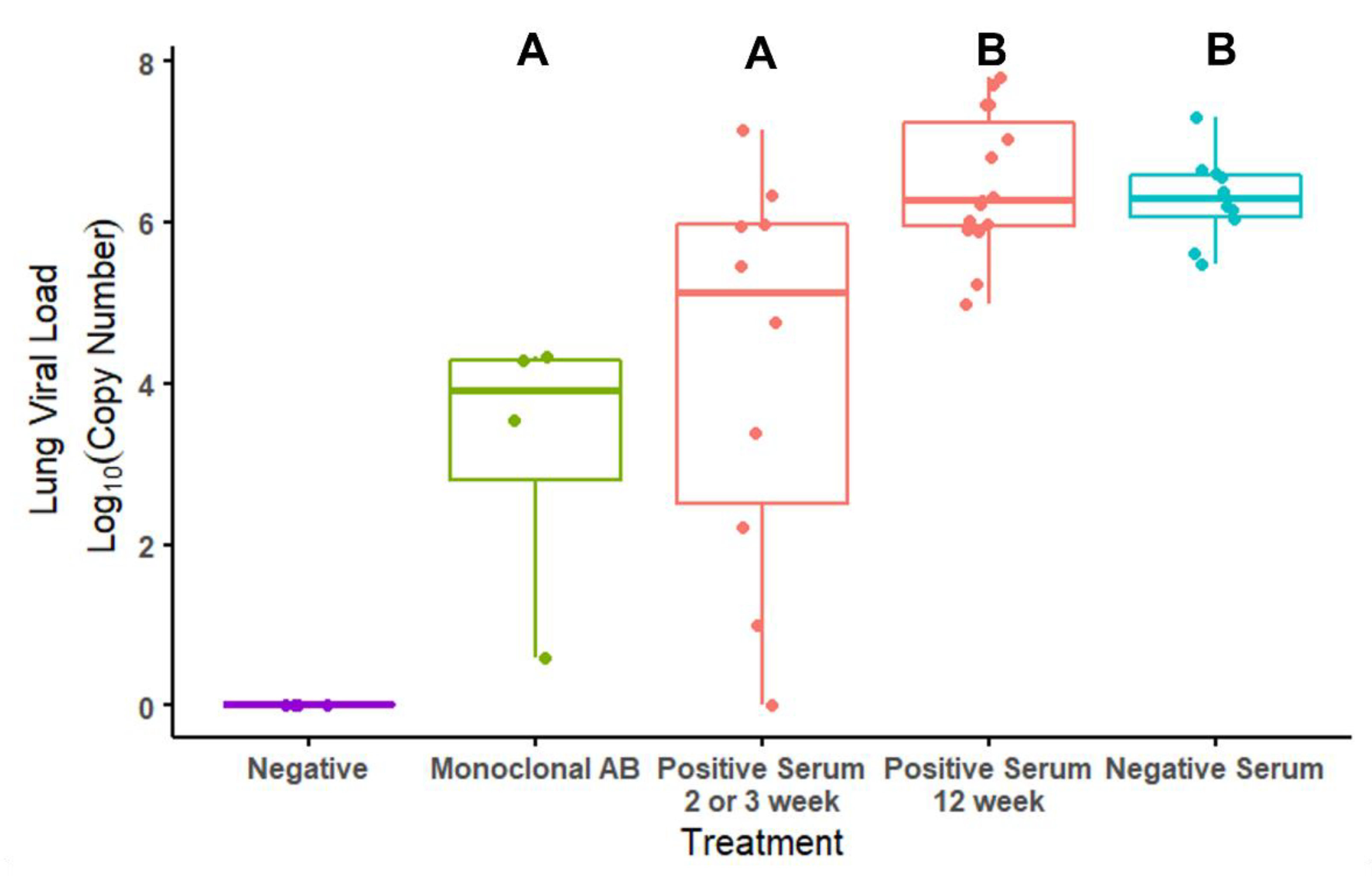
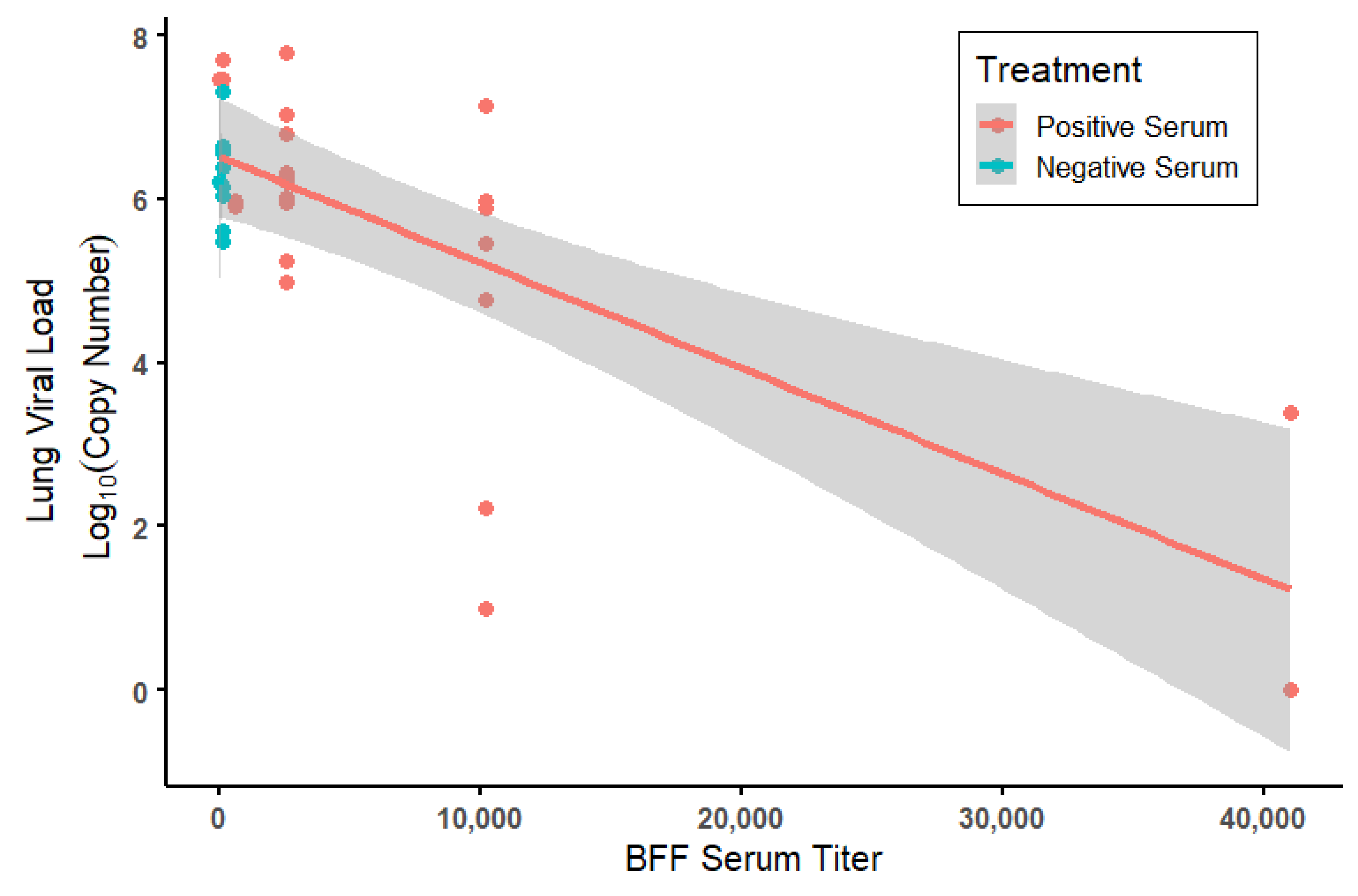
| ELISA Titers | PRNT50 Titers | ||||||
|---|---|---|---|---|---|---|---|
| Ferret | Age (Years) | Treatment | Pre-Vac | Pre-Boost | 2–3 Wk Post-Boost | 12 Wk Post-Boost | 2–3 Wk Post-Boost |
| 8593 | 4 | Vaccine | NA | 640 | 2560 | 640 | Neg |
| 8847 | 4 | Vaccine | 160 | 160 | 10,240 | 2560 | Neg |
| 8995 * | 3 | Vaccine | 160 | 2560 | 10,240 | 2560 | <32 |
| 9135 | 2 | Vaccine | 160 | 2560 | 10,240 | 640 | <16 |
| 9189 | 2 | Vaccine | 160 | 10,240 | 10,240 | 2560 | 256 |
| 9219 | 2 | Vaccine | 160 | 10,240 | 10,240 | 10,240 | <64 |
| 9337 | 1 | Vaccine | 40 | 10,240 | 10,240 | 2560 | <64 |
| 9371 | 1 | Vaccine | 40 | 10,240 | 10,240 | 2560 | 256 |
| 9422 | 1 | Vaccine | 160 | 10,240 | 40,960 | 2560 | 256 |
| 9469 | 1 | Vaccine | 160 | 10,240 | 10,240 | 2560 | <64 |
| 9476 | 1 | Vaccine | 160 | 640 | 10,240 | 2560 | Neg |
| 9568 | 1 | Vaccine | 40 | 2560 | 10,240 | 2560 | <64 |
| 9571 | 1 | Vaccine | 40 | 10,240 | 40,960 | 160 | <64 |
| 9576 | 1 | Vaccine | 40 | 10,240 | 40,960 | 160 | 256 |
| 9598 * | 1 | Vaccine | 160 | 160 | 2560 | 40 | Neg |
| 8710 * | 4 | Placebo | 160 | 160 | 160 | 160 | Neg |
| 8721 | 4 | Placebo | 160 | 160 | 160 | 160 | Neg |
| 8970 | 3 | Placebo | 160 | 160 | 160 | 160 | Neg |
| 9247 | 2 | Placebo | 160 | 160 | 160 | 160 | Neg |
| 9311 | 2 | Placebo | 160 | 160 | 160 | 160 | Neg |
| 9388 | 1 | Placebo | 40 | 40 | 40 | 40 | Neg |
| 9425 | 1 | Placebo | 160 | 160 | 160 | 160 | Neg |
| 9519 | 1 | Placebo | 40 | 40 | 40 | 40 | Neg |
| 9592 | 1 | Placebo | 160 | 160 | 160 | 40 | Neg |
| Ferret | Vaccinated | PFU/mL—Oropharyngeal Swab | PFU/mL—Nasal Flush Fluid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 1 | Day 3 | Day 5 | Day 7 | ||
| 8822 | No | <10 | <10 | NA | NA | 40 | 1600 | NA | NA |
| 8305 | Yes | <10 | 5400 | NA | NA | 630 | 29,000 | NA | NA |
| 8847 | Yes | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| 8226 | No | <10 | 20 | <10 | 120 | 1500 | 18,000 | 1200 | 270 |
| 9072 | No | <10 | 140 | 10 | 130 | 90 | 1900 | <10 | 60 |
| 8215 | No | <10 | 60 | <10 | 10 | 60 | 18,000 | 470 | 280 |
| Ferret | Vaccinated | Sex | Age | PRNT90 Titer | PFU/Gram—Day 3 Post-Challenge | |||
|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 14 | Turbinates | Cranial Lung | Caudal Lung | ||||
| 8822 | No | M | 5 | <10 | NA | 2.8 × 104 | <100 | <100 |
| 8305 | Yes | F | 7 | <10 | NA | 4.8 × 107 | <100 | 1.8 × 107 |
| 8847 | Yes | M | 5 | 320 | 10,240 | NA | NA | NA |
| 8226 | No | F | 7 | <10 | 40 | NA | NA | NA |
| 9072 | No | M | 4 | <10 | 10 | NA | NA | NA |
| 8215 | No | M | 7 | <10 | 40 | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leon, A.E.; Garelle, D.; Hartwig, A.; Falendysz, E.A.; Ip, H.S.; Lankton, J.S.; Tretten, T.N.; Spraker, T.R.; Bowen, R.; Rocke, T.E. Immunogenicity, Safety, and Anti-Viral Efficacy of a Subunit SARS-CoV-2 Vaccine Candidate in Captive Black-Footed Ferrets (Mustela nigripes) and Their Susceptibility to Viral Challenge. Viruses 2022, 14, 2188. https://doi.org/10.3390/v14102188
Leon AE, Garelle D, Hartwig A, Falendysz EA, Ip HS, Lankton JS, Tretten TN, Spraker TR, Bowen R, Rocke TE. Immunogenicity, Safety, and Anti-Viral Efficacy of a Subunit SARS-CoV-2 Vaccine Candidate in Captive Black-Footed Ferrets (Mustela nigripes) and Their Susceptibility to Viral Challenge. Viruses. 2022; 14(10):2188. https://doi.org/10.3390/v14102188
Chicago/Turabian StyleLeon, Ariel E., Della Garelle, Airn Hartwig, Elizabeth A. Falendysz, Hon S. Ip, Julia S. Lankton, Tyler N. Tretten, Terry R. Spraker, Richard Bowen, and Tonie E. Rocke. 2022. "Immunogenicity, Safety, and Anti-Viral Efficacy of a Subunit SARS-CoV-2 Vaccine Candidate in Captive Black-Footed Ferrets (Mustela nigripes) and Their Susceptibility to Viral Challenge" Viruses 14, no. 10: 2188. https://doi.org/10.3390/v14102188
APA StyleLeon, A. E., Garelle, D., Hartwig, A., Falendysz, E. A., Ip, H. S., Lankton, J. S., Tretten, T. N., Spraker, T. R., Bowen, R., & Rocke, T. E. (2022). Immunogenicity, Safety, and Anti-Viral Efficacy of a Subunit SARS-CoV-2 Vaccine Candidate in Captive Black-Footed Ferrets (Mustela nigripes) and Their Susceptibility to Viral Challenge. Viruses, 14(10), 2188. https://doi.org/10.3390/v14102188






