Epidemiological Analysis of Cassava Mosaic and Brown Streak Diseases, and Bemisia tabaci in the Comoros Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Sites and Dates
2.2. Foliar Viral Disease Symptoms and Vector Abundance Assessment
2.3. CBSIs and CMBs Detection by (RT) PCR Testing
2.4. High Throughput Sequencing for CBSIs and CMBs
2.5. Virus Detection
2.6. Viral Genome Assembly
2.7. Genetic Identification of B. tabaci and Other Whiteflies
3. Results
3.1. Distribution of Cassava Varieties and Disease Symptoms
3.1.1. Distribution of Cassava Varieties
3.1.2. Distribution of CBSD and CMD Symptoms
3.2. CBSIs and CMBs Detection by (RT) PCR Testing and Next-Generation Sequencing
3.3. Virus Detection and Discovery by VirusDetect
3.4. Characterization of B. tabaci and Other Whiteflies
3.4.1. Vector Abundance
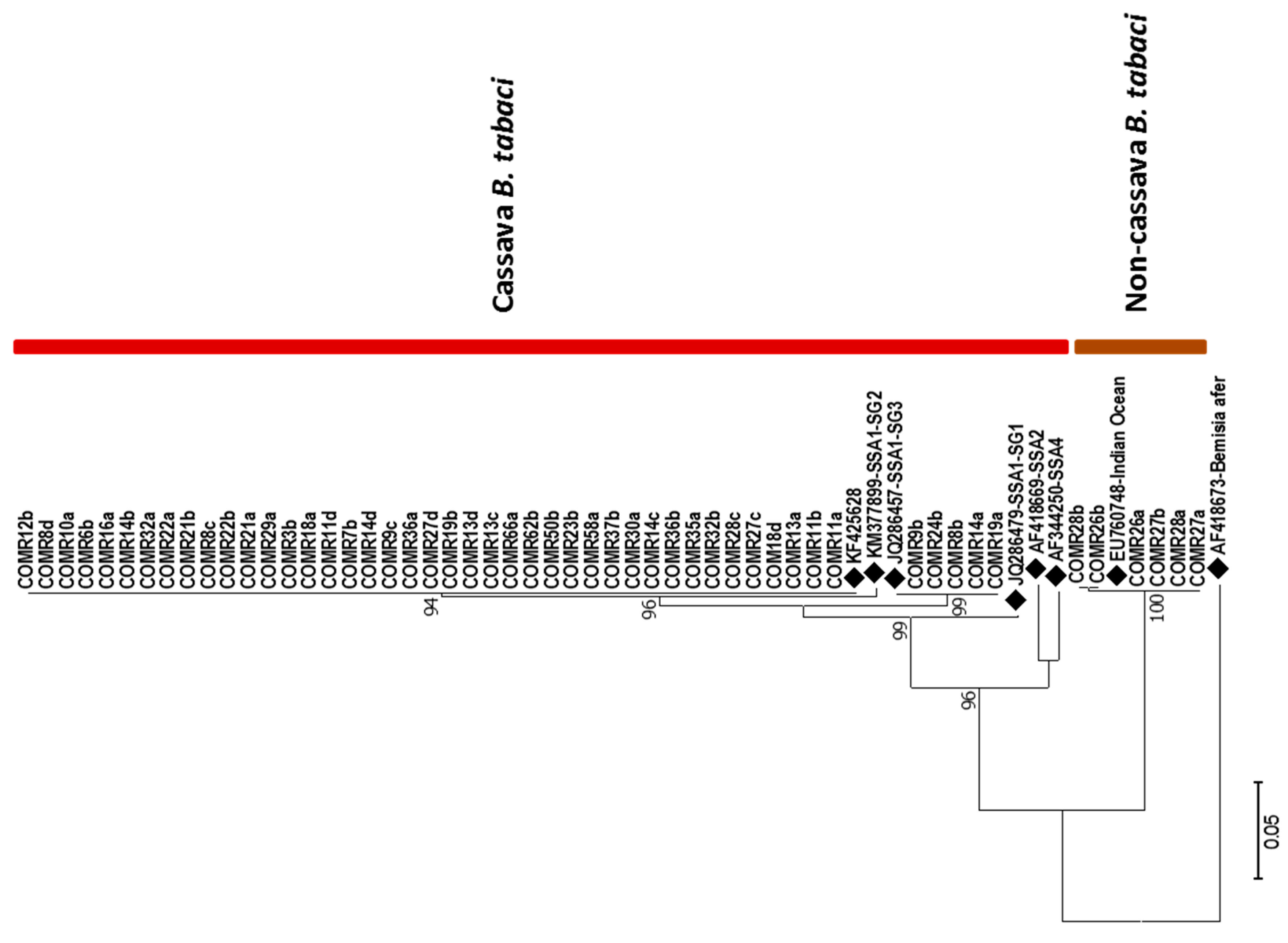
3.4.2. Genetic Diversity of Whiteflies
4. Discussion
4.1. Varietal Response to Cassava Viruses
4.2. Characterization of Cassava Brown Streak Ipomoviruses and Cassava Mosaic Begomoviruses
4.3. Mismatch between Disease Incidence and Virus Incidence
4.4. Characterization of Bemisia tabaci Whiteflies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The WIRE. Why Cassava Has So Much Potential in Sub-Saharan Africa. 2020. Available online: https://thewire.in/agriculture/food-security-climate-change-cassava (accessed on 19 April 2022).
- The Food and Agriculture Organization Statistics. FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 7 March 2022).
- Tarawali, G.; Ilona, P.; Ojiako, I.A.; Iyangbe, C.; Ogundijo, D.S.; Asumugha, G.; Udensi, U.E. A Comprehensive Training Module on Competitive Cassava Production; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2013; Available online: https://www.iita.org/wp-content/uploads/2016/06/A_comprehensive_training_module_on_competitive_cassava_production.pdf (accessed on 19 April 2022).
- Storey, H.H. Virus diseases of East African plants: VI-A progress report on studies of the diseases of cassava. East Afr. Agric. J. 1936, 2, 34–39. [Google Scholar]
- Owor, B.; Legg, J.P.; Okao-Okuja, G.; Obonyo, R.; Ogenga-Latigo, M.W. The effect of cassava mosaic geminiviruses on symptom severity, growth and root yield of a cassava mosaic virus disease-susceptible variety in Uganda. Ann. Appl. Biol. 2004, 145, 331–337. [Google Scholar] [CrossRef]
- Legg, J.P.; Owor, B.; Sseruwagi, P.; Ndunguru, J. Cassava mosaic virus disease in east and central Africa: Epidemiology and management of a regional pandemic. Adv. Virus Res. 2006, 67, 355–418. [Google Scholar] [PubMed]
- Mbanzibwa, D.R.; Tian, Y.P.; Mukasa, S.B.; Volkonen, Y.P.T. Cassava brown streak virus (Potyviridae) encodes a putative Maf/HAM1 pyrophosphatase implicated in reduction of mutations and a P1 proteinase that suppresses RNA silencing but contains no HC-Pro. J. Virol. 2009, 83, 6934–6940. [Google Scholar] [CrossRef] [PubMed]
- Mbanzibwa, D.R.; Tian, Y.P.; Tugume, A.K.; Mukasa, S.B.; Tairo, F.; Kyamanywa, S.; Kullaya, A.; Valkonen, J.P.T. Simultaneous virus-specific detection of the two cassava brown streak-associated viruses by RT-PCR reveals wide distribution in East Africa, mixed infections, and infections in Manihot glaziovii. J. Virol. Methods 2011, 171, 394–400. [Google Scholar] [CrossRef]
- Monger, W.A.; Alicai, T.; Ndunguru, J.; Kinyua, Z.M.; Potts, M.; Reeder, R.H.; Miano, D.W.; Adams, I.P.; Boonham, N.; Glover, R.H.; et al. The complete genome sequence of the Tanzanian strain of Cassava brown streak virus and comparison with the Ugandan strain sequence. Arch. Virol. 2010, 155, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Koebler, M.; Stein, B.; Pietruszka, A.; Paape, M.; Butgereitt, A. The analysis of cassava brown streak viruses reveals the presence of a distinct virus species causing cassava brown streak disease in East Africa. J. Gen. Virol. 2010, 91, 365–376. [Google Scholar] [CrossRef]
- Bock, K.; Woods, R. The etiology of African cassava mosaic disease. Plant Dis. 1983, 67, 994–996. [Google Scholar] [CrossRef]
- Legg, J.P.; Fauquet, C.M. Cassava mosaic geminiviruses in Africa. Plant Mol. Biol. 2004, 56, 585–599. [Google Scholar] [CrossRef]
- Thresh, J.M.; Otim-Nape, G.W.; Fargette, D. The Control of African Cassava Mosaic Virus Disease: Phytosanitation and/or Resistance? In Plant Virus Disease Control; Hadidi, A., Khetaspal, R.K., Koganezawa, H., Eds.; APS Press: St. Paul, MN, USA, 1998; pp. 670–677. [Google Scholar]
- Maruthi, M.N.; Hillocks, R.J.; Mtunda, K.; Raya, M.D.; Muhanna, M.; Kiozia, H.; Rekha, A.R.; Colvin, J.; Thresh, J.M. Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 2005, 153, 307–312. [Google Scholar] [CrossRef]
- Storey, H.H.; Nichols, R.F.W. Studies on the mosaic of cassava. Ann. Appl. Biol. 1938, 25, 790–806. [Google Scholar] [CrossRef]
- De Barro, P.J. The Bemisia species complex: Questions to guide future research. J. Integ. Agri. 2012, 11, 187–196. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Ghosh, S.; Bouvaine, S.; Maruthi, M. Prevalence and genetic diversity of endosymbiotic bacteria infecting cassava whiteflies in Africa. BMC Microbiol. 2015, 15, 93. [Google Scholar] [CrossRef]
- Legg, J.P.; Sseruwagi, P.; Boniface, S.; Okao-Okuja, G.; Shirima, R.; Bigirimana, S.; Gashaka, G.; Herrmann, H.W.; Jeremiah, S.; Obiero, H.; et al. Spatio-temporal patterns of genetic change amongst populations of cassava Bemisia tabaci whiteflies driving virus pandemics in East and Central Africa. Virus Res. 2014, 186, 61–75. [Google Scholar] [CrossRef]
- Esterhuizen, L.L.; Mabasa, K.G.; van Heerden, S.W.; Czosnek, H.; Brown, J.K.; van Heerden, H.; Rey, M.E.C. Genetic identification of members of the Bemisia tabaci cryptic species complex from South Africa reveals native and introduced haplotypes. J. Appl. Ent. 2013, 137, 122–135. [Google Scholar] [CrossRef]
- Berry, S.D.; Fondong, V.N.; Rey, C.; Rogan, D.; Fauquet, C.M.; Brown, J.K. Molecular evidence for five distinct Bemisia tabaci (Homoptera: Aleyrodidae) geographic haplotypes associated with cassava plants in sub-Saharan Africa. Ann. Entomol. Soc. Am. 2004, 97, 852–859. [Google Scholar] [CrossRef]
- Wosula, E.N.; Chen, W.; Amour, M.; Fei, Z.; Legg, J.P. KASP Genotyping as a molecular tool for diagnosis of cassava colonizing Bemisia tabaci. Insects 2020, 11, 305. [Google Scholar] [CrossRef]
- De Bruyn, A.; Villemot, J.; Lefeuvre, P.; Villar, E.; Hoareau, M.; Harimalala, M.; Abdoul-Karime, A.L.; Abdou-Chakour, C.; Reynaud, B.; Harkins, G.W.; et al. East African cassava mosaic-like viruses from Africa to Indian Ocean islands: Molecular diversity, evolutionary history and geographical dissemination of a bipartite begomovirus. BMC Evol. Biol. 2012, 12, 228. [Google Scholar] [CrossRef]
- Tomlinson, K.R.; Bailey, A.M.; Alicai, T.; Seal, S.; Foster, G.D. Cassava brown streak disease: Historical timeline, current knowledge and future prospects. Mol. Plant Pathol. 2018, 19, 1282–1294. [Google Scholar] [CrossRef]
- Alicai, T.; Omongo, C.A.; Maruthi, M.N.; Hillocks, R.J.; Baguma, Y.; Kawuki, R.; Bua, A.; Otim-Nape, G.W.; Colvin, J. Re-emergence of cassava brown streak disease in Uganda. Plant Dis. 2007, 91, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mulenga, R.M.; Boykin, L.M.; Chikoti, P.C.; Sichilima, S.; Ng’uni, D. Cassava brown streak disease and Ugandan cassava brown streak virus reported for the first time in Zambia. Plant Dis. 2018, 102, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Mulimbi, W.; Phemba, X.; Assumani, B.; Kasereka, P.; Muyisa, S.; Ugentho, H.; Reeder, R.; Legg, J.P.; Laurenson, L.; Weekes, R.; et al. First report of Ugandan cassava brown streak virus on cassava in Democratic Republic of Congo. New Dis. Rep. 2012, 26, 11. [Google Scholar] [CrossRef]
- Bigirimana, S.; Barumbanze, P.; Ndayihanzamaso, P.; Shirima, R.; Legg, J.P. First report of cassava brown streak disease and associated Ugandan cassava brown streak virus in Burundi. New Dis. Rep. 2011, 24, 26. [Google Scholar] [CrossRef]
- Hillocks, R.J.; Raya, M.D.; Mtunda, K.; Kiozia, H. Effects of brown streak virus disease on yield and quality of cassava in Tanzania. J. Phytopathol. 2008, 149, 389–394. [Google Scholar] [CrossRef]
- Azali, H.A.; Maillot, V.; Cassam, N.; Chesneau, T.; Soulezelle, J.; Scussel, S.; Abdoul-Karime, A.L.; Hostachy, B.; Reynaud, B.; Roux-Cuvelier, M.; et al. Occurrence of cassava brown streak disease and associated Cassava brown streak virus and Ugandan cassava brown streak virus in the Comoros Islands. New Dis. Rep. 2017, 36, 19. [Google Scholar] [CrossRef]
- Sseruwagi, P.; Sserubombwe, W.S.; Legg, J.P.; Ndunguru, J.; Thresh, J.M. Methods of surveying the incidence and severity of cassava mosaic disease and whitefly vector populations on cassava in Africa: A review. Virus Res. 2004, 100, 129–142. [Google Scholar] [CrossRef]
- Cours, G. Manioc in Madagascar. Mémoires L’institut Sci. Madagascar. Série B Biol. Végétale 1951, 3, 203–400. [Google Scholar]
- Gondwe, F.M.T.; Mahungu, N.M.; Hillocks, R.J.; Raya, M.D.; Moyo, C.C.; Soko, M.M.; Chipungu, F.B.; Benesi, I.R.M. Economic losses experienced by small-scale farmers in Malawi due to cassava brown streak virus disease. In ‘Development of a Co-ordinated Plan of African Action for CBSD Research in Eastern and Southern Africa’, Proceedings of a Workshop held at the Whitesands Hotel, Mombasa, Keya, 27–30 October 2002; DFID Crop Protection Programme: Aylesford, UK, 2003; pp. 28–35. [Google Scholar]
- Fargette, D. Epidémiologie de la Mosaïque Africaine du Manioc en Côte d’Ivoire. Ph.D. Thesis, Université des Sciences et Techniques du Languedoc, Montpellier, France, 1985. [Google Scholar]
- Maruthi, M.N.; Colvin, J.; Seal, S.; Gibson, G.; Cooper, J. Co-adaptation between cassava mosaic geminiviruses and their local vector populations. Virus Res. 2002, 86, 71–85. [Google Scholar] [CrossRef]
- Adams, I.P.; Abidrabo, P.; Miano, D.W.; Alicai, T.; Kinyua, Z.M.; Clarke, J.; Macarthur, R.; Weekes, R.; Laurenson, L.; Hany, U.; et al. High throughput real-time RT-PCR assays for specific detection of cassava brown streak disease causal viruses and their application to testing planting material. Plant Pathol. 2013, 62, 233–242. [Google Scholar] [CrossRef]
- Shirima, R.R.; Maeda, D.G.; Kanju, E.; Ceasar, G.; Tibazarwa, F.I.; Legg, J.P. Absolute quantification of cassava brown streak virus mRNA by real-time qPCR. J. Virol. Methods 2017, 245, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Alabi, O.J.; Kumar, P.L.; Naidu, R.A. Multiplex PCR method for the detection of African cassava mosaic virus and East African cassava mosaic Cameroon virus in cassava. J. Virol. Methods 2008, 154, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, S.; Padmanabhan, C.; Li, R.; Galvez, M.; Gutierrez, D.; Fuentes, S.; Ling, K.-S.; Kreuze, J.; Fei, Z. VirusDetect: An automated pipeline for efficient virus discovery using deep sequencing of small RNAs. Virology 2017, 500, 130–138. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. The short read alignment component (bwa-short) has been published: Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Mugerwa, H.; Seal, S.; Wang, H.-L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P.; et al. African ancestry of New World, Bemisia tabaci whitefly species. Sci. Rep. 2018, 8, 2734. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Hoeh, W.R.; Black, M.B.; Vrijenhoek, R.C. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wosula, E.N.; Chen, W.; Fei, Z.; Legg, J.P. Unravelling the genetic diversity among cassava Bemisia tabaci whiteflies using NextRAD sequencing. Genome Biol. Evol. 2017, 9, 2958–2973. [Google Scholar] [CrossRef]
- Chen, W.; Wosula, E.N.; Hasegawa, D.K.; Casinga, C.; Shirima, R.R.; Fiaboe, K.K.M.; Hanna, R.; Fosto, A.; Goergen, G.; Tamò, M.; et al. Genome of the African cassava whitefly Bemisia tabaci and distribution and genetic diversity of cassava-colonizing whiteflies in Africa. Insect Biochem. Mol. Biol. 2019, 110, 112–120. [Google Scholar] [CrossRef]
- Ano, C.U.; Ochwo-Ssemakula, M.; Ibanda, A.; Ozimati, A.; Gibson, P.; Onyeka, J.; Njoku, D.; Egesi, C.; Kawuki, R.S. Cassava Brown Streak Disease Response and Association with Agronomic Traits in Elite Nigerian Cassava Cultivars. Front. Plant Sci. 2021, 12, 720532. [Google Scholar] [CrossRef]
- World Bank. The Union of the Comoros: Jumpstarting Agricultural Transformation. 2017. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32398/The-Union-of-the-Comoros-Jumpstarting-Agricultural-Transformation.pdf?sequence=1 (accessed on 8 February 2022).
- Shirima, R.R.; Maeda, D.G.; Kanju, E.E.; Tumwegamire, S.; Ceasar, G.; Mushi, E.; Sichalwe, C.; Mtunda, K.; Mkamilo, G.; Legg, J.P. Assessing the degeneration of cassava under high-virus inoculum conditions in coastal Tanzania. Plant Dis. 2019, 103, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Shirima, R.R.; Legg, J.P.; Maeda, D.G.; Tumwegamire, S.; Mkamilo, G.; Mtunda, K.; Kulembeka, H.; Ndyetabula, I.; Kimata, B.P.; Matondo, D.G.; et al. Genotype by environment cultivar evaluation for cassava brown streak disease resistance in Tanzania. Viruses 2020, 286, 198017. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Agoti, C.N.; Lewa, C.S.; Oketch, J.; Owor, B.E.; Otieno, G.P.; Bett, A.; Cane, P.A.; Nokes, D.J. Recent sequence variation in probe binding site affected detection of respiratory syncytial virus group B by real-time RT-PCR. J. Clin. Virol. 2017, 88, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Scussel, S.; Candresse, T.; Marais, A.; Claverie, S.; Hoareau, M.; Azali, H.A.; Verdin, E.; Tepfer, M.; Filloux, D.; Fernandez, E.; et al. High-throughput sequencing of complete genomes of ipomoviruses associated with an epidemic of cassava brown streak disease in the Comoros Archipelago. Arch. Virol. 2019, 164, 2193–2196. [Google Scholar] [CrossRef] [PubMed]
- Legg, J.P.; Kumar, P.L.; Makeshkumar, T.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Tripathi, L.; Cuellar, W. Cassava virus diseases: Biology, epidemiology and management. Adv. Virus Res. 2015, 91, 85–142. [Google Scholar]
- Brown, J.K.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef]
- Alabi, O.J.; Mulenga, R.M.; Legg, J.P. Cassava mosaic. In Virus Diseases of Tropical and Subtropical Crops; Tennant, P., Fermin, G., Eds.; CAB International: Wallingford, UK, 2015; pp. 56–72. [Google Scholar]
- Thottappilly, G.; Thresh, J.M.; Calvert, L.A.; Winter, S. Cassava. In Virus and Virus-like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 107–165. [Google Scholar]
- Berry, S.; Rey, M.E.C. Molecular evidence for diverse populations of cassava-infecting begomoviruses in southern Africa. Arch. Virol. 2001, 146, 1795–1802. [Google Scholar] [CrossRef]
- Harimalala, M.; Chiroleu, F.; Giraud-Carrier, C.; Hoareau, M.; Zinga, I.; Randriamampianina, J.A.; Velombola, S.; Ranomenjanahary, S.; Andrianjaka, A.; Reynaud, B.; et al. Molecular epidemiology of cassava mosaic disease in Madagascar. Plant Pathol. 2014, 64, 501–507. [Google Scholar] [CrossRef]
- Fondong, V.N.; Pita, J.S.; Rey, C.M.; de Kochko, A.; Beachy, R.N.; Fauquet, C.M. Evidence of synergism between African cassava mosaic virus and new double-recombinant geminivirus infecting cassava in Cameroon. J. Gen. Virol. 2000, 81, 287–297. [Google Scholar] [CrossRef]
- Harrison, B.D.; Zhou, X.; Otim-Nape, G.W.; Liu, Y.; Robinson, D.J. Role of a novel type of double infection in the geminivirus-induced epidemic of severe cassava mosaic in Uganda. Ann. Appl. Biol. 1997, 131, 437–448. [Google Scholar] [CrossRef]
- Legg, J.; Winter, S. Cassava mosaic viruses (Geminiviridae). In Reference Module in Life Sciences; Roitberg, B.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–12. [Google Scholar]
- Maruthi, M.N.; Jeremiah, S.C.; Mohammed, I.U.; Legg, J.P. The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J. Phytopathol. 2017, 165, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Tocko-Marabena, B.K.; Silla, S.; Simiand, C.; Zinga, I.; Legg, J.P.; Reynaud, B.; Delatte, H. Genetic diversity of Bemisia tabaci species colonizing cassava in Central African Republic characterized by analysis of cytochrome oxidase subunit I. PLoS ONE 2017, 12, e0182749. [Google Scholar] [CrossRef]
- Misaka, B.C.; Wosula, E.N.; Marchelo-d’Ragga, P.W.; Hvoslef-Eide, T.; Legg, J.P. Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) colonizing sweet potato and cassava in South Sudan. Insects 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Munguti, F.M.; Kilalo, D.C.; Nyaboga, E.N.; Wosula, E.N.; Macharia, I.; Mwango’mbe, A.W. Distribution and molecular diversity of whitefly species colonizing cassava in Kenya. Insects 2021, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Chikoti, P.C.; Tembo, M.; Legg, J.P.; Shirima, R.R.; Mugerwa, H.; Sseruwagi, P. Genetic diversity of mitochondrial DNA of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) associated with cassava and the occurrence of cassava mosaic disease in Zambia. Insects 2020, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Sseruwagi, P.; Maruthi, M.N.; Colvin, J.; Rey, M.E.C.; Brown, J.K.; Legg, J.P. Colonization of non-cassava plant species by cassava whiteflies (Bemisia tabaci) in Uganda. Entomol. Exp. Appl. 2006, 119, 145–153. [Google Scholar] [CrossRef]
- Mugerwa, H.; Rey, M.E.; Alicai, T.; Ateka, E.; Atuncha, H.; Ndunguru, J.; Sseruwagi, P. Genetic diversity and geographic distribution of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) genotypes associated with cassava in East Africa. Ecol. Evol. 2012, 2, 2749–2762. [Google Scholar] [CrossRef]
- Legg, J.P. Host-associated strains within Ugandan populations of the whitefly Bemisia tabaci (Genn.), (Hom., Aleyrodidae). J. App. Ent. 1996, 120, 523–527. [Google Scholar] [CrossRef]
- Milenovic, M.; Wosula, E.N.; Rapisarda, C.; Legg, J.P. Impact of host plant species and whitefly species on feeding behavior of Bemisia tabaci. Front. Plant Sci. 2019, 10, 1. [Google Scholar] [CrossRef]
- Maruthi, M.N.; Rekha, A.R.; Sseruwagi, P.; Hillocks, R.J. Mitochondrial DNA variability and development of a PCR diagnostic test for populations of the whitefly Bemisia afer (Priesner and Hosny). Mol. Biotech. 2007, 35, 31–40. [Google Scholar] [CrossRef]
- Gamarra, H.; Carhuapoma, P.; Kreuze, J.; Kroschel, J. White fly, Bemisia afer (Priesner & Hosny 1934). In Pest Distribution and Risk Atlas for Africa. Potential Global and Regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents under Current and Future Climates; Kroschel, J., Mujica, N., Carhuapoma, P., Sporleder, M., Eds.; International Potato Center (CIP): Lima, Peru, 2016; pp. 100–113. [Google Scholar]
- Sundararaj, R.; Krishnan, S.; Sumalatha, B.V. Invasion and expansion of exotic whiteflies (Hemiptera: Aleyrodidae) in India and their economic importance. Phytoparasitica 2021, 49, 851–863. [Google Scholar] [CrossRef]
- Akinlosotu, T.A.; Jackai, L.E.N.; Ntonifor, N.N.; Hassan, A.T.; Agyakwa, C.W.; Odebiyi, J.A.; Akingbohungbe, A.E.; Rossel, H.W. Spiralling whitefly Aleurodicus dispersus in Nigeria. FAO Plant Prot. Bull. 1993, 41, 127–129. [Google Scholar]
- Neuenschwander, P. Spiralling whitefly, Aleurodicus dispersus, a recent invader and new cassava pest. Afr. Crop Sci. J. 1994, 2, 419–421. [Google Scholar]
- Hazell, S.P.; Vel, T.; Fellowes, M.D.E. The role of exotic plants in the invasion of Seychelles by the polyphagous insect Aleurodicus dispersus: A phylogenetically controlled analysis. Biol. Invasions 2008, 10, 169–175. [Google Scholar] [CrossRef]
- Mware, B.; Olubayo, F.; Narla, R.; Songa, J.; Amata, R.; Kyamanywa, S.; Ateka, E.M. First record of spiraling whitefly in coastal Kenya: Emergence, host range, distribution and association with cassava brown streak virus disease. Int. J. Agric. Biol. 2010, 12, 411–415. [Google Scholar]
- Guastella, D.; Lulah, H.; Tajebe, L.S.; Cavalieri, V.; Evans, G.A.; Pedata, P.A.; Rapisarda, C.; Legg, J.P. Survey on whiteflies and their parasitoids in cassava mosaic pandemic areas of Tanzania using morphological and molecular techniques. Pest Manag. Sci. 2015, 71, 383–394. [Google Scholar] [CrossRef]
- Omongo, C.A.; Namuddu, A.; Okao-Okuja, G.; Alicai, T.; van Brunschot, S.; Ouvrard, D.; Colvin, J. Occurrence of Bondar’s nesting whitefly, Paraleyrodes bondari (Hemiptera: Aleyrodidae), on cassava in Uganda. Rev. Bras Entomol. 2018, 62, 257–259. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Kawuki, R.S.; Kaweesi, T.; Esuma, W.; Pariyo, A.; Kayondo, I.S.; Ozimati, A.; Kyaligonza, V.; Abaca, A.; Orone, J.; Tumuhimbise, R.; et al. Eleven years of breeding efforts to combat cassava brown streak disease. Breed. Sci. 2016, 66, 560–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manyong, V.M.; Dixon, A.G.O.; Makinde, K.O.; Bokanga, M.; Whyte, J. The Contribution of IITA-Improved Cassava to Food Security in Sub-Saharan Africa: An Impact Study; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2000; Available online: https://www.researchgate.net/publication/265656112_The_Contribution_of_IITA-Improved_Cassava_to_Food_Security_in_Sub-Saharan_Africa_An_Impact_Study (accessed on 22 July 2022).
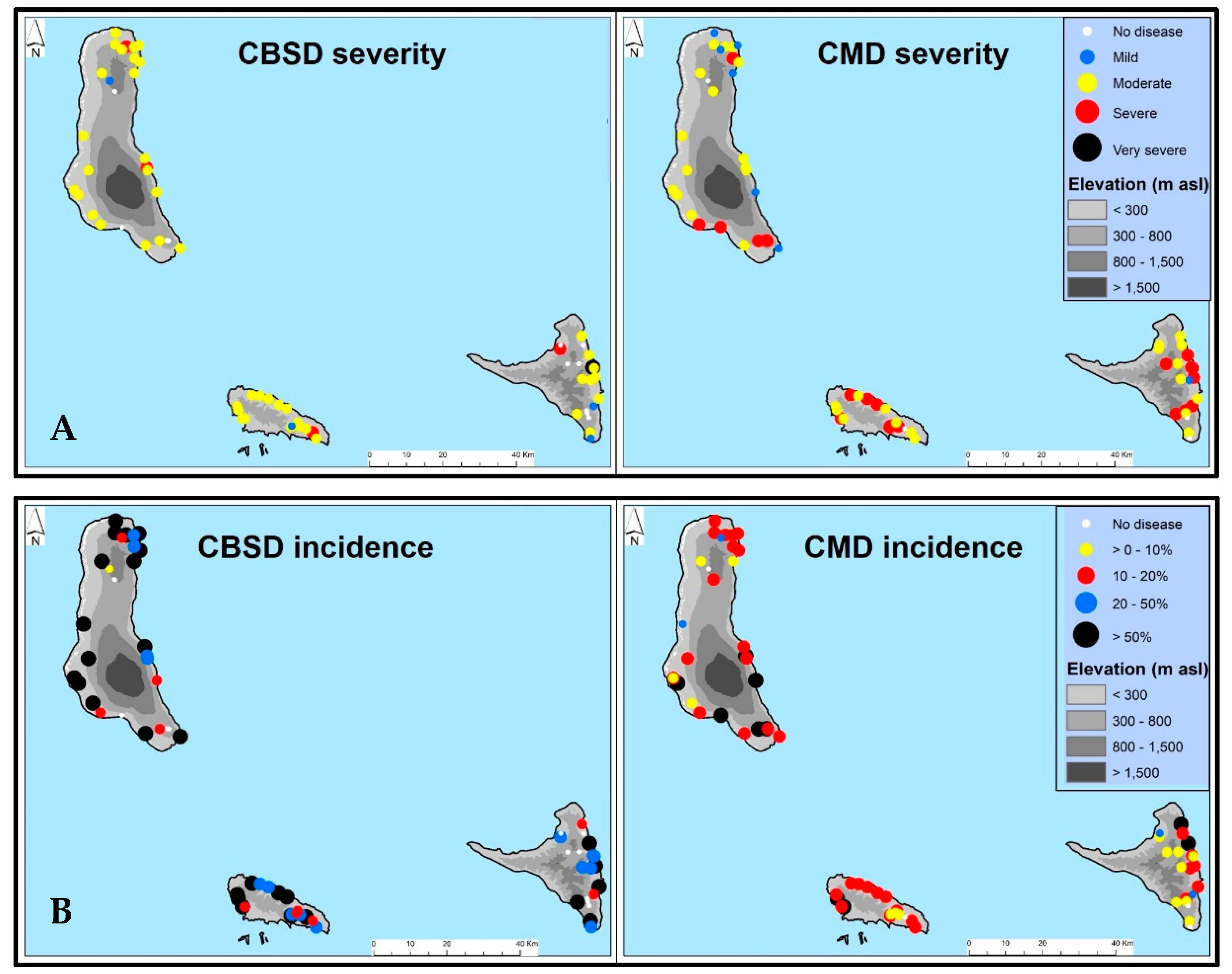

| Island | Variety | Sites | $Bemisia tabaci | # Leaf CBSD Severity | & Leaf CBSD Incidence | # CMD Severity | & CMD Incidence | ^ CBSD Prevalence | ^ CMD Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Ndzwani | Chihawati | 2 | 0.92 | 2.33 | 10.0 | 3.90 | 25.0 | 65.0 | 95.0 |
| Java | 2 | 0.17 | 3.04 | 41.7 | 2.71 | 20.0 | |||
| Mdja | 8 | 2.05 | 2.79 | 43.3 | 3.18 | 35.8 | |||
| Meladi | 2 | 3.02 | * | 0.0 | 2.80 | 8.3 | |||
| Mkoudu | 1 | 0.57 | * | 0.0 | 2.56 | 30.0 | |||
| Unknown | 1 | 1.77 | * | 0.0 | 3.00 | 3.3 | |||
| Wachididri | 4 | 1.26 | 2.97 | 35.0 | 3.05 | 21.7 | |||
| Mwali | Chihawati | 2 | 3.85 | 2.77 | 66.7 | 3.25 | 26.7 | 100.0 | 94.1 |
| Mdja | 6 | 2.97 | 2.99 | 51.1 | 3.01 | 41.7 | |||
| Mdjomani | 1 | 4.87 | 2.60 | 16.7 | 3.00 | 53.3 | |||
| Meladi | 3 | 1.23 | 2.72 | 24.4 | 2.96 | 23.3 | |||
| Mweou | 4 | 0.29 | 2.91 | 71.7 | 3.01 | 25.0 | |||
| Unknown | 1 | 0.47 | 2.60 | 16.7 | 3.83 | 20.0 | |||
| Ngazidja | Java | 3 | 1.82 | 2.85 | 24.4 | 3.18 | 54.4 | 86.2 | 96.6 |
| Mdja | 18 | 1.37 | 2.86 | 56.3 | 2.73 | 30.6 | |||
| Mdjema | 2 | 3.97 | * | 0.0 | 3.53 | 56.7 | |||
| Mdjomani | 3 | 1.89 | 2.98 | 47.8 | 2.95 | 21.1 | |||
| Mkoudu | 1 | 2.13 | 2.92 | 40.0 | 3.07 | 50.0 | |||
| Nkatsa | 1 | 1.37 | 2.76 | 56.7 | 2.91 | 36.7 | |||
| Unknown | 1 | 0.77 | 2.71 | 23.3 | 2.86 | 46.7 | |||
| Total/Mean | 66 | 1.75 | 2.86 | 42.0 | 3.00 | 31.6 | 83.3 | 95.5 | |
| Island | Sites | Bemisia tabaci | CMD Sev. | CBSD Sev. | CMD Wf | CMD Cut | CMD Total | fCBSD Inc. | stCBSD |
|---|---|---|---|---|---|---|---|---|---|
| Mwali | 17 | 2.10 | 3.08 | 2.85 | 10.2 | 22.0 | 32.2 | 49.0 | 10.4 |
| Ngazidja | 29 | 1.66 | 2.88 | 2.86 | 9.2 | 26.1 | 35.3 | 46.6 | 7.8 |
| Ndzwani | 20 | 1.60 | 3.12 | 2.84 | 5.0 | 20.7 | 25.7 | 29.5 | 3.2 |
| Average/Total | 66 | 1.75 | 3.00 | 2.86 | 8.2 | 23.4 | 31.6 | 42.0 | 7.07 |
| Real-Time RT-PCR Testing for Symptomatic Samples | |||||
| Island | Number of samples collected | CBSV positive | UCBSV positive | CBSIs negative | %Symptomatic positive |
| Mwali | 69 | 63 | 0 | 6 | 91.3 |
| Ngazidja | 99 | 73 | 0 | 27 | 73.7 |
| Ndzwani | 53 | 27 | 0 | 26 | 50.9 |
| Total | 221 | 163 | 0 | 59 | |
| Real-time RT-PCR testing for asymptomatic samples | |||||
| Island | Number of samples collected | CBSV positive | UCBSV positive | CBSIs negative | %Asymptomatic positive |
| Mwali | 16 | 4 | 0 | 12 | 25.0 |
| Ngazidja | 46 | 9 | 0 | 36 | 19.6 |
| Ndzwani | 47 | 0 | 0 | 47 | 0.0 |
| Total | 109 | 13 | 0 | 95 | |
| PCR testing for CMD symptomatic samples | |||||
| Island | Number of samples collected | ACMV positive | EACMV positive | CMBs negative | %Symptomatic positive |
| Mwali | 61 | 0 | 6 | 55 | 9.8 |
| Ngazidja | 95 | 0 | 14 | 81 | 14.7 |
| Ndzwani | 66 | 0 | 8 | 58 | 12.1 |
| Total | 222 | 0 | 28 | 194 | |
| PCR testing for CMD asymptomatic samples | |||||
| Island | Number of samples collected | ACMV positive | EACMV positive | CMBs negative | %Asymptomatic positive |
| Mwali | 24 | 0 | 0 | 24 | 0.0 |
| Ngazidja | 50 | 0 | 1 | 49 | 2.0 |
| Ndzwani | 34 | 0 | 0 | 34 | 0.0 |
| Total | 108 | 0 | 1 | 107 | |
| Sample ID | BankIt Submission ID | GenBank Accession Number | Sites ID | Virus |
|---|---|---|---|---|
| AQHM1C | BankIt2465160 | MZ362877 | NgB1.1 | CBSV |
| AQHM2U | BankIt2465160 | MZ362878 | NgB19.5 | UCBSV |
| AQHM1EA4-B | BankIt2470742 | MZ494476 | NgB1.1 | EACMV |
| AQHM20EA2-B | BankIt2470742 | MZ494477 | NgM16.1 | EACMV |
| AQHM23EA4-B | BankIt2470742 | MZ494478 | AnM19.4 | EACMV |
| AQHM25EA1-B | BankIt2470742 | MZ494479 | AnM13.5 | EACMV |
| AQHM26EA1-B | BankIt2470742 | MZ494480 | MoM3.3 | EACMV |
| AQHM29EA3-B | BankIt2470742 | MZ494481 | MoM12.2 | EACMV |
| AQHM1EA1-A | BankIt2473359 | MZ494482 | NgB1.1 | EACMV |
| AQHM20EA1-A | BankIt2473359 | MZ494483 | NgM16.1 | EACMV |
| AQHM23EA3-A | BankIt2473359 | MZ494484 | AnM19.4 | EACMV |
| AQHM25EA2-A | BankIt2473359 | MZ494485 | Anm13.5 | EACMV |
| AQHM26EA2-A | BankIt2473359 | MZ494486 | MoM3.3 | EACMV |
| AQHM27EA1-A | BankIt2473359 | MZ494487 | MoM3.4 | EACMV |
| Accession Number | * Primer or Probe | ^ Mismatch | $ Mismatch Position | Number of Mismatches |
|---|---|---|---|---|
| MZ362877 | CBSV probe | A/T | 14 | 1 |
| MK103392 | CBSV probe | A/G | 2 | 1 |
| MK103392 | CBSV reverse | T/C and G/A | 5 and 15 | 2 |
| MK103391 and MZ362877 | UCBSV forward | A/G | 2 | 1 |
| MK103391 and MZ362877 | UCBSV probe | A/T and T/A | 6 and 21 | 2 |
| MK103391 and MZ362877 | UCBSV reverse | A/G | 4 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirima, R.R.; Wosula, E.N.; Hamza, A.A.; Mohammed, N.A.; Mouigni, H.; Nouhou, S.; Mchinda, N.M.; Ceasar, G.; Amour, M.; Njukwe, E.; et al. Epidemiological Analysis of Cassava Mosaic and Brown Streak Diseases, and Bemisia tabaci in the Comoros Islands. Viruses 2022, 14, 2165. https://doi.org/10.3390/v14102165
Shirima RR, Wosula EN, Hamza AA, Mohammed NA, Mouigni H, Nouhou S, Mchinda NM, Ceasar G, Amour M, Njukwe E, et al. Epidemiological Analysis of Cassava Mosaic and Brown Streak Diseases, and Bemisia tabaci in the Comoros Islands. Viruses. 2022; 14(10):2165. https://doi.org/10.3390/v14102165
Chicago/Turabian StyleShirima, Rudolph Rufini, Everlyne Nafula Wosula, Abdou Azali Hamza, Nobataine Ali Mohammed, Hadji Mouigni, Salima Nouhou, Naima Mmadi Mchinda, Gloria Ceasar, Massoud Amour, Emmanuel Njukwe, and et al. 2022. "Epidemiological Analysis of Cassava Mosaic and Brown Streak Diseases, and Bemisia tabaci in the Comoros Islands" Viruses 14, no. 10: 2165. https://doi.org/10.3390/v14102165
APA StyleShirima, R. R., Wosula, E. N., Hamza, A. A., Mohammed, N. A., Mouigni, H., Nouhou, S., Mchinda, N. M., Ceasar, G., Amour, M., Njukwe, E., & Legg, J. P. (2022). Epidemiological Analysis of Cassava Mosaic and Brown Streak Diseases, and Bemisia tabaci in the Comoros Islands. Viruses, 14(10), 2165. https://doi.org/10.3390/v14102165







