Lessons Learned from the COVID-19 Pandemic and How Blood Operators Can Prepare for the Next Pandemic
Abstract
1. Introduction
2. Lessons Learned
2.1. Emerging Zoonotic Viruses Should Be Routinely Tracked by Blood Operators
2.2. Understand How the Emerging Virus of Interest Is Transmitted in the Community
2.3. Determining Whether the Emerging Virus Is a Direct Threat to the Blood Supply
2.4. Be Vigilant for Viral Genetic Changes in Viruses That Might Impact on Blood Operator Practices
2.5. Blood Operators May Be Involved in Creating New Blood Products
2.6. Blood Operators May Be Asked to Play a Public Health Role
3. How Blood Operators Can Prepare for the Next Pandemic
3.1. Be Prepared for Changes in Activities, Policies, and Procedures
3.2. Activities
3.3. Policies
3.4. Procedures
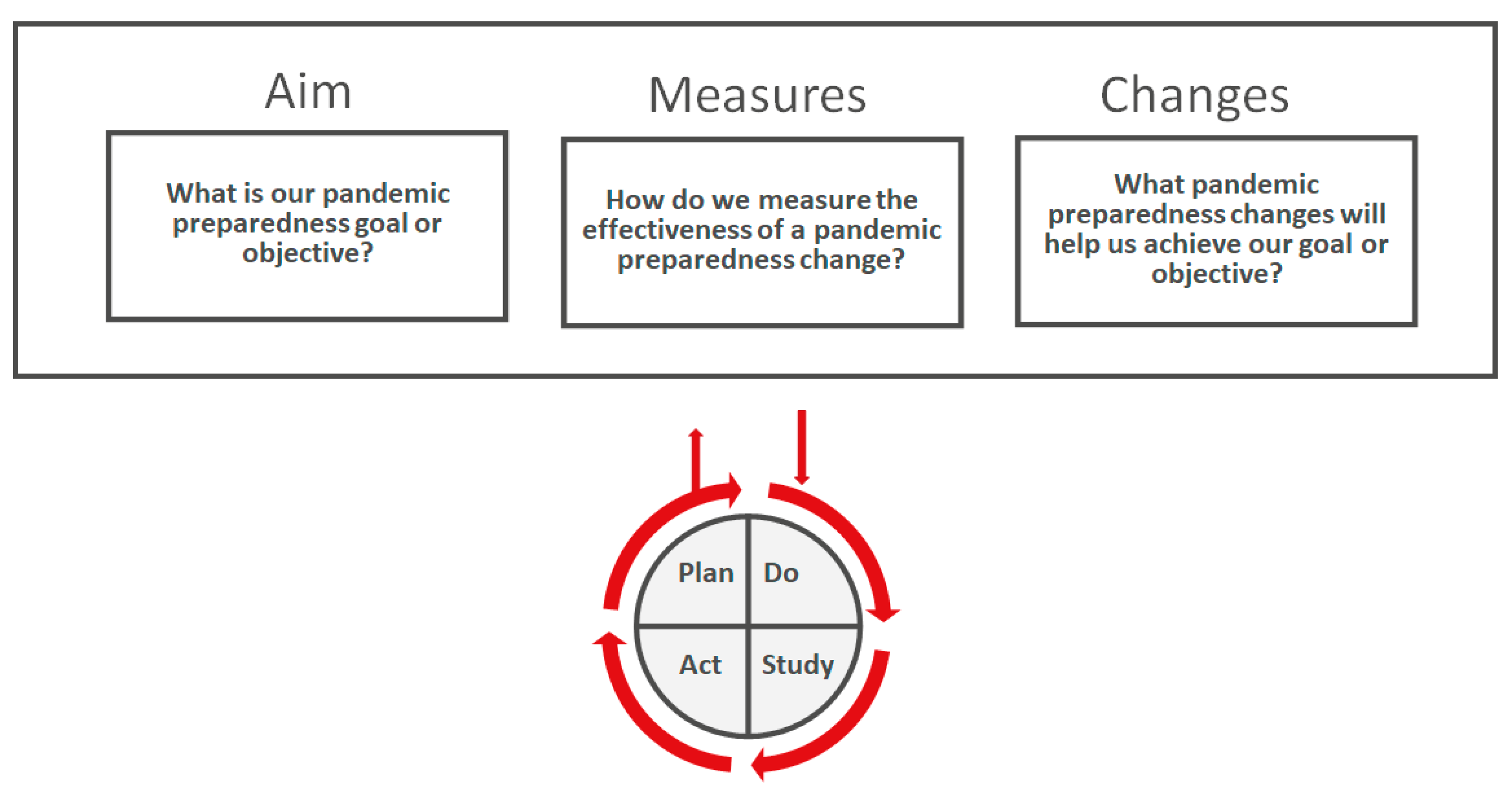
3.5. Models for Pandemic Planning Improvement
3.6. Approaches to Protect the Blood Supply
- vigilance for emerging viruses
- surveillance, donor studies and risk assessments for novel viruses that have emerged
- ensuring that operations continue
- donor engagement and trust
- initiation of laboratory testing for emerged virus
3.7. Hardwiring Vigilance by Creating or Updating a Pandemic Plan?
3.8. Plan for the Long Term: The End May Not Be Near
3.9. Plan Broadly Even if the Next Pandemic Is Not Blood-Borne and/or Transfusion-Transmitted
3.10. Consider Candidate Agents for the Next Pandemic
4. What to Watch for in a Worst-Case Scenario
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, E.C. COVID-19-lessons for zoonotic disease. Science 2022, 375, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.L.; Wang, Y.M.; Wu, Z.Q.; Xiang, Z.C.; Guo, L.; Xu, T.; Jiang, Y.Z.; Xiong, Y.; Li, Y.J.; Li, X.W.; et al. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. 2020, 133, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Shi, Z.; Zhang, S.; Field, H.; Daszak, P.; Eaton, B.T. Review of bats and SARS. Emerg. Infect. Dis. 2006, 12, 1834–1840. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Jones, J.; Sun, X.; Jang, Y.; Thor, S.; Belser, J.A.; Zanders, N.; Creager, H.M.; Ridenour, C.; Wang, L.; et al. Antigenically Diverse Swine Origin H1N1 Variant Influenza Viruses Exhibit Differential Ferret Pathogenesis and Transmission Phenotypes. J. Virol. 2018, 92, e00095-18. [Google Scholar] [CrossRef] [PubMed]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V.; et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef]
- Bermingham, A.; Chand, M.A.; Brown, C.S.; Aarons, E.; Tong, C.; Langrish, C.; Hoschler, K.; Brown, K.; Galiano, M.; Myers, R.; et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012, 17, 20290. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Liu, Y.; Gao, G.F.; Li, S.; Bi, Y. MERS in South Korea and China: A potential outbreak threat? Lancet 2015, 385, 2349–2350. [Google Scholar] [CrossRef]
- AABB. Emerging Infectious Disease Agents and Their Potential Threat to Transfusion Safety. Available online: https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/infectious-diseases/emerging-infectious-disease-agents (accessed on 27 June 2022).
- Leblanc, J.F.; Germain, M.; Delage, G.; O’Brien, S.; Drews, S.J.; Lewin, A. Risk of transmission of severe acute respiratory syndrome coronavirus 2 by transfusion: A literature review. Transfusion 2020, 60, 3046–3054. [Google Scholar] [CrossRef]
- Abe, K.T.; Rathod, B.; Colwill, K.; Gingras, A.C.; Tuite, A.; Robbins, N.F.; Orjuela, G.; Jenkins, C.; Conrod, V.; Yi, Q.L.; et al. A Qualitative Comparison of the Abbott SARS-CoV-2 IgG II Quant Assay against Commonly Used Canadian SARS-CoV-2 Enzyme Immunoassays in Blood Donor Retention Specimens, April 2020 to March 2021. Microbiol. Spectr. 2022, 10, e0113422. [Google Scholar] [CrossRef]
- Drews, S.J.; Hu, Q.; Samson, R.; Abe, K.T.; Rathod, B.; Colwill, K.; Gingras, A.C.; Yi, Q.L.; O’Brien, S.F. SARS-CoV-2 Virus-Like Particle Neutralizing Capacity in Blood Donors Depends on Serological Profile and Donor-Declared SARS-CoV-2 Vaccination History. Microbiol. Spectr. 2022, 10, e0226221. [Google Scholar] [CrossRef]
- Reedman, C.N.; Drews, S.J.; Yi, Q.L.; Pambrun, C.; O’Brien, S.F. Changing Patterns of SARS-CoV-2 Seroprevalence among Canadian Blood Donors during the Vaccine Era. Microbiol. Spectr. 2022, 10, e0033922. [Google Scholar] [CrossRef] [PubMed]
- Prokopchuk-Gauk, O.; Petraszko, T.; Nahirniak, S.; Doncaster, C.; Levy, I. Blood shortages planning in Canada: The National Emergency Blood Management Committee experience during the first 6 months of the COVID-19 pandemic. Transfusion 2021, 61, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Dibernardo, A.; Toledo, N.P.; Robinson, A.; Osiowy, C.; Giles, E.; Day, J.; Lindsay, L.R.; Drebot, M.A.; Booth, T.F.; Pidduck, T.; et al. Evaluation of the performance of multiple immunoassay diagnostic platforms on the National Microbiology Laboratory SARS-CoV-2 National Serology Panel. Off. J. Assoc. Med Microbiol. Infect. Dis. Can. 2022, e20210026. [Google Scholar] [CrossRef]
- Reperant, L.A.; Cornaglia, G.; Osterhaus, A.D. The importance of understanding the human-animal interface: From early hominins to global citizens. Curr. Top. Microbiol. Immunol. 2013, 365, 49–81. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Watts, A.; Thomas-Bachli, A.; Huber, C.; Kraemer, M.U.G.; Khan, K. Potential for global spread of a novel coronavirus from China. J. Travel. Med. 2020, 27, taaa011. [Google Scholar] [CrossRef]
- Ennab, F.; Nawaz, F.A.; Narain, K.; Nchasi, G.; Essar, M.Y. Rise of monkeypox: Lessons from COVID-19 pandemic to mitigate global health crises. Ann. Med. Surg. 2022, 79, 104049. [Google Scholar] [CrossRef]
- Wilson, M.E.; Chen, L.H. Travellers give wings to novel coronavirus (2019-nCoV). J. Travel. Med. 2020, 27, taaa015. [Google Scholar] [CrossRef]
- Centers for Disease Control. Possible transfusion-associated acquired immune deficiency syndrome (AIDS)-California. MMWR Morb. Mortal. Wkly. Rep. 1982, 31, 652–654. [Google Scholar]
- Institute of Medicine Committee to Study HIV Transmission Through Blood Products. HIV And The Blood Supply: An Analysis Of Crisis Decisionmaking; Leveton, L.B., Sox, H.C., Jr., Stoto, M.A., Eds.; National Academies Press (US): Washington, DC, USA, 1995. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). HIV transmission through transfusion---Missouri and Colorado, 2008. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1335–1339. [Google Scholar]
- O’Brien, S.F.; Yi, Q.L.; Fan, W.; Scalia, V.; Goldman, M.; Fearon, M.A. Residual risk of HIV, HCV and HBV in Canada. Transfus. Apher. Sci. 2017, 56, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E. Population biology of emerging and re-emerging pathogens. Trend. Microbiol. 2002, 10, S3–S7. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef]
- Li, W.; Wong, S.K.; Li, F.; Kuhn, J.H.; Huang, I.C.; Choe, H.; Farzan, M. Animal origins of the severe acute respiratory syndrome coronavirus: Insight from ACE2-S-protein interactions. J. Virol. 2006, 80, 4211–4219. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Pourrut, X.; Leroy, E. Ebolavirus and other filoviruses. Curr. Top. Microbiol. Immunol. 2007, 315, 363–387. [Google Scholar] [CrossRef]
- Smith, G.J.; Vijaykrishna, D.; Bahl, J.; Lycett, S.J.; Worobey, M.; Pybus, O.G.; Ma, S.K.; Cheung, C.L.; Raghwani, J.; Bhatt, S.; et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009, 459, 1122–1125. [Google Scholar] [CrossRef]
- Wang, W.; Tian, J.H.; Chen, X.; Hu, R.X.; Lin, X.D.; Pei, Y.Y.; Lv, J.X.; Zheng, J.J.; Dai, F.H.; Song, Z.G.; et al. Coronaviruses in wild animals sampled in and around Wuhan at the beginning of COVID-19 emergence. Virus Evol. 2022, 8, veac046. [Google Scholar] [CrossRef]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses Viruses. Master Species List 2021.V2. Available online: https://talk.ictvonline.org/files/master-species-lists/m/msl/13425 (accessed on 8 September 2022).
- Chang, L.; Yan, Y.; Wang, L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus. Med. Rev. 2020, 34, 75–80. [Google Scholar] [CrossRef]
- Pagano, M.B.; Hess, J.R.; Tsang, H.C.; Staley, E.; Gernsheimer, T.; Sen, N.; Clark, C.; Nester, T.; Bailey, C.; Alcorn, K. Prepare to adapt: Blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID-19) pandemic affecting Washington State. Transfusion 2020, 60, 908–911. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Zhang, S.; Huang, N.; Zhao, T.; Lu, Q.B.; Cui, F. Differences in incidence and fatality of COVID-19 by SARS-CoV-2 Omicron variant versus Delta variant in relation to vaccine coverage: A world-wide review. J. Med. Virol. 2022. [Google Scholar] [CrossRef]
- Dol, J.; Boulos, L.; Somerville, M.; Saxinger, L.; Doroshenko, A.; Hastings, S.; Reynolds, B.; Gallant, A.; Shin, H.D.; Wong, H.; et al. Health system impacts of SARS-CoV - 2 variants of concern: A rapid review. BMC Health Serv. Res. 2022, 22, 544. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis Following COVID-19 Vaccine: Incidence, Presentation, Diagnosis, Pathophysiology, Therapy, and Outcomes put into Perspective. Eur. J. Heart Fail. 2022. [Google Scholar] [CrossRef]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Zheng, S.S.; Rashid, F.N.; Enjeti, A.; Ting, S.B.; Chong, J.J.H.; Chong, B.H. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2022, 13, 5206. [Google Scholar] [CrossRef]
- Miyauchi, S.; Hiyama, T.; Nakano, Y.; Yoshida, M.; Yoshino, A.; Miyake, Y.; Okamoto, Y. Real-World Effectiveness of a Booster Dose of the COVID-19 Vaccines among Japanese University Students. Vaccines 2022, 10, 1283. [Google Scholar] [CrossRef]
- Cappy, P.; Legrain-Jbilou, S.; Chabli, L.; N’Debi, M.; Gallian, P.; Brisbarre, N.; Pillonel, J.; Morel, P.; Laperche, S. SARS-CoV-2 and post-donation information: A one-year experience of the French haemovigilance network. Blood Transfus. 2022, 20, 362–373. [Google Scholar] [CrossRef]
- Le Cam, S.; Gallian, P.; Ricard, C.; Narboux, C.; Barlet, V.; Maugard, C.; Hauser, L.; Brisbarre, N.; Cappy, P.; Pillonel, J.; et al. Low rate of RNAemia in blood donations collected during the first wave of COVID-19 in France. Transfusion 2022, 62, 633–640. [Google Scholar] [CrossRef]
- Bégin, P.; Callum, J.; Jamula, E.; Cook, R.; Heddle, N.M.; Tinmouth, A.; Zeller, M.P.; Beaudoin-Bussières, G.; Amorim, L.; Bazin, R.; et al. Convalescent plasma for hospitalized patients with COVID-19: An open-label, randomized controlled trial. Nat. Med. 2021, 27, 2012–2024. [Google Scholar] [CrossRef]
- Estcourt, L.J.; Turgeon, A.F.; McQuilten, Z.K.; McVerry, B.J.; Al-Beidh, F.; Annane, D.; Arabi, Y.M.; Arnold, D.M.; Beane, A.; Bégin, P.; et al. Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Al-Riyami, A.Z.; Burnouf, T.; Wood, E.M.; Devine, D.V.; Oreh, A.; Apelseth, T.O.; Goel, R.; Bloch, E.M.; van Den Berg, K.; Getshen, M.; et al. International Society of Blood Transfusion survey of experiences of blood banks and transfusion services during the COVID-19 pandemic. Vox Sang. 2022, 117, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, W.P.; Bhakta, V.; Howell, A.; Jenkins, C.; Serrano, K.; Johnson, N.; Lin, Y.J.; Colwill, K.; Rathod, B.; Greenberg, B.; et al. Retention of hemostatic and immunological properties of frozen plasma and COVID-19 convalescent apheresis fresh-frozen plasma produced and freeze-dried in Canada. Transfusion 2022, 62, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Drews, S.J.; Devine, D.V.; McManus, J.; Mendoza, E.; Manguiat, K.; Wood, H.; Girardin, R.; Dupuis, A.; McDonough, K.; Drebot, M. A trend of dropping anti-SARS-CoV-2 plaque reduction neutralization test titers over time in Canadian convalescent plasma donors. Transfusion 2021, 61, 1440–1446. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Drews, S.J.; Lewin, A.; Osiowy, C.; Drebot, M.A.; Renaud, C. Canadian blood suppliers: An expanding role in public health surveillance? Can. Commun. Dis. Rep. 2022, 48, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Stone, M.; Sulaeman, H.; Fink, R.V.; Dave, H.; Levy, M.E.; Di Germanio, C.; Green, V.; Notari, E.; Saa, P.; et al. Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020–May 2021. JAMA 2021, 326, 1400–1409. [Google Scholar] [CrossRef]
- Cong, Y.; Ren, X. Coronavirus entry and release in polarized epithelial cells: A review. Rev. Med. Virol. 2014, 24, 308–315. [Google Scholar] [CrossRef]
- Tuite, A.R.; Fisman, D.; Abe, K.T.; Rathod, B.; Pasculescu, A.; Colwill, K.; Gingras, A.C.; Yi, Q.L.; O’Brien, S.F.; Drews, S.J. Estimating SARS-CoV-2 Seroprevalence in Canadian Blood Donors, April 2020 to March 2021: Improving Accuracy with Multiple Assays. Microbiol. Spectr. 2022, 10, e0256321. [Google Scholar] [CrossRef]
- Eurosurveillance Editorial Team. Latest updates on COVID-19 from the European Centre for Disease Prevention and Control. Euro Surveill. 2020, 25, 2002131. [Google Scholar] [CrossRef]
- Chen, Z.M.; Fu, J.F.; Shu, Q.; Chen, Y.H.; Hua, C.Z.; Li, F.B.; Lin, R.; Tang, L.F.; Wang, T.L.; Wang, W.; et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatr. 2020, 16, 240–246. [Google Scholar] [CrossRef]
- Biscayart, C.; Angeleri, P.; Lloveras, S.; Chaves, T.; Schlagenhauf, P.; Rodríguez-Morales, A.J. The next big threat to global health? 2019 novel coronavirus (2019-nCoV): What advice can we give to travellers?—Interim recommendations January 2020, from the Latin-American society for Travel Medicine (SLAMVI). Travel Med. Infect. Dis. 2020, 33, 101567. [Google Scholar] [CrossRef]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P., 2nd; Girardin, R.C.; Rathod, B.; et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, Y.; Uchida, Y.; Takemae, N.; Hayashi, T.; Tsuda, T.; Saito, T. Real-time reverse transcription-PCR assay for differentiating the Pandemic H1N1 2009 influenza virus from swine influenza viruses. J. Virol. Methods 2010, 170, 169–172. [Google Scholar] [CrossRef]
- Katz, L.M. Is SARS-CoV-2 transfusion transmitted? Transfusion 2020, 60, 1111–1114. [Google Scholar] [CrossRef]
- Bakkour, S.; Saá, P.; Groves, J.A.; Montalvo, L.; Di Germanio, C.; Best, S.M.; Grebe, E.; Livezey, K.; Linnen, J.M.; Strauss, D.; et al. Minipool testing for SARS-CoV-2 RNA in United States blood donors. Transfusion 2021, 61, 2384–2391. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Ward, S.; Gallian, P.; Fabra, C.; Pillonel, J.; Kitchen, A.D.; Davison, K.; Seed, C.R.; Delage, G.; Steele, W.R.; et al. Malaria blood safety policy in five non-endemic countries: A retrospective comparison through the lens of the ABO risk-based decision-making framework. Blood Transfus. 2019, 17, 94–102. [Google Scholar] [CrossRef]
- Drews, S.J. The taxonomy, classification, and characterization of medically important viruses. In Clinical Virology Manual, 5th ed.; Loeffelholz, M., Hodinka, R.L., Young, S.A., Pinksy, B.A., Eds.; ASM Press: Washington, DC, USA, 2016; pp. 1–26. [Google Scholar]
- Worobey, M. Dissecting the early COVID-19 cases in Wuhan. Science 2021, 374, 1202–1204. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Rambaut, A.; Pybus, O.G.; Holmes, E.C. Rates of molecular evolution in RNA viruses: A quantitative phylogenetic analysis. J. Mol. Evol. 2002, 54, 156–165. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef]
- Saad-Roy, C.M.; Metcalf, C.J.E.; Grenfell, B.T. Immuno-epidemiology and the predictability of viral evolution. Science 2022, 376, 1161–1162. [Google Scholar] [CrossRef]
- Jain, R.; Mallya, M.V.; Amoncar, S.; Palyekar, S.; Adsul, H.P.; Kumar, R.; Chawla, S. Seroprevalence of SARS-CoV-2 among potential convalescent plasma donors and analysis of their deferral pattern: Experience from tertiary care hospital in western India. Transfus. Clin. Biol. 2022, 29, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Drews, S.J.; Pambrun, C.; Yi, Q.L.; Osmond, L.; O’Brien, S.F. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion 2021, 61, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Greer, A.L.; Brankston, G.; Hillmer, M.; O’Brien, S.F.; Drews, S.J.; Tuite, A.R. COVID-19 Case Age Distribution: Correction for Differential Testing by Age. Ann. Intern. Med. 2021, 174, 1430–1438. [Google Scholar] [CrossRef]
- Korves, C.; Izurieta, H.S.; Smith, J.; Zwain, G.M.; Powell, E.I.; Balajee, A.; Ryder, K.M.; Young-Xu, Y. Relative effectiveness of booster vs. 2-dose mRNA COVID-19 vaccination in the Veterans Health Administration: Self-controlled risk interval analysis. Vaccine 2022, 61, 862–872. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Chen, Z.; Huang, X.; Xu, M.; He, T.; Zhang, Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J. Med. Virol. 2020, 92, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.M.; Ip, J.D.; Chu, A.W.; Yip, C.C.; Lo, L.S.; Chan, K.H.; Ng, A.C.; Poon, R.W.; To, W.K.; Tsang, O.T.; et al. Identification of nsp1 gene as the target of SARS-CoV-2 real-time RT-PCR using nanopore whole-genome sequencing. J. Med. Virol. 2020, 92, 2725–2734. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality: Model for Improvement and PDSA Cycles. Available online: https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/job-aid-model-pdsa.pdf (accessed on 7 September 2022).
- Fabbro, M., 2nd; Patel, P.A.; Henderson, R.A., Jr.; Bolliger, D.; Tanaka, K.A.; Mazzeffi, M.A. Coagulation and Transfusion Updates From 2021. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3447–3458. [Google Scholar] [CrossRef]
- Rodrigues, D.O.W.; Magalhães, N.N.S.; Silva-Malta, M.C.F.; Chaves, D.G.; Freire de Carvalho, R.V.; Ribeiro, M.A.; Cioffi, J.G.M.; Martins, M.L. Impact of COVID-19 on the efficacy of meeting the transfusion demand by a Brazilian blood banks network. Transfus. Apher. Sci. 2022, 103439. [Google Scholar] [CrossRef]
- Canada, G.O. Canadian Pandemic Influenza Preparedness Planning Guidance for the Health Sector; Her Majesty the Queen in Right of Canada, as Represented by the Minister of Health: Ottawa, ON, Canada, 2018; p. 64. [Google Scholar]
- Henry, B. Canadian Pandemic Influenza Preparedness: Health sector planning guidance. Can. Commun. Dis. Rep. 2018, 44, 6–9. [Google Scholar] [CrossRef]
- Liang, F.; Guan, P.; Wu, W.; Liu, J.; Zhang, N.; Zhou, B.S.; Huang, D.S. A review of documents prepared by international organizations about influenza pandemics, including the 2009 pandemic: A bibliometric analysis. BMC Infect. Dis. 2018, 18, 383. [Google Scholar] [CrossRef]
- Risk.net. Time to Dust off Business Continuity Plans in Preparation for a Global Pandemic. Available online: https://www.risk.net/risk-management/1521054/time-to-dust-off-business-continuity-plans-in-preparation-for-a-global-pandemic (accessed on 8 July 2022).
- Matthews, S. GPs Need to ‘Dust Off’ Their Pandemic Plans ahead of the Winter Flu Season, Warns Leading Medic. Dally Mail, Published Online, 09 September 2018. Available online: https://www.dailymail.co.uk/health/article-6193165/GPs-dust-pandemic-plans-flu-season-warns-leading-medic.html (accessed on 20 September 2022).
- Koonin, L.M. Novel coronavirus disease (COVID-19) outbreak: Now is the time to refresh pandemic plans. J. Bus. Contin. Emer. Plan. 2020, 13, 298–312. [Google Scholar] [PubMed]
- Fisman, D.N.; Amoako, A.; Tuite, A.R. Impact of population mixing between vaccinated and unvaccinated subpopulations on infectious disease dynamics: Implications for SARS-CoV-2 transmission. CMAJ 2022, 194, E573–E580. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Savage, R.; Whelan, M.; Rosella, L.C.; Crowcroft, N.S.; Willison, D.; Winter, A.L.; Davies, R.; Gemmill, I.; Mucchal, P.K.; et al. Assessing the relative timeliness of Ontario’s syndromic surveillance systems for early detection of the 2009 influenza H1N1 pandemic waves. Can. J. Public Health 2013, 104, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Elderfield, R.A.; Watson, S.J.; Godlee, A.; Adamson, W.E.; Thompson, C.I.; Dunning, J.; Fernandez-Alonso, M.; Blumenkrantz, D.; Hussell, T.; Zambon, M.; et al. Accumulation of human-adapting mutations during circulation of A(H1N1)pdm09 influenza virus in humans in the United Kingdom. J. Virol. 2014, 88, 13269–13283. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, F.; Jankowiak, M.; Barkas, N.; Schaffner, S.F.; Pyle, J.D.; Yurkovetskiy, L.; Bosso, M.; Park, D.J.; Babadi, M.; MacInnis, B.L.; et al. Analysis of 6.4 million SARS-CoV-2 genomes identifies mutations associated with fitness. Science 2022, 376, 1327–1332. [Google Scholar] [CrossRef]
- Fierro, A.; Romano, S.; Liccardo, A. Vaccination and variants: Retrospective model for the evolution of COVID-19 in Italy. PLoS ONE 2022, 17, e0265159. [Google Scholar] [CrossRef]
- Bardi, T.; Gómez-Rojo, M.; Candela-Toha, A.M.; de Pablo, R.; Martinez, R.; Pestaña, D. Rapid response to COVID-19, escalation and de-escalation strategies to match surge capacity of Intensive Care beds to a large scale epidemic. Rev. Esp. Anestesiol. Reanim. 2021, 68, 21–27. [Google Scholar] [CrossRef]
- Moreno-Sueskun, I.; Díaz-González, J.A.; Acuña Juanbeltz, A.; Pérez-Murillo, A.; Garasa Jiménez, A.; García-Osés, V.; Extramiana Cameno, E. Return to work in the context of the COVID-19 pandemic in the industrial and construction sectors in Navarre (Spain). Arch. Prev. Riesgos. Labor. 2020, 23, 443–457. [Google Scholar] [CrossRef]
- Rueda-Garrido, J.C.; Vicente-Herrero, M.T.; Del Campo, M.T.; Reinoso-Barbero, L.; de la Hoz, R.E.; Delclos, G.L.; Kales, S.N.; Fernandez-Montero, A. Return to work guidelines for the COVID-19 pandemic. Occup. Med. 2020, 70, 300–305. [Google Scholar] [CrossRef]
- Fragala, M.S.; Goldberg, Z.N.; Goldberg, S.E. Return to Work: Managing Employee Population Health During the COVID-19 Pandemic. Popul. Health Manag. 2021, 24, S3–S15. [Google Scholar] [CrossRef]
- Baptista, M.C.; Burton, W.N.; Pawlecki, B.; Pransky, G. A Physician’s Guide for Workers’ Return to Work During COVID-19 Pandemic. J. Occup. Environ. Med. 2021, 63, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M. The Ethics of Selective Mandatory Vaccination for COVID-19. Public Health Ethics. 2022, 15, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, Z.; Khan, M.A.; Asghar, M.S.; Zaman, M.; Ahmed, M.; Tahir, M.J. COVID-19 Omicron variant-Time for airborne precautions. Ann. Med. Surg. 2022, 78, 103919. [Google Scholar] [CrossRef]
- Rhee, C.; Baker, M.A.; Klompas, M. Survey of coronavirus disease 2019 (COVID-19) infection control policies at leading US academic hospitals in the context of the initial pandemic surge of the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) omicron variant. Infect. Control. Hosp. Epidemiol. 2022, 78, 1–7. [Google Scholar] [CrossRef]
- Kiely, P.; Hoad, V.C.; Seed, C.R.; Gosbell, I.B. Severe Acute Respiratory Syndrome Coronavirus 2 and Blood Safety: An Updated Review. Transfus. Med. Hemother. 2022, 5, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Oreh, A.; Bozegha, T.; Ihimekpen, A.; Biyama, F.; Irechukwu, C.; Aliu, S.; Oshiame, D.; Nnabuihe, A.; Ndanitsa, A.; Nnachi, O.; et al. Effect of the COVID-19 pandemic on blood donations and transfusions in Nigeria - A multi-facility study of 34 tertiary hospitals. Niger. J. Clin. Pract. 2022, 25, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Routray, S.S.; Ray, G.K.; Prakash, S.; Sahu, A.; Naik, A.; Mukherjee, S. Impact of COVID-19 on blood donor deferral patterns during the COVID-19 pandemic: A retrospective analysis. Vox Sang. 2022, 117, 656–663. [Google Scholar] [CrossRef]
- Gupta, A.M.; Jain, P. Blood donor deferral periods after COVID-19 vaccination. Transfus. Apher. Sci. 2021, 60, 103179. [Google Scholar] [CrossRef]
- Monaco, L.G.V. The Nxt Pandemic will be Arriving Shortly. Available online: https://foreignpolicy.com/2018/09/28/the-next-pandemic-will-be-arriving-shortly-global-health-infectious-avian-flu-ebola-zoonotic-diseases-trump/ (accessed on 18 July 2022).
- Heymann, D.R.E.; Wallace, J. The Next Pandemic–When Could it Be? Available online: https://www.chathamhouse.org/2022/02/next-pandemic-when-could-it-be (accessed on 18 July 2022).
- Zhu, H.; Zhou, B.; Fan, X.; Lam, T.T.; Wang, J.; Chen, A.; Chen, X.; Chen, H.; Webster, R.G.; Webby, R.; et al. Novel reassortment of Eurasian avian-like and pandemic/2009 influenza viruses in swine: Infectious potential for humans. J. Virol. 2011, 85, 10432–10439. [Google Scholar] [CrossRef]
- Charlton, C.L.; Babady, E.; Ginocchio, C.C.; Hatchette, T.F.; Jerris, R.C.; Li, Y.; Loeffelholz, M.; McCarter, Y.S.; Miller, M.B.; Novak-Weekley, S.; et al. Practical Guidance for Clinical Microbiology Laboratories: Viruses Causing Acute Respiratory Tract Infections. Clin. Microbiol. Rev. 2019, 32, e00042-18. [Google Scholar] [CrossRef]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Global outbreak puts spotlight on neglected virus. Science 2022, 376, 1032–1033. [Google Scholar] [CrossRef] [PubMed]
- Berting, A.; Goerner, W.; Spruth, M.; Kistner, O.; Kreil, T.R. Effective poxvirus removal by sterile filtration during manufacture of plasma derivatives. J. Med. Virol. 2005, 75, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Sagripanti, J.L.; Marschall, H.J.; Voss, L.; Hülseweh, B. Photochemical inactivation of alpha- and poxviruses. Photochem. Photobiol. 2011, 87, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Ragan, I.; Hartson, L.; Pidcoke, H.; Bowen, R.; Goodrich, R. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. PLoS ONE 2020, 15, e0233947. [Google Scholar] [CrossRef]
- Dhawan, M.; Priyanka; Choudhary, O.P. Emergence of monkeypox: Risk assessment and containment measures. Travel. Med. Infect. Dis. 2022, 49, 102392. [Google Scholar] [CrossRef]
- Hoteit, R.; Yassine, H.M. Biological Properties of SARS-CoV-2 Variants: Epidemiological Impact and Clinical Consequences. Vaccines 2022, 10, 919. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Anderson, R.M.; Vegvari, C.; Hollingsworth, T.D.; Pi, L.; Maddren, R.; Ng, C.W.; Baggaley, R.F. The SARS-CoV-2 pandemic: Remaining uncertainties in our understanding of the epidemiology and transmission dynamics of the virus, and challenges to be overcome. Interface Focus 2021, 11, 20210008. [Google Scholar] [CrossRef]
- Millet, J.K.; Jaimes, J.A.; Whittaker, G.R. Molecular diversity of coronavirus host cell entry receptors. FEMS Microbiol. Rev. 2021, 45, fuaa057. [Google Scholar] [CrossRef]
- Hu, Z.; Song, C.; Xu, C.; Jin, G.; Chen, Y.; Xu, X.; Ma, H.; Chen, W.; Lin, Y.; Zheng, Y.; et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020, 63, 706–711. [Google Scholar] [CrossRef] [PubMed]

| Issue | Possible Cause | Blood Operator Lesson for Future Emerging Event or Pandemic | References |
|---|---|---|---|
| Rapid emergence and spread of a zoonotic agent even in presence of public health controls. | Closer interactions between humans and infected host animals. Unclear epidemiology and transmissibility. Human populations travel globally. | Continue to track zoonotic virus activity and risks for human emergence and spread. | [1,25,31] |
| Early growing evidence that SARS-CoV-2 was spread between people via the respiratory route. | Unclear understanding of ease of virus transmission. | Look for taxonomic and early epidemiologic clues for patterns of transmission. | [32,33] |
| Questions from transfusion community on transfusion-transmission of SARS-CoV-2. | Unclear evidence on transmissibility. Questions from transfusion community, regulators, and public. | Undertake risk assessments for transfusion transmission. Continue to review even after first assessments done. | [9,34,35] |
| Evidence for small scale (single nucleotide polymorphisms) and large scale (lineage replacement) changes in virus | Nature of virus genome and responses to evolutionary pressures. | Be prepared to change practices and processes focused on staff and donor safety as viruses evolve. Consider if transfusion-transmission risk changes. | [36,37,38,39,40,41,42] |
| Blood operators asked to help assess new blood products | New emerging agent had no known effective treatment and vaccines were not yet available. | Be prepared to be involved in clinical trials that involve the blood operator. Be prepared to be involved in the development of new donor testing approaches. | [43,44,45,46,47] |
| Blood operators asked to engage with public health on surveillance initiatives | Public health may focus on data from unwell populations. Public health may focus regionally or may use aggregate data across regions collected using different approaches. | Be prepared to operationalize new surveillance strategies. Be prepared to advise how blood donors can represent the general population. | [48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drews, S.J.; O’Brien, S.F. Lessons Learned from the COVID-19 Pandemic and How Blood Operators Can Prepare for the Next Pandemic. Viruses 2022, 14, 2126. https://doi.org/10.3390/v14102126
Drews SJ, O’Brien SF. Lessons Learned from the COVID-19 Pandemic and How Blood Operators Can Prepare for the Next Pandemic. Viruses. 2022; 14(10):2126. https://doi.org/10.3390/v14102126
Chicago/Turabian StyleDrews, Steven J., and Sheila F. O’Brien. 2022. "Lessons Learned from the COVID-19 Pandemic and How Blood Operators Can Prepare for the Next Pandemic" Viruses 14, no. 10: 2126. https://doi.org/10.3390/v14102126
APA StyleDrews, S. J., & O’Brien, S. F. (2022). Lessons Learned from the COVID-19 Pandemic and How Blood Operators Can Prepare for the Next Pandemic. Viruses, 14(10), 2126. https://doi.org/10.3390/v14102126





