Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review
Abstract
1. Introduction
2. Structure and Role of NS3 and NS3 Protease Domain in Flavivirus Replication
3. Structure and Role of NS2B and NS2B Hydrophilic Domain in Flavivirus Replication
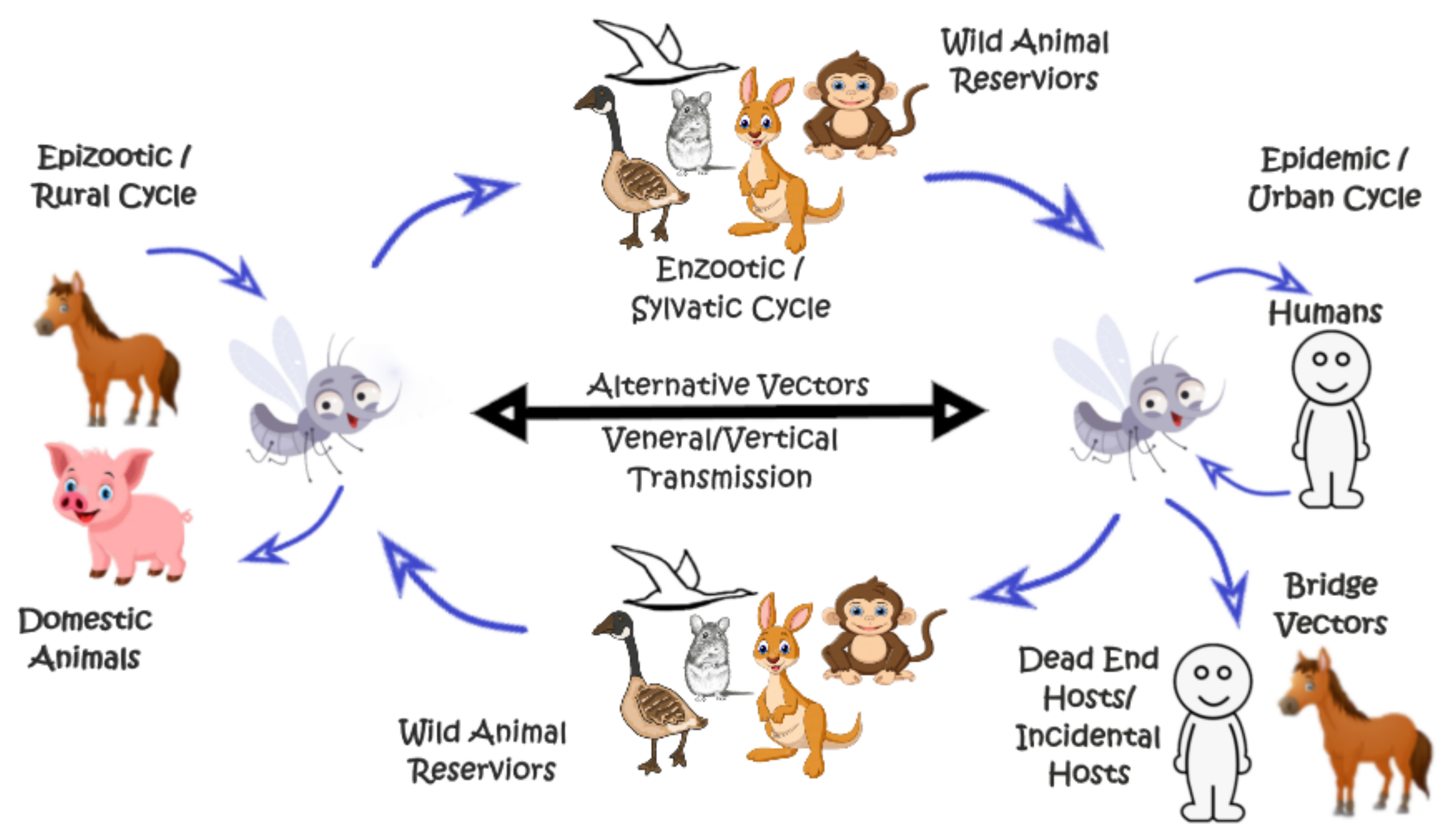
4. Dengue Virus (DENV)
5. Yellow Fever Virus (YFV)
6. Zika Virus (ZIKV)
7. Japanese Encephalitis Virus (JEV)
8. West Nile Virus (WNV)
9. Interaction of Flavivirus NS2B-NS3 Proteases with Cellular Proteins
10. Interactions of Flavivirus NS3 with Host Cell NPC and Nucleus
11. Characterization of Flavivirus NS2B-NS3 Proteases
12. NS2B-NS3 Proteases as a Potential Viral Inhibition Drug Target
13. Proposing Role of STING in Development of In Vitro and In Vivo Models for Studying Flavivirus Pathogenesis and Antiviral Drug Screens
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westaway, E.G.; Brinton, M.A.; Gaidamovich, S.Y.; Horzinek, M.C.; Igarashi, A.; Kääriäinen, L.; Lvov, D.K.; Porterfield, J.S.; Russell, P.K.; Trent, D.W. Flaviviridae. Intervirology 1985, 24, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Gould, E.A. Taxonomy of the virus family Flaviviridae. Adv. Virus Res. 2003, 59, 1–19. [Google Scholar] [PubMed]
- Bessaud, M.; Pastorino, B.A.M.; Peyrefitte, C.N.; Rolland, D.; Grandadam, M.; Tolou, H.J. Functional characterization of the NS2B/NS3 protease complex from seven viruses belonging to different groups inside the genus Flavivirus. Virus Res. 2006, 120, 79–90. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, N.; Taylor-Robinson, A.W.; Bradbury, R.S.; Potter, A.; Aaskov, J.G. Infection of Western Gray Kangaroos (Macropus fuliginosus) with Australian Arboviruses Associated with Human Infection. Vector-Borne Zoonotic Dis. 2020, 20, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Angsubhakorn, S.; Moe, J.B.; Latendresse, J.R.; Ward, G.S.; Ngamprochana, M.; Sahaphong, S.; Bhamarapravati, N. The neurovirulence of flaviviruses in crab-eating monkeys (Macaca fascicularis). S. Asian J. Trop. Med. Public Health 1986, 17, 604–612. [Google Scholar]
- Valentine, M.J.; Murdock, C.C.; Kelly, P.J. Sylvatic cycles of arboviruses in non-human primates. Parasites Vectors 2019, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Hubálek, Z.; Bakonyi, T.; Nowotny, N. Zoonotic mosquito-borne flaviviruses: Worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Veter Microbiol. 2010, 140, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tien, T.; Lundkvist, Å.; Lindahl, J. Urban transmission of mosquito-borne flaviviruses—A review of the risk for humans in Vietnam. Infect. Ecol. Epidemiol. 2019, 9, 1660129. [Google Scholar] [CrossRef]
- Vasilakis, N.; Weaver, S.C. Flavivirus transmission focusing on Zika. Curr. Opin. Virol. 2017, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, T.; Foy, B.D.; Marques, E.T.; Ebel, G.D.; Weger-Lucarelli, J. Mosquito-borne and sexual transmission of Zika virus: Recent developments and future directions. Virus Res. 2018, 254, 1–9. [Google Scholar] [CrossRef]
- Pandit, P.S.; Doyle, M.M.; Smart, K.M.; Young, C.C.W.; Drape, G.W.; Johnson, C.K. Predicting wildlife reservoirs and global vulnerability to zoonotic Flaviviruses. Nat. Commun. 2018, 9, 5425. [Google Scholar] [CrossRef] [PubMed]
- Blahove, M.R.; Carter, J.R. Flavivirus Persistence in Wildlife Populations. Viruses 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Host range specificity of flaviviruses: Correlation with in vitro replication. J. Med. Entomol. 2007, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Migné, C.; Moutailler, S.; Attoui, H. Strategies for Assessing Arbovirus Genetic Variability in Vectors and/or Mammals. Pathogens 2020, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, Y.-P.; Wang, M.; Miao, M.; Zhou, H.; Xu, J.; Kong, J.; Zheng, D.; Li, R.-T.; Zhang, R.-R.; et al. Flavivirus induces and antagonizes antiviral RNA interference in both mammals and mosquitoes. Sci. Adv. 2020, 6, eaax7989. [Google Scholar] [CrossRef] [PubMed]
- Lannes, N.; Garcia-Nicolàs, O.; Démoulins, T.; Summerfield, A.; Filgueira, L. CX3CR1-CX3CL1-dependent cell-to-cell Japanese encephalitis virus transmission by human microglial cells. Sci. Rep. 2019, 9, 4833. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Liu, K.; Anwar, N.; Wahaab, A.; Safdar, A.; Di, D.; Boruah, P.; Xu, J.; Wang, X.; Li, B.; et al. The emerged genotype I of Japanese encephalitis virus shows an infectivity similar to genotype III in Culex pipiens mosquitoes from China. PLoS Negl. Trop. Dis. 2019, 13, e0007716. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Wahaab, A.; Shan, T.; Wang, X.; Khan, S.; Di, D.; Xiqian, L.; Zhang, J.-J.; Anwar, M.N.; Nawaz, M.; et al. A Metagenomic Analysis of Mosquito Virome Collected From Different Animal Farms at Yunnan–Myanmar Border of China. Front. Microbiol. 2021, 11, 591478. [Google Scholar] [CrossRef] [PubMed]
- Daep, C.A.; Muñoz-Jordán, J.L.; Eugenin, E.A. Flaviviruses, an expanding threat in public health: Focus on dengue, West Nile, and Japanese encephalitis virus. J. Neurovirol. 2014, 20, 539–560. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.-J.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the Genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef]
- Leung, J.Y.; Pijlman, G.; Kondratieva, N.; Hyde, J.; Mackenzie, J.M.; Khromykh, A.A. Role of Nonstructural Protein NS2A in Flavivirus Assembly. J. Virol. 2008, 82, 4731–4741. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Bisaillon, M.; Geiss, B.J. Organization of the Flavivirus RNA replicase complex. Wiley Interdiscip. Rev. RNA 2017, 8, e1437. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus Genome Organization, Expression, and Replication. Ann. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Clum, S.; You, S.; Ebner, K.E.; Padmanabhan, R. The Serine Protease and RNA-Stimulated Nucleoside Triphosphatase and RNA Helicase Functional Domains of Dengue Virus Type 2 NS3 Converge within a Region of 20 Amino Acids. J. Virol. 1999, 73, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Utama, A.; Shimizu, H.; Morikawa, S.; Hasebe, F.; Morita, K.; Igarashi, A.; Hatsu, M.; Takamizawa, K.; Miyamura, T. Identification and characterization of the RNA helicase activity of Japanese encephalitis virus NS3 protein. FEBS Lett. 2000, 465, 74–78. [Google Scholar] [CrossRef]
- Preugschat, F.; Yao, C.W.; Strauss, J.H. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J. Virol. 1990, 64, 4364–4374. [Google Scholar] [CrossRef]
- Wengler, G.; Wengler, G. The NS 3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology 1993, 197, 265–273. [Google Scholar] [CrossRef]
- Takegami, T.; Sakamuro, D.; Furukawa, T. Japanese encephalitis virus nonstructural protein NS3 has RNA binding and ATPase activities. Virus Genes 1995, 9, 105–112. [Google Scholar] [CrossRef]
- Wengler, G.; Czaya, G.; Färber, P.M.; Hegemann, J.H. In vitro synthesis of West Nile virus proteins indicates that the amino-terminal segment of the NS3 protein contains the active centre of the protease which cleaves the viral polyprotein after multiple basic amino acids. J. General Virol. 1991, 72 Pt 4, 851–858. [Google Scholar] [CrossRef]
- Warrener, P.; Tamura, J.K.; Collett, M.S. RNA-stimulated NTPase activity associated with yellow fever virus NS3 protein expressed in bacteria. J. Virol. 1993, 67, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Wahaab, A.; Liu, K.; Hameed, M.; Anwar, M.; Kang, L.; Li, C.; Ma, X.; Wajid, A.; Yang, Y.; Khan, U.; et al. Identification of Cleavage Sites Proteolytically Processed by NS2B-NS3 Protease in Polyprotein of Japanese Encephalitis Virus. Pathogens 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, K.J.; Westaway, E.G.; Khromykh, A.A. Expression and purification of enzymatically active recombinant RNA-dependent RNA polymerase (NS5) of the flavivirus Kunjin. J. Virol. Methods 2001, 92, 37–44. [Google Scholar] [CrossRef]
- Tan, B.H.; Fu, J.; Sugrue, R.J.; Yap, E.H.; Chan, Y.C.; Tan, Y.H. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology 1996, 216, 317–325. [Google Scholar] [CrossRef]
- Egloff, M.; Benarroch, D.; Selisko, B.; Romette, J.; Canard, B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: Crystal structure and functional characterization. EMBO J. 2002, 21, 2757–2768. [Google Scholar] [CrossRef] [PubMed]
- Issur, M.; Geiss, B.J.; Bougie, I.; Picard-Jean, F.; Despins, S.; Mayette, J.; Hobdey, S.E.; Bisaillon, M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA 2009, 15, 2340–2350. [Google Scholar] [CrossRef]
- Ray, D.; Shah, A.; Tilgner, M.; Guo, Y.; Zhao, Y.; Dong, H.; Deas, T.S.; Zhou, Y.; Li, H.; Shi, P.-Y. West Nile Virus 5′-Cap Structure Is Formed by Sequential Guanine N-7 and Ribose 2′-O Methylations by Nonstructural Protein 5. J. Virol. 2006, 80, 8362–8370. [Google Scholar] [CrossRef]
- Krishnan, M.N.; Sukumaran, B.; Pal, U.; Agaisse, H.; Murray, J.L.; Hodge, T.W.; Fikrig, E. Rab 5 Is Required for the Cellular Entry of Dengue and West Nile Viruses. J. Virol. 2007, 81, 4881–4885. [Google Scholar] [CrossRef]
- Marianneau, P.; Steffan, A.M.; Royer, C.; Drouet, M.T.; Jaeck, D.; Kirn, A.; Deubel, V. Infection of primary cultures of human Kupffer cells by Dengue virus: No viral progeny synthesis, but cytokine production is evident. J. Virol. 1999, 73, 5201–5206. [Google Scholar] [CrossRef]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef]
- Hackett, B.A.; Cherry, S. Flavivirus internalization is regulated by a size-dependent endocytic pathway. Proc. Natl. Acad. Sci. USA 2018, 115, 4246–4251. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Puerta-Guardo, H.; Biering, S.B.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.N.; Espinosa, D.A.; et al. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef]
- Carro, S.D.; Cherry, S. Beyond the Surface: Endocytosis of Mosquito-Borne Flaviviruses. Viruses 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J. Wrapping Things up about Virus RNA Replication. Traffic 2005, 6, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Westaway, E.G. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 2001, 75, 10787–10799. [Google Scholar] [CrossRef]
- Uchil, P.D.; Satchidanandam, V. Architecture of the flaviviral replication complex. Protease, nuclease, and detergents reveal encasement within double-layered membrane compartments. J. Biol. Chem. 2003, 278, 24388–24398. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis. Microbiol. Mol. Biol. Rev. 2017, 81, e00055-16. [Google Scholar] [CrossRef]
- Wang, P.-G.; Kudelko, M.; Lo, J.; Siu, L.Y.L.; Kwok, K.T.H.; Sachse, M.; Nicholls, J.M.; Bruzzone, R.; Altmeyer, R.M.; Nal, B. Efficient Assembly and Secretion of Recombinant Subviral Particles of the Four Dengue Serotypes Using Native prM and E Proteins. PLoS ONE 2009, 4, e8325. [Google Scholar] [CrossRef]
- Gruba, N.; Rodriguez Martinez, J.I.; Grzywa, R.; Wysocka, M.; Skoreński, M.; Burmistrz, M.; Łęcka, M.; Lesner, A.; Sieńczyk, M.; Pyrć, K. Substrate profiling of Zika virus NS2B-NS3 protease. FEBS Lett. 2016, 590, 3459–3468. [Google Scholar] [CrossRef]
- Luo, D.; Xu, T.; Hunke, C.; Grüber, G.; Vasudevan, S.; Lescar, J. Crystal Structure of the NS3 Protease-Helicase from Dengue Virus. J. Virol. 2008, 82, 173–183. [Google Scholar] [CrossRef]
- Luo, D.; Wei, N.; Doan, D.N.; Paradkar, P.N.; Chong, Y.; Davidson, A.D.; Kotaka, M.; Lescar, J.; Vasudevan, S.G. Flexibility between the Protease and Helicase Domains of the Dengue Virus NS3 Protein Conferred by the Linker Region and Its Functional Implications. J. Biol. Chem. 2010, 285, 18817–18827. [Google Scholar] [CrossRef]
- Benzaghou, I.; Bougie, I.; Picard-Jean, F.; Bisaillon, M. Energetics of RNA binding by the West Nile virus RNA triphosphatase. FEBS Lett. 2006, 580, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xu, T.; Watson, R.P.; Scherer-Becker, D.; Sampath, A.; Jahnke, W.; Yeong, S.S.; Wang, C.H.; Lim, S.P.; Strongin, A.; et al. Insights into RNA unwinding and ATP hydrolysis by the flavivirus NS3 protein. EMBO J. 2008, 27, 3209–3219. [Google Scholar] [CrossRef]
- Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.; Vasudevan, S.G.; Hommel, U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F.; Fletterick, R.J. Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology 1989, 171, 637–639. [Google Scholar] [CrossRef]
- Yotmanee, P.; Rungrotmongkol, T.; Wichapong, K.; Choi, S.B.; Wahab, H.A.; Kungwan, N.; Hannongbua, S. Binding specificity of polypeptide substrates in NS2B/NS3pro serine protease of dengue virus type 2: A molecular dynamics Study. J. Mol. Gr. Model. 2015, 60, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Nestorowicz, A.; Amberg, S.M.; Rice, C.M. Mutagenesis of the yellow fever virus NS2B protein: Effects on proteolytic processing, NS2B-NS3 complex formation, and viral replication. J. Virol. 1993, 67, 6797–6807. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, A.S.; Li, Q.; Kang, C. Expression, purification, and initial structural characterization of nonstructural protein 2B, an integral membrane protein of Dengue-2 virus, in detergent micelles. Protein Expr. Purif. 2011, 80, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.F.; Preugschat, F.; Strauss, J.H. Dengue 2 Virus NS2B and NS3 Form a Stable Complex That Can Cleave NS3 within the Helicase Domain. Virology 1993, 193, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Nestorowicz, A.; Rice, C.M. Mutagenesis of the yellow fever virus NS2B/3 cleavage site: Determinants of cleavage site specificity and effects on polyprotein processing and viral replication. J. Virol. 1995, 69, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Droll, D.A.; Tang, Y.; Liang, Y.; Ganesh, V.K.; Murthy, K.H.M.; Nickells, M. Yellow fever virus NS2B–NS3 protease: Characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J. Gen. Virol. 2005, 86 Pt 5, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Clum, S.; Ebner, K.E.; Padmanabhan, R. Cotranslational Membrane Insertion of the Serine Proteinase Precursor NS2B-NS3(Pro) of Dengue Virus Type 2 Is Required for Efficient in Vitro Processing and Is Mediated through the Hydrophobic Regions of NS2B. J. Biol. Chem. 1997, 272, 30715–30723. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.; Padmanabhan, R. Molecular targets for flavivirus drug discovery. Antivir. Res. 2009, 81, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Liew, O.W.; Chen, G.; Chong, P.C.J.; Lee, S.H.; Chen, K.; Jiang, H.; Puah, C.M.; Zhu, W. Mechanism of NS2B-Mediated Activation of NS3pro in Dengue Virus: Molecular Dynamics Simulations and Bioassays. J. Virol. 2009, 83, 1060–1070. [Google Scholar] [CrossRef][Green Version]
- Falgout, B.; Miller, R.H.; Lai, C.J. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: Identification of a domain required for NS2B-NS3 protease activity. J. Virol. 1993, 67, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.K.; Kuhn, R.J.; Smith, J.L. Functional Characterization of cis and trans Activity of the Flavivirus NS2B-NS3 Protease. J. Biol. Chem. 2007, 282, 12883–12892. [Google Scholar] [CrossRef]
- Aleshin, A.E.; Shiryaev, S.A.; Strongin, A.Y.; Liddington, R.C. Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold. Protein Sci. 2007, 16, 795–806. [Google Scholar] [CrossRef]
- Chambers, T.J.; Grakoui, A.; Rice, C.M. Processing of the yellow fever virus nonstructural polyprotein: A catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J. Virol. 1991, 65, 6042–6050. [Google Scholar] [CrossRef]
- Lin, C.; Amberg, S.M.; Chambers, T.J.; Rice, C.M. Cleavage at a novel site in the NS4A region by the yellow fever virus NS2B-3 proteinase is a prerequisite for processing at the downstream 4A/4B signalase site. J. Virol. 1993, 67, 2327–2335. [Google Scholar] [CrossRef]
- Lobigs, M. Flavivirus premembrane protein cleavage and spike heterodimer secretion require the function of the viral proteinase NS3. Proc. Natl. Acad. Sci. USA 1993, 90, 6218–6222. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Phoo, W.W.; Luo, D. Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol. Sin. 2014, 29, 74–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Westaway, E.G.; Mackenzie, J.M.; Kenney, M.T.; Jones, M.K.; Khromykh, A.A. Ultrastructure of Kunjin virus-infected cells: Colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 1997, 71, 6650–6661. [Google Scholar] [CrossRef] [PubMed]
- Wichapong, K.; Pianwanit, S.; Sippl, W.; Kokpol, S. Homology modeling and molecular dynamics simulations of Dengue virus NS2B/NS3 protease: Insight into molecular interaction. J. Mol. Recognit. 2009, 23, 283–300. [Google Scholar] [CrossRef]
- Junaid, M.; Chalayut, C.; Torrejon, A.S.; Angsuthanasombat, C.; Shutava, I.; Lapins, M.; Wikberg, J.E.S.; Katzenmeier, G. Enzymatic Analysis of Recombinant Japanese Encephalitis Virus NS2B(H)-NS3pro Protease with Fluorogenic Model Peptide Substrates. PLoS ONE 2012, 7, e36872. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Schroder, K.; White, H.; Fang, N.X.; Stoermer, M.J.; Abbenante, G.; Martin, J.L.; Young, P.R.; Fairlie, D.P. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J. Biol. Chem. 2001, 276, 45762–45771. [Google Scholar] [CrossRef]
- Yusof, R.; Clum, S.; Wetzel, M.; Murthy, H.M.K.; Padmanabhan, R. Purified NS2B/NS3 Serine Protease of Dengue Virus Type 2 Exhibits Cofactor NS2B Dependence for Cleavage of Substrates with Dibasic Amino Acids in Vitro. J. Biol. Chem. 2000, 275, 9963–9969. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-bound structures of the dengue virus protease reveal the active conformation. J. Virol. 2012, 86, 438–446. [Google Scholar] [CrossRef]
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991, 65, 2467–2475. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wong, Y.L.; Liew, L.S.Y.; Kang, C. Membrane topology of NS2B of dengue virus revealed by NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2015, 1848 Pt A, 2244–2252. [Google Scholar] [CrossRef]
- Choksupmanee, O.; Hodge, K.; Katzenmeier, G.; Chimnaronk, S. Structural Platform for the Autolytic Activity of an Intact NS2B–NS3 Protease Complex from Dengue Virus. Biochemistry 2012, 51, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Holloway, S.; Schirmeister, T.; Klein, C. Biochemistry and Medicinal Chemistry of the Dengue Virus Protease. Chem. Rev. 2014, 114, 11348–11381. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.G.; Juárez, M.L.; González-Y-Merchand, J.A.; Barrón, L.C.; Castañeda, B.G. Caveolin-1 in Lipid Rafts Interacts with Dengue Virus NS3 during Polyprotein Processing and Replication in HMEC-1 Cells. PLoS ONE 2014, 9, e90704. [Google Scholar]
- Wu, R.-H.; Tsai, M.-H.; Tsai, K.-N.; Ni Tian, J.; Wu, J.-S.; Wu, S.-Y.; Chern, J.-H.; Chen, C.-H.; Yueh, A. Mutagenesis of Dengue Virus Protein NS2A Revealed a Novel Domain Responsible for Virus-Induced Cytopathic Effect and Interactions between NS2A and NS2B Transmembrane Segments. J. Virol. 2017, 91, e01836-16. [Google Scholar] [CrossRef]
- León-Juárez, M.; Martínez-Castillo, M.; Shrivastava, G.; García-Cordero, J.; Villegas-Sepulveda, N.; Mondragón-Castelán, M.; Mondragón-Flores, R.; Cedillo-Barrón, L. Recombinant Dengue virus protein NS2B alters membrane permeability in different membrane models. Virol. J. 2016, 13, 1. [Google Scholar] [CrossRef]
- Niyomrattanakit, P.; Winoyanuwattikun, P.; Chanprapaph, S.; Angsuthanasombat, C.; Panyim, S.; Katzenmeier, G. Identification of residues in the dengue virus type 2 NS2B cofactor that are critical for NS3 protease activation. J. Virol. 2004, 78, 13708–13716. [Google Scholar] [CrossRef]
- Aguirre, S.; Luthra, P.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Patel, J.; Lamothe, F.; Fredericks, A.C.; Tripathi, S.; Zhu, T.; Pintado-Silva, J.; et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017, 2, 17037. [Google Scholar] [CrossRef] [PubMed]
- Falgout, B.; Markoff, L. Evidence that flavivirus NS1-NS2A cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J. Virol. 1995, 69, 7232–7243. [Google Scholar] [CrossRef]
- Amberg, S.M.; Nestorowicz, A.; McCourt, D.W.; Rice, C.M. NS2B-3 proteinase-mediated processing in the yellow fever virus structural region: In vitro and in vivo studies. J. Virol. 1994, 68, 3794–3802. [Google Scholar] [CrossRef]
- Cahour, A.; Falgout, B.; Lai, C.J. Cleavage of the dengue virus polyprotein at the NS3/NS4A and NS4B/NS5 junctions is mediated by viral protease NS2B-NS3, whereas NS4A/NS4B may be processed by a cellular protease. J. Virol. 1992, 66, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Yamshchikov, V.F.; Compans, R.W. Formation of the flavivirus envelope: Role of the viral NS2B-NS3 protease. J. Virol. 1995, 69, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Nestorowicz, A.; Chambers, T.J.; Rice, C.M. Mutagenesis of the yellow fever virus NS2A/2B cleavage site: Effects on proteolytic processing, viral replication, and evidence for alternative processing of the NS2A protein. Virology 1994, 199, 114–123. [Google Scholar] [CrossRef]
- Droll, D.A.; Murthy, H.K.; Chambers, T.J. Yellow Fever Virus NS2B–NS3 Protease: Charged-to-Alanine Mutagenesis and Deletion Analysis Define Regions Important for Protease Complex Formation and Function. Virology 2000, 275, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, K.; Wu, C.; Chen, C.; Hu, C.; Buzovetsky, O.; Wang, Z.; Ji, X.; Xiong, Y.; Yang, H. Mechanisms of activation and inhibition of Zika virus NS2B-NS3 protease. Cell Res. 2016, 26, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Hansen, G.; Nitsche, C.; Klein, C.D.; Zhang, L.; Hilgenfeld, R. Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science 2016, 353, 503–505. [Google Scholar] [CrossRef]
- Lee, H.; Ren, J.; Nocadello, S.; Rice, A.J.; Ojeda, I.; Light, S.; Minasov, G.; Vargas, J.; Nagarathnam, D.; Anderson, W.F.; et al. Identification of novel small molecule inhibitors against NS2B/NS3 serine protease from Zika virus. Antivir. Res. 2017, 139, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.; Kumar, D.; Sharma, N.; Uversky, V.N. Intrinsically Disordered Side of the Zika Virus Proteome. Front. Cell. Infect. Microbiol. 2016, 6, 144. [Google Scholar] [CrossRef]
- Goh, G.K.-M.; Dunker, A.K.; Uversky, V.N. Correlating Flavivirus virulence and levels of intrinsic disorder in shell proteins: Protective roles vs. immune evasion. Mol. BioSyst. 2016, 12, 1881–1891. [Google Scholar] [CrossRef]
- Hameed, M.; Wahaab, A.; Nawaz, M.; Khan, S.; Nazir, J.; Liu, K.; Wei, J.; Ma, Z. Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses 2021, 13, 357. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935–2017. Vector Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef]
- Anwar, N.; Wang, X.; Hameed, M.; Wahaab, A.; Li, C.; Sharma, M.; Pang, L.; Malik, M.I.; Liu, K.; Li, B.; et al. Phenotypic and Genotypic Comparison of a Live-Attenuated Genotype I Japanese Encephalitis Virus SD12-F120 Strain with Its Virulent Parental SD12 Strain. Viruses 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.N.; Jiang, C.; Di, D.; Zhang, J.; Guo, S.; Wang, X.; Hameed, M.; Wahaab, A.; Shao, D.; Li, Z.; et al. A Novel Recombinant Virus-Like Particles Displaying B and T Cell Epitopes of Japanese Encephalitis Virus Offers Protective Immunity in Mice and Guinea Pigs. Vaccines 2021, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.D.; Monaghan, S.; Flint, M. Virus-encoded proteinases of the Flaviviridae. J. Gen. Virol. 1998, 79, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Deng, C.-L.; Ye, H.-Q.; Zhang, H.-L.; Zhang, Q.-Y.; Chen, D.-D.; Zhang, P.-T.; Shi, P.-Y.; Yuan, Z.-M.; Zhang, B. Transmembrane Domains of NS2B Contribute to both Viral RNA Replication and Particle Formation in Japanese Encephalitis Virus. J. Virol. 2016, 90, 5735–5749. [Google Scholar] [CrossRef]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- Liang, J.-J.; Liao, C.-L.; Liao, J.-T.; Lee, Y.-L.; Lin, Y.-L. A Japanese encephalitis virus vaccine candidate strain is attenuated by decreasing its interferon antagonistic ability. Vaccine 2009, 27, 2746–2754. [Google Scholar] [CrossRef]
- Fan, Y.-C.; Liang, J.-J.; Chen, J.-M.; Lin, J.-W.; Chen, Y.-Y.; Su, K.-H.; Lin, C.-C.; Tu, W.-C.; Chiou, M.-T.; Ou, S.-C.; et al. NS2B/NS3 mutations enhance the infectivity of genotype I Japanese encephalitis virus in amplifying hosts. PLoS Pathog. 2019, 15, e1007992. [Google Scholar] [CrossRef]
- Lin, C.-W.; Huang, H.-D.; Shiu, S.-Y.; Chen, W.-J.; Tsai, M.-H.; Huang, S.-H.; Wan, L.; Lin, Y.-J. Functional determinants of NS2B for activation of Japanese encephalitis virus NS3 protease. Virus Res. 2007, 127, 88–94. [Google Scholar] [CrossRef]
- Chappell, K.J.; Stoermer, M.; Fairlie, D.; Young, P.R. Mutagenesis of the West Nile virus NS2B cofactor domain reveals two regions essential for protease activity. J. Gen. Virol. 2008, 89 Pt 4, 1010–1014. [Google Scholar] [CrossRef]
- Zhou, H.; Singh, N.J.; Kim, K.S. Homology modeling and molecular dynamics study of West Nile virus NS3 protease: A molecular basis for the catalytic activity increased by the NS2B cofactor. Proteins 2006, 65, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Chappell, K.J.; Stoermer, M.; Fairlie, D.; Young, P. West Nile Virus NS2B/NS3 Protease As An Antiviral Target. Curr. Med. Chem. 2008, 15, 2771–2784. [Google Scholar] [CrossRef]
- Jia, F.; Fan, J.; Zhang, B.; Yuan, Z. Mutagenesis of D80-82 and G83 residues in West Nile Virus NS2B: Effects on NS2B-NS3 activity and viral replication. Virol. Sin. 2013, 28, 16–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chernov, A.V.; Shiryaev, S.A.; Aleshin, A.E.; Ratnikov, B.I.; Smith, J.W.; Liddington, R.C.; Strongin, A.Y. The Two-component NS2B-NS3 Proteinase Represses DNA Unwinding Activity of the West Nile Virus NS3 Helicase. J. Biol. Chem. 2008, 283, 17270–17278. [Google Scholar] [CrossRef]
- Klema, V.J.; Padmanabhan, R.; Choi, K.H. Flaviviral Replication Complex: Coordination between RNA Synthesis and 5′-RNA Capping. Viruses 2015, 7, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Huang, Z.-S.; Chiang, P.-L.; Chen, C.-T.; Wu, H.-N. Analysis of the nucleoside triphosphatase, RNA triphosphatase, and unwinding activities of the helicase domain of dengue virus NS3 protein. FEBS Lett. 2009, 583, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-C.; Ozawa, K.; Qi, R.; Vasudevan, S.; Lim, S.P.; Otting, G. NMR Analysis of the Dynamic Exchange of the NS2B Cofactor between Open and Closed Conformations of the West Nile Virus NS2B-NS3 Protease. PLoS Negl. Trop. Dis. 2009, 3, e561. [Google Scholar] [CrossRef] [PubMed]
- Robin, G.; Chappell, K.; Stoermer, M.J.; Hu, S.-H.; Young, P.R.; Fairlie, D.P.; Martin, J.L. Structure of West Nile Virus NS3 Protease: Ligand Stabilization of the Catalytic Conformation. J. Mol. Biol. 2009, 385, 1568–1577. [Google Scholar] [CrossRef]
- Nall, T.A.; Chappell, K.J.; Stoermer, M.; Fang, N.-X.; Tyndall, J.; Young, P.; Fairlie, D. Enzymatic Characterization and Homology Model of a Catalytically Active Recombinant West Nile Virus NS3 Protease. J. Biol. Chem. 2004, 279, 48535–48542. [Google Scholar] [CrossRef]
- Wu, C.-F.; Wang, S.-H.; Sun, C.-M.; Hu, S.-T.; Syu, W.-J. Activation of dengue protease autocleavage at the NS2B–NS3 junction by recombinant NS3 and GST–NS2B fusion proteins. J. Virol. Methods 2003, 114, 45–54. [Google Scholar] [CrossRef]
- Pastorino, B.; Peyrefitte, C.N.; Grandadam, M.; Thill, M.C.E.; Tolou, H.J.; Bessaud, M. Mutagenesis analysis of the NS2B determinants of the Alkhurma virus NS2B–NS3 protease activation. J. Gen. Virol. 2006, 87 Pt 11, 3279–3283. [Google Scholar] [CrossRef]
- Yang, T.-C.; Shiu, S.-L.; Chuang, P.-H.; Lin, Y.-J.; Wan, L.; Lan, Y.-C.; Lin, C.-W. Japanese encephalitis virus NS2B-NS3 protease induces caspase 3 activation and mitochondria-mediated apoptosis in human medulloblastoma cells. Virus Res. 2009, 143, 77–85. [Google Scholar] [CrossRef]
- Chappell, K.J.; Stoermer, M.J.; Fairlie, D.P.; Young, P.R. Insights to substrate binding and processing by West Nile Virus NS3 protease through combined modeling, protease mutagenesis, and kinetic studies. J. Biol. Chem. 2006, 281, 38448–38458. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Davis, K.A.; Dowd, K.A.; Akey, D.L.; Smith, J.L.; Pierson, T.C. Context-Dependent Cleavage of the Capsid Protein by the West Nile Virus Protease Modulates the Efficiency of Virus Assembly. J. Virol. 2015, 89, 8632–8642. [Google Scholar] [CrossRef] [PubMed]
- Preugschat, F.; Lenches, E.M.; Strauss, J.H. Flavivirus enzyme-substrate interactions studied with chimeric proteinases: Identification of an intragenic locus important for substrate recognition. J. Virol. 1991, 65, 4749–4758. [Google Scholar] [CrossRef] [PubMed]
- Constant, D.A.; Mateo, R.; Nagamine, C.M.; Kirkegaard, K. Targeting intramolecular proteinase NS2B/3 cleavages for trans-dominant inhibition of dengue virus. Proc. Natl. Acad. Sci. USA 2018, 115, 10136–10141. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.F.; Wright, P.J. Internal proteolysis of the NS3 protein specified by dengue virus 2. J. Gen. Virol. 1997, 78 Pt 2, 337–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiryaev, S.A.; Farhy, C.; Pinto, A.; Huang, C.-T.; Simonetti, N.; Ngono, A.E.; Dewing, A.; Shresta, S.; Pinkerton, A.B.; Cieplak, P.; et al. Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists. Antivir. Res. 2017, 143, 218–229. [Google Scholar] [CrossRef]
- Heaton, N.S.; Perera, R.; Berger, K.L.; Khadka, S.; Lacount, D.J.; Kuhn, R.J.; Randall, G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 17345–17350. [Google Scholar] [CrossRef]
- Tang, W.-C.; Lin, R.-J.; Liao, C.-L.; Lin, Y.-L. Rab18 Facilitates Dengue Virus Infection by Targeting Fatty Acid Synthase to Sites of Viral Replication. J. Virol. 2014, 88, 6793–6804. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Conde, J.N.; Allonso, D.; Ventura, G.T.; Coelho, D.R.; Carneiro, P.H.; Silva, M.L.; Paes, M.V.; Rabelo, K.; Weissmuller, G.; et al. Dengue virus nonstructural 3 protein interacts directly with human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and reduces its glycolytic activity. Sci. Rep. 2019, 9, 2651. [Google Scholar] [CrossRef]
- Bonafé, N.; Gilmore-Hebert, M.; Folk, N.L.; Azodi, M.; Zhou, Y.; Chambers, S.K. Glyceraldehyde-3-Phosphate Dehydrogenase Binds to the AU-Rich 3′ Untranslated Region of Colony-Stimulating Factor–1 (CSF-1) Messenger RNA in Human Ovarian Cancer Cells: Possible Role in CSF-1 Posttranscriptional Regulation and Tumor Phenotype. Cancer Res. 2005, 65, 3762–3771. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Yamaji, R.; Irie, K.; Kioka, N.; Murakami, A. Glyceraldehyde-3-phosphate dehydrogenase regulates cyclooxygenase-2 expression by targeting mRNA stability. Arch. Biochem. Biophys. 2012, 528, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Riedl, W.; Acharya, D.; Lee, J.-H.; Liu, G.; Serman, T.; Chiang, C.; Chan, Y.K.; Diamond, M.S.; Gack, M.U. Zika Virus NS3 Mimics a Cellular 14-3-3-Binding Motif to Antagonize RIG-I- and MDA5-Mediated Innate Immunity. Cell Host Microbe 2019, 26, 493–503.e6. [Google Scholar] [CrossRef] [PubMed]
- Tzivion, G.; Shen, Y.H.; Zhu, J. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 2001, 20, 6331–6338. [Google Scholar] [CrossRef]
- Lennemann, N.J.; Coyne, C.B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 2017, 13, 322–332. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Yuan, L.; Ross, D.; Johansen, A.; Sands, D.; Stanley, V.; Guemez-Gamboa, A.; Gregor, A.; Evans, T.; et al. Zika Virus Protease Cleavage of Host Protein Septin-2 Mediates Mitotic Defects in Neural Progenitors. Neuron 2019, 101, 1089–1098.e4. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ye, W.; Yang, J.; Han, P.; Wang, Y.; Ye, C.; Weng, D.; Zhang, F.; Xu, Z.; Lei, Y. DDX21 translocates from nucleus to cytoplasm and stimulates the innate immune response due to dengue virus infection. Biochem. Biophys. Res. Commun. 2016, 473, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-Q.; Yuan, L.; Zhao, Q.; Yuan, J.-L.; Miao, C.; Chang, Y.-F.; Wen, X.-T.; Wu, R.; Huang, X.-B.; Wen, Y.-P.; et al. Hsp40 Protein DNAJB6 Interacts with Viral NS3 and Inhibits the Replication of the Japanese Encephalitis Virus. Int. J. Mol. Sci. 2019, 20, 5719. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Genet. 2018, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Liang, J.J.; Li, J.K.; Lee, Y.L.; Chang, B.L.; Su, C.I.; Huang, W.J.; Lai, M.M.; Lin, Y.L. Dengue Virus Impairs Mitochondrial Fusion by Cleaving Mitofusins. PLoS Pathog. 2015, 11, e1005350. [Google Scholar] [CrossRef] [PubMed]
- Gandikota, C.; Mohammed, F.; Gandhi, L.; Maisnam, D.; Mattam, U.; Rathore, D.; Chatterjee, A.; Mallick, K.; Billoria, A.; Prasad, V.S.V.; et al. Mitochondrial Import of Dengue Virus NS3 Protease and Cleavage of GrpEL1, a Cochaperone of Mitochondrial Hsp70. J. Virol. 2020, 94, e01178-20. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Breaching the Barrier—The Nuclear Envelope in Virus Infection. J. Mol. Biol. 2016, 428, 1949–1961. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Palacios-Rápalo, S.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; Del Ángel, R.M. The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication. Viruses 2021, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- De Jesús-González, L.A.; Cervantes-Salazar, M.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfán-Morales, C.N.; Palacios-Rápalo, S.N.; Pérez-Olais, J.H.; Cordero-Rivera, C.D.; Hurtado-Monzón, A.M.; Ruíz-Jiménez, F.; et al. The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses. Viruses 2020, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cruz-Cosme, R.; Armstrong, N.; Obwolo, L.A.; Wen, F.; Hu, W.; Luo, M.H.; Tang, Q. Molecular cloning and characterization of the genes encoding the proteins of Zika virus. Gene 2017, 628, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Goellner, S.; Acosta, E.G.; Neufeldt, C.; Oleksiuk, O.; Lampe, M.; Haselmann, U.; Funaya, C.; Schieber, N.; Ronchi, P.; et al. Ultrastructural Characterization of Zika Virus Replication Factories. Cell Rep. 2017, 18, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Cervantes-Salazar, M.; Guillen, A.E.L.; Chávez-Munguía, B.; Salas-Benito, J.S.; Del Ángel, R.M. Strand-like structures and the nonstructural proteins 5, 3 and 1 are present in the nucleus of mosquito cells infected with dengue virus. Virology 2018, 515, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. Nuclear localization of non-structural protein 3 (NS3) during dengue virus infection. Arch. Virol. 2021, 166, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Ratnikov, B.I.; Aleshin, A.E.; Kozlov, I.A.; Nelson, N.A.; Lebl, M.; Smith, J.W.; Liddington, R.C.; Strongin, A.Y. Switching the Substrate Specificity of the Two-Component NS2B-NS3 Flavivirus Proteinase by Structure-Based Mutagenesis. J. Virol. 2007, 81, 4501–4509. [Google Scholar] [CrossRef]
- Jan, L.-R.; Yang, C.-S.; Trent, D.W.; Falgout, B.; Lai, C.-J. Processing of Japanese encephalitis virus non-structural proteins: NS2B-NS3 complex and heterologous proteases. J. Gen. Virol. 1995, 76 Pt 3, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, H.; Mori, C.; Fuke, I.; Morita, K.; Kuhara, S.; Kondou, J.; Kikuchi, Y.; Nagamatu, H.; Igarashi, A. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology 1987, 161, 497–510. [Google Scholar] [CrossRef]
- Rice, C.M.; Lenches, E.M.; Eddy, S.R.; Shin, S.J.; Sheets, R.L.; Strauss, J.H. Nucleotide sequence of yellow fever virus: Implications for flavivirus gene expression and evolution. Science 1985, 229, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G.; Castle, E.; Leidner, U.; Nowak, T.; Wengler, G. Sequence analysis of the membrane protein V3 of the flavivirus west nile virus and of its gene. Virology 1985, 147, 264–274. [Google Scholar] [CrossRef]
- Castle, E.; Leidner, U.; Nowak, T.; Wengler, G.; Wengler, G. Primary structure of the West Nile flavivirus genome region coding for all nonstructural proteins. Virology 1986, 149, 10–26. [Google Scholar] [CrossRef]
- Wengler, G.; Wengler, G. Cell-associated West Nile flavivirus is covered with E+pre-M protein heterodimers which are destroyed and reorganized by proteolytic cleavage during virus release. J. Virol. 1989, 63, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Mundt, E.; Muller, H. Complete Nucleotide Sequences of 5′- and 3′-Noncoding Regions of Both Genome Segments of Different Strains of Infectious Bursal Disease Virus. Virology 1995, 209, 10–18. [Google Scholar] [CrossRef]
- Morazzani, E.M.; Compton, J.R.; Leary, D.H.; Berry, A.V.; Hu, X.; Marugan, J.J.; Glass, P.J.; Legler, P.M. Proteolytic cleavage of host proteins by the Group IV viral proteases of Venezuelan equine encephalitis virus and Zika virus. Antivir. Res. 2019, 164, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.W.; McAda, P.C.; Mason, T.L.; Fournier, M.J. Sequence of the dengue-1 virus genome in the region encoding the three structural proteins and the major nonstructural protein NS1. Virology 1987, 161, 262–267. [Google Scholar] [CrossRef]
- Hahn, Y.S.; Caller, R.; Hunkapiller, T.; Dalrymple, J.M.; Strauss, J.H.; Strauss, E.G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology 1988, 162, 167–180. [Google Scholar] [CrossRef]
- Osatomi, K.; Fuke, I.; Tsuru, D.; Shiba, T.; Sakaki, Y.; Sumiyoshi, H. Nucleotide sequence of dengue type 3 virus genomic RNA encoding viral structural proteins. Virus Genes 1988, 2, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mackow, E.; Makino, Y.; Zhao, B.T.; Zhang, Y.M.; Markoff, L.; Buckler-White, A.; Guiler, M.; Chanock, R.; Lai, C.J. The nucleotide sequence of dengue type 4 virus: Analysis of genes coding for nonstructural proteins. Virology 1987, 159, 217–228. [Google Scholar] [CrossRef]
- Zhao, B.; Mackow, E.; Buckler-White, A.; Markoff, L.; Chanock, R.M.; Lai, C.-J.; Making, Y. Cloning full-length dengue type 4 viral DNA sequences: Analysis of genes coding for structural proteins. Virology 1986, 155, 77–88. [Google Scholar] [CrossRef]
- Mueller, N.H.; Yon, C.; Ganesh, V.K.; Padmanabhan, R. Characterization of the West Nile virus protease substrate specificity and inhibitors. Int. J. Biochem. Cell Biol. 2007, 39, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Phoo, W.W.; Loh, Y.R.; Wang, W.; Liu, S.; Chen, M.W.; Hung, A.W.; Keller, T.H.; Luo, D.; et al. Structural Dynamics of Zika Virus NS2B-NS3 Protease Binding to Dipeptide Inhibitors. Structure 2017, 25, 1242–1250.e3. [Google Scholar] [CrossRef] [PubMed]
- Phoo, W.W.; Li, Y.; Zhang, Z.; Lee, M.Y.; Loh, Y.R.; Tan, Y.B.; Ng, E.Y.; Lescar, J.; Kang, C.; Luo, D. Structure of the NS2B-NS3 protease from Zika virus after self-cleavage. Nat. Commun. 2016, 7, 13410. [Google Scholar] [CrossRef]
- Kondo, M.Y.; Oliveira, L.C.; Okamoto, D.N.; De Araujo, M.R.; Dos Santos, C.N.D.; Juliano, M.A.; Juliano, L.; Gouvea, I.E. Yellow fever virus NS2B/NS3 protease: Hydrolytic Properties and Substrate Specificity. Biochem. Biophys. Res. Commun. 2011, 407, 640–644. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Wang, Q.-Y.; Noble, C.G.; Chen, Y.-L.; Dong, H.; Zou, B.; Yokokawa, F.; Nilar, S.; Smith, P.; Beer, D.; et al. Ten years of dengue drug discovery: Progress and prospects. Antivir. Res. 2013, 100, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Niyomrattanakit, P.; Yahorava, S.; Mutule, I.; Mutulis, F.; Petrovska, R.; Prusis, P.; Katzenmeier, G.; Wikberg, J.E.S. Probing the substrate specificity of the dengue virus type 2 NS3 serine protease by using internally quenched fluorescent peptides. Biochem. J. 2006, 397, 203–211. [Google Scholar] [CrossRef]
- Yin, Z.; Patel, S.J.; Wang, W.-L.; Chan, W.-L.; Rao, K.R.; Wang, G.; Ngew, X.; Patel, V.; Beer, D.; Knox, J.E.; et al. Peptide inhibitors of dengue virus NS3 protease. Part 2: SAR study of tetrapeptide aldehyde inhibitors. Bioorganic Med. Chem. Lett. 2006, 16, 40–43. [Google Scholar] [CrossRef]
- Adamek, R.N.; Maniquis, R.V.; Khakoo, S.; Bridges, M.D.; Salzameda, N.T. A FRET-based assay for the discovery of West Nile Virus NS2B-NS3 protease inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 4848–4850. [Google Scholar] [CrossRef] [PubMed]
- Behnam, M.A.M.; Graf, D.; Bartenschlager, R.; Zlotos, D.P.; Klein, C.D. Discovery of Nanomolar Dengue and West Nile Virus Protease Inhibitors Containing a 4-Benzyloxyphenylglycine Residue. J. Med. Chem. 2015, 58, 9354–9370. [Google Scholar] [CrossRef]
- Nitsche, C.; Zhang, L.; Weigel, L.F.; Schilz, J.; Graf, D.; Bartenschlager, R.; Hilgenfeld, R.; Klein, C.D. Peptide–Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. J. Med. Chem. 2017, 60, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Schüller, A.; Yin, Z.; Brian Chia, C.S.; Doan, D.N.; Kim, H.K.; Shang, L.; Loh, T.P.; Hill, J.; Vasudevan, S.G. Tripeptide inhibitors of dengue and West Nile virus NS2B-NS3 protease. Antivir. Res. 2011, 92, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Ratnikov, B.I.; Chekanov, A.V.; Sikora, S.; Rozanov, D.V.; Godzik, A.; Wang, J.; Smith, J.W.; Huang, Z.; Lindberg, I.; et al. Cleavage targets and the D-arginine-based inhibitors of the West Nile virus NS3 processing proteinase. Biochem. J. 2006, 393 Pt 2, 503–511. [Google Scholar] [CrossRef]
- Stoermer, M.J.; Chappell, K.J.; Liebscher, S.; Jensen, C.M.; Gan, C.H.; Gupta, P.K.; Xu, W.J.; Young, P.R.; Fairlie, D.P. Potent cationic inhibitors of West Nile virus NS2B/NS3 protease with serum stability, cell permeability and antiviral activity. J. Med. Chem. 2008, 51, 5714–5721. [Google Scholar] [CrossRef]
- Knox, J.E.; Ma, N.L.; Yin, Z.; Patel, S.J.; Wang, W.-L.; Chan, W.-L.; Rao, K.R.R.; Wang, G.; Ngew, X.; Patel, V.; et al. Peptide Inhibitors of West Nile NS3 Protease: SAR Study of Tetrapeptide Aldehyde Inhibitors. J. Med. Chem. 2006, 49, 6585–6590. [Google Scholar] [CrossRef]
- Behnam, M.; Nitsche, C.; Vechi, S.M.; Klein, C.D. C-Terminal Residue Optimization and Fragment Merging: Discovery of a Potent Peptide-Hybrid Inhibitor of Dengue Protease. ACS Med. Chem. Lett. 2014, 5, 1037–1042. [Google Scholar] [CrossRef]
- Bastos Lima, A.; Behnam, M.A.; El Sherif, Y.; Nitsche, C.; Vechi, S.M.; Klein, C.D. Dual inhibitors of the dengue and West Nile virus NS2B-NS3 proteases: Synthesis, biological evaluation and docking studies of novel peptide-hybrids. Bioorg. Med. Chem. 2015, 23, 5748–5755. [Google Scholar] [CrossRef]
- Jia, F.; Zou, G.; Fan, J.; Yuan, Z. Identification of palmatine as an inhibitor of West Nile virus. Arch. Virol. 2010, 155, 1325–1329. [Google Scholar] [CrossRef]
- Cregar-Hernandez, L.; Jiao, G.-S.; Johnson, A.T.; Lehrer, A.T.; Wong, T.A.S.; Margosiak, S.A. Small Molecule Pan-Dengue and West Nile Virus NS3 Protease Inhibitors. Antivir. Chem. Chemother. 2011, 21, 209–217. [Google Scholar] [CrossRef]
- Kouretova, J.; Hammamy, M.Z.; Epp, A.; Hardes, K.; Kallis, S.; Zhang, L.; Hilgenfeld, R.; Bartenschlager, R.; Steinmetzer, T. Effects of NS2B-NS3 protease and furin inhibition on West Nile and Dengue virus replication. J. Enzym. Inhib. Med. Chem. 2017, 32, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Schreier, V.N.; Behnam, M.A.M.; Kumar, A.; Bartenschlager, R.; Klein, C.D. Thiazolidinone–Peptide Hybrids as Dengue Virus Protease Inhibitors with Antiviral Activity in Cell Culture. J. Med. Chem. 2013, 56, 8389–8403. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Mohamed, Z.; Suhaeb, A.M.; Rahman, N.A.; Yusof, R. Antiviral Cationic Peptides as a Strategy for Innovation in Global Health Therapeutics for Dengue Virus: High Yield Production of the Biologically Active Recombinant Plectasin Peptide. OMICS J. Integr. Biol. 2013, 17, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.F.; Nitsche, C.; Graf, D.; Bartenschlager, R.; Klein, C.D. Phenylalanine and Phenylglycine Analogues as Arginine Mimetics in Dengue Protease Inhibitors. J. Med. Chem. 2015, 58, 7719–7733. [Google Scholar] [CrossRef]
- Tambunan, U.S.F.; Alamudi, S. Designing cyclic peptide inhibitor of dengue virus NS3-NS2B protease by using molecular docking approach. Bioinformation 2010, 5, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hsieh, Y.C.; Lee, S.J.; Wu, S.H.; Liao, C.L.; Tsao, C.H.; Chao, Y.S.; Chern, J.H.; Wu, C.P.; Yueh, A. Novel dengue virus-specific NS2B/NS3 protease inhibitor, BP2109, discovered by a high-throughput screening assay. Antimicrob. Agents Chemother. 2011, 55, 229–238. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hu, H.-S.; Wu, R.-H.; Wu, S.-H.; Lee, S.-J.; Jiaang, W.-T.; Chern, J.-H.; Huang, Z.-S.; Wu, H.-N.; Chang, C.-M.; et al. A Novel Dengue Virus Inhibitor, BP13944, Discovered by High-Throughput Screening with Dengue Virus Replicon Cells Selects for Resistance in the Viral NS2B/NS3 Protease. Antimicrob. Agents Chemother. 2014, 58, 110–119. [Google Scholar] [CrossRef]
- Beesetti, H.; Tyagi, P.; Medapi, B.; Krishna, V.S.; Sriram, D.; Khanna, N.; Swaminathan, S. A quinoline compound inhibits the replication of dengue virus serotypes 1–4 in Vero cells. Antivir. Ther. 2018, 23, 385–394. [Google Scholar] [CrossRef]
- Aravapalli, S.; Lai, H.; Teramoto, T.; Alliston, K.R.; Lushington, G.H.; Ferguson, E.L.; Padmanabhan, R.; Groutas, W.C. Inhibitors of Dengue virus and West Nile virus proteases based on the aminobenzamide scaffold. Bioorg. Med. Chem. 2012, 20, 4140–4148. [Google Scholar] [CrossRef]
- Nie, S.; Yao, Y.; Wu, F.; Wu, X.; Zhao, J.; Hua, Y.; Wu, J.; Huo, T.; Lin, Y.L.; Kneubehl, A.R.; et al. Synthesis, Structure-Activity Relationships, and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J. Med. Chem. 2021, 64, 2777–2800. [Google Scholar] [CrossRef]
- Rothan, H.A.; Abdulrahman, A.Y.; Sasikumar, P.G.; Othman, S.; Rahman, N.A.; Yusof, R. Protegrin-1 Inhibits Dengue NS2B-NS3 Serine Protease and Viral Replication in MK2 Cells. J. Biomed. Biotechnol. 2012, 2012, 251482. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Han, H.C.; Ramasamy, T.S.; Othman, S.; Rahman, N.A.; Yusof, R. Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect. Dis. 2012, 12, 314. [Google Scholar] [CrossRef]
- Kiat, T.S.; Pippen, R.; Yusof, R.; Ibrahim, H.; Khalid, N.; Rahman, N.A. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg. Med. Chem. Lett. 2006, 16, 3337–3340. [Google Scholar] [CrossRef]
- Tomlinson, S.M.; Watowich, S.J. Use of parallel validation high-throughput screens to reduce false positives and identify novel dengue NS2B-NS3 protease inhibitors. Antivir. Res. 2012, 93, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Behnam, M.; Steuer, C.; Klein, C.D. Retro peptide-hybrids as selective inhibitors of the Dengue virus NS2B-NS3 protease. Antivir. Res. 2012, 94, 72–79. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Chik, K.K.-H.; Yuan, S.; Yip, C.C.-Y.; Zhu, Z.; Tee, K.-M.; Tsang, J.O.-L.; Chan, C.C.-S.; Poon, V.K.-M.; Lu, G.; et al. Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antivir. Res. 2017, 141, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chan, J.F.; den-Haan, H.; Chik, K.K.; Zhang, A.J.; Chan, C.C.; Poon, V.K.; Yip, C.C.; Mak, W.W.; Zhu, Z.; et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antivir. Res. 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Liang, B.; Aarthy, M.; Singh, S.K.; Garg, N.; Mysorekar, I.U.; Giri, R. Hydroxychloroquine Inhibits Zika Virus NS2B-NS3 Protease. ACS Omega 2018, 3, 18132–18141. [Google Scholar] [CrossRef]
- Li, Z.; Sakamuru, S.; Huang, R.; Brecher, M.; Koetzner, C.A.; Zhang, J.; Chen, H.; Qin, C.-F.; Zhang, Q.-Y.; Zhou, J.; et al. Erythrosin B is a potent and broad-spectrum orthosteric inhibitor of the flavivirus NS2B-NS3 protease. Antivir. Res. 2017, 150, 217–225. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, R.; Huang, C.; Zhang, R.; Wang, J.; Zhang, Y.; Ding, J.; Li, X.; Zhou, J.; Cen, S. Identification of Theaflavin-3,3’-Digallate as a Novel Zika Virus Protease Inhibitor. Front. Pharmacol. 2020, 11, 514313. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, D.; Chinthakindi, P.K.; Båhlström, A.; Palanisamy, N.; Sandström, A.; Lundkvist, Å.; Lennerstrand, J. Identification of a C2-symmetric diol based human immunodeficiency virus protease inhibitor targeting Zika virus NS2B-NS3 protease. J. Biomol. Struct. Dyn. 2020, 38, 5526–5536. [Google Scholar] [CrossRef] [PubMed]
- Brecher, M.; Li, Z.; Liu, B.; Zhang, J.; Koetzner, C.A.; Alifarag, A.; Jones, S.A.; Lin, Q.; Kramer, L.D.; Li, H. A conformational switch high-throughput screening assay and allosteric inhibition of the flavivirus NS2B-NS3 protease. PLoS Pathog. 2017, 13, e1006411. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kimura-Kuroda, J.; Hirabayashi, Y.; Yasui, K. Development of a novel mouse model for dengue virus infection. Virology 1999, 263, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bente, D.A.; Melkus, M.W.; Garcia, J.V.; Rico-Hesse, R. Dengue Fever in Humanized NOD/SCID Mice. J. Virol. 2005, 79, 13797–13799. [Google Scholar] [CrossRef]
- Cox, J.; Mota, J.; Sukupolvi-Petty, S.; Diamond, M.S.; Rico-Hesse, R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012, 86, 7637–7649. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Pearson, T.; Friberg, H.; Shultz, L.D.; Greiner, D.L.; Rothman, A.L.; Mathew, A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS ONE 2009, 4, e7251. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Pazoles, P.; Woda, M.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; Mathew, A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology 2012, 136, 334–343. [Google Scholar] [CrossRef]
- Kuruvilla, J.G.; Troyer, R.M.; Devi, S.; Akkina, R. Dengue virus infection and immune response in humanized RAG2(-/-)gamma(c)(-/-) (RAG-hu) mice. Virology 2007, 369, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Liao, C.-L.; Chen, L.-K.; Yeh, C.-T.; Liu, C.-I.; Ma, S.-H.; Huang, Y.-Y.; Huang, Y.-L.; Kao, C.-L.; King, C.-C. Study of Dengue Virus Infection in SCID Mice Engrafted with Human K562 Cells. J. Virol. 1998, 72, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Rico-Hesse, R. Humanized Mice Show Clinical Signs of Dengue Fever according to Infecting Virus Genotype. J. Virol. 2009, 83, 8638–8645. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Chen, Q.; Tang, K.F.; Ooi, E.E.; Hibberd, M.L.; Chen, J. Inhibition of Megakaryocyte Development in the Bone Marrow Underlies Dengue Virus-Induced Thrombocytopenia in Humanized Mice. J. Virol. 2013, 87, 11648–11658. [Google Scholar] [CrossRef] [PubMed]
- Alves dos Santos, E.; Fink, K. Animal Models for Dengue and Zika Vaccine Development. In Dengue and Zika: Control and Antiviral Treatment Strategies; Hilgenfeld, R., Vasudevan, S.G., Eds.; Springer: Singapore, 2018; pp. 215–239. [Google Scholar]
- Coronel-Ruiz, C.; Gutiérrez-Barbosa, H.; Medina-Moreno, S.; Velandia-Romero, M.L.; Chua, J.V.; Castellanos, J.E.; Zapata, J.C. Humanized Mice in Dengue Research: A Comparison with Other Mouse Models. Vaccines 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Gaska, J.M.; Douam, F.; Wei, L.; Kim, D.; Balev, M.; Heller, B.; Ploss, A. Species-specific disruption of STING-dependent antiviral cellular defenses by the Zika virus NS2B3 protease. Proc. Natl. Acad. Sci. USA 2018, 115, E6310–E6318. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Vance, R.E. STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 2013, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef]
- Zhong, B.; Yang, Y.; Li, S.; Wang, Y.-Y.; Li, Y.; Diao, F.; Lei, C.; He, X.; Zhang, L.; Tien, P.; et al. The Adaptor Protein MITA Links Virus-Sensing Receptors to IRF3 Transcription Factor Activation. Immunity 2008, 29, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, B.; Bao, M.; Lu, N.; Kim, T.; Liu, Y.-J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011, 12, 959–965. [Google Scholar] [CrossRef]
- Holm, C.K.; Rahbek, S.H.; Gad, H.H.; Bak, R.O.; Jakobsen, M.R.; Jiang, Z.; Hansen, A.L.; Jensen, S.K.; Sun, C.; Thomsen, M.K.; et al. Influenza A virus targets a cGAS-independent STING pathway that controls enveloped RNA viruses. Nature Commun. 2016, 7, 10680. [Google Scholar] [CrossRef]
- Aguirre, S.; Maestre, A.M.; Pagni, S.; Patel, J.R.; Savage, T.; Gutman, D.; Maringer, K.; Bernal-Rubio, D.; Shabman, R.S.; Simon, V.; et al. DENV Inhibits Type I IFN Production in Infected Cells by Cleaving Human STING. PLoS Pathog. 2012, 8, e1002934. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.; Sakamoto, N.; Nakagawa, M.; Kakinuma, S.; Mishima, K.; Kusano-Kitazume, A.; Kiyohashi, K.; Murakawa, M.; Nishimura-Sakurai, Y.; Azuma, S.; et al. Hepatitis C virus NS4B protein targets STING and abrogates RIG-I-mediated type I interferon-dependent innate immunity. Hepatology 2013, 57, 46–58. [Google Scholar] [CrossRef]
- Sun, L.; Xing, Y.; Chen, X.; Zheng, Y.; Yang, Y.; Nichols, D.B.; Clementz, M.A.; Banach, B.S.; Li, K.; Baker, S.C.; et al. Coronavirus Papain-like Proteases Negatively Regulate Antiviral Innate Immune Response through Disruption of STING-Mediated Signaling. PLoS ONE 2012, 7, e30802. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Chang, T.-H.; Liang, J.-J.; Chiang, R.-L.; Lee, Y.-L.; Liao, C.-L.; Lin, Y.-L. Dengue Virus Targets the Adaptor Protein MITA to Subvert Host Innate Immunity. PLoS Pathog. 2012, 8, e1002780. [Google Scholar] [CrossRef] [PubMed]
- Stabell, A.C.; Meyerson, N.R.; Gullberg, R.C.; Gilchrist, A.R.; Webb, K.J.; Old, W.M.; Perera, R.; Sawyer, S.L. Dengue viruses cleave STING in humans but not in nonhuman primates, their presumed natural reservoir. eLife 2018, 7, e31919. [Google Scholar] [CrossRef]
- Ashour, J.; Morrison, J.; Laurent-Rolle, M.; Belicha-Villanueva, A.; Plumlee, C.R.; Bernal-Rubio, D.; Williams, K.L.; Harris, E.; Fernandez-Sesma, A.; Schindler, C.; et al. Mouse STAT2 Restricts Early Dengue Virus Replication. Cell Host Microbe 2010, 8, 410–421. [Google Scholar] [CrossRef]
- Jones, M.; Davidson, A.; Hibbert, L.; Gruenwald, P.; Schlaak, J.; Ball, S.; Foster, G.R.; Jacobs, M. Dengue Virus Inhibits Alpha Interferon Signaling by Reducing STAT2 Expression. J. Virol. 2005, 79, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Jones, M.; Davidson, A.; Chain, B.; Jacobs, M. Dengue Virus NS5 Inhibits Interferon-α Signaling by Blocking Signal Transducer and Activator of Transcription 2 Phosphorylation. J. Infect. Dis. 2009, 200, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Aguirre, S.; Fernandez-Sesma, A. Innate Immunity Evasion by Dengue Virus. Viruses 2012, 4, 397–413. [Google Scholar] [CrossRef]
- Zompi, S.; Harris, E. Animal Models of Dengue Virus Infection. Viruses 2012, 4, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Putnak, R.; Barvir, D.A.; Burrous, J.M.; Dubois, D.R.; D’Andrea, V.M.; Hoke, C.H.; Sadoff, J.C.; Eckels, K.H. Development of a Purified, Inactivated, Dengue-2 Virus Vaccine Prototype in Vero Cells: Immunogenicity and Protection in Mice and Rhesus Monkeys. J. Infect. Dis. 1996, 174, 1176–1184. [Google Scholar] [CrossRef]
- Men, R.; Wyatt, L.; Tokimatsu, I.; Arakaki, S.; Shameem, G.; Elkins, R.; Chanock, R.; Moss, B.; Lai, C.-J. Immunization of rhesus monkeys with a recombinant of modified vaccinia virus Ankara expressing a truncated envelope glycoprotein of dengue type 2 virus induced resistance to dengue type 2 virus challenge. Vaccine 2000, 18, 3113–3122. [Google Scholar] [CrossRef]
- Putnak, R.; Fuller, J.; VanderZanden, L.; Innis, B.L.; Vaughn, D.W. Vaccination of rhesus macaques against dengue-2 virus with a plasmid DNA vaccine encoding the viral pre-membrane and envelope genes. Am. J. Trop. Med. Hyg. 2003, 68, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Raviprakash, K.; Porter, K.R.; Kochel, T.J.; Ewing, D.; Simmons, M.; Phillips, I.; Murphy, G.S.; Weiss, W.R.; Hayes, C.G. Dengue virus type 1 DNA vaccine induces protective immune responses in rhesus macaques. Microbiology 2000, 81 Pt 7, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Raviprakash, K.; Apt, D.; Brinkman, A.; Skinner, C.; Yang, S.; Dawes, G.; Ewing, D.; Wu, S.-J.; Bass, S.; Punnonen, J.; et al. A chimeric tetravalent dengue DNA vaccine elicits neutralizing antibody to all four virus serotypes in rhesus macaques. Virology 2006, 353, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Raviprakash, K.; Wang, D.; Ewing, D.; Holman, D.H.; Block, K.; Woraratanadharm, J.; Chen, L.; Hayes, C.; Dong, J.Y.; Porter, K. A Tetravalent Dengue Vaccine Based on a Complex Adenovirus Vector Provides Significant Protection in Rhesus Monkeys against All Four Serotypes of Dengue Virus. J. Virol. 2008, 82, 6927–6934. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Zhang, X.; Cai, H.; Niewiesk, S.; Li, J. Rational Design of Human Metapneumovirus Live Attenuated Vaccine Candidates by Inhibiting Viral mRNA Cap Methyltransferase. J. Virol. 2014, 88, 11411–11429. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Thomas, S.J.; De La Barrera, R.; Im-Erbsin, R.; Jarman, R.G.; Baras, B.; Toussaint, J.-F.; Mossman, S.; Innis, B.; Schmidt, A.; et al. An Adjuvanted, Tetravalent Dengue Virus Purified Inactivated Vaccine Candidate Induces Long-Lasting and Protective Antibody Responses Against Dengue Challenge in Rhesus Macaques. Am. J. Trop. Med. Hyg. 2015, 92, 698–708. [Google Scholar] [CrossRef]
- McBurney, S.P.; Sunshine, J.E.; Gabriel, S.; Huynh, J.P.; Sutton, W.F.; Fuller, D.; Haigwood, N.; Messer, W.B. Evaluation of protection induced by a dengue virus serotype 2 envelope domain III protein scaffold/DNA vaccine in non-human primates. Vaccine 2016, 34, 3500–3507. [Google Scholar] [CrossRef]
- Velzing, J.; Groen, J.; Drouet, M.T.; van Amerongen, G.; Copra, C.; Osterhaus, A.D.; Deubel, V. Induction of protective immunity against Dengue virus type 2: Comparison of candidate live attenuated and recombinant vaccines. Vaccine 1999, 17, 1312–1320. [Google Scholar] [CrossRef]
- Butrapet, S.; Rabablert, J.; Angsubhakorn, S.; Wiriyarat, W.; Huang, C.; Kinney, R.; Punyim, S.; Bhamarapravati, N. Chimeric dengue type 2/type 1 viruses induce immune responses in cynomolgus monkeys. S. Asian J. Trop. Med. Public Health 2002, 33, 589–599. [Google Scholar]
- Hermida, L.; Bernardo, L.; Martín, J.; Alvarez, M.; Prado, I.; López, C.; Sierra, B.D.L.C.; Martínez, R.; Rodríguez, R.; Zulueta, A. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 2006, 24, 3165–3171. [Google Scholar] [CrossRef]
- Izquierdo, A.; Bernardo, L.; Martin, J.; Santana, E.; Hermida, L.; Guillén, G.; Guzmán, M.G. Serotype-specificity of recombinant fusion proteins containing domain III of dengue virus. Virus Res. 2008, 138, 135–138. [Google Scholar] [CrossRef]
- Osorio, J.E.; Brewoo, J.N.; Powell, T.D.; Arguello, J.; Huang, C.Y.-H.; Kinney, R.M.; Tary-Lehmann, M.; Silengo, S.J.; Livengood, J.A.; Moldovan, I.R.; et al. Efficacy of a Tetravalent Chimeric Dengue Vaccine (DENVax) in Cynomolgus Macaques. Am. J. Trop. Med. Hyg. 2011, 84, 978–987. [Google Scholar] [CrossRef]
- Strouts, F.R.; Popper, S.J.; Partidos, C.D.; Stinchcomb, D.T.; Osorio, J.E.; Relman, D.A. Early Transcriptional Signatures of the Immune Response to a Live Attenuated Tetravalent Dengue Vaccine Candidate in Non-human Primates. PLoS Negl. Trop. Dis. 2016, 10, e0004731. [Google Scholar] [CrossRef] [PubMed]
- Suphatrakul, A.; Yasanga, T.; Keelapang, P.; Sriburi, R.; Roytrakul, T.; Pulmanausahakul, R.; Utaipat, U.; Kawilapan, Y.; Puttikhunt, C.; Kasinrerk, W.; et al. Generation and preclinical immunogenicity study of dengue type 2 virus-like particles derived from stably transfected mosquito cells. Vaccine 2015, 33, 5613–5622. [Google Scholar] [CrossRef]
- Huang, K.-J.; Li, S.-Y.J.; Chen, S.-C.; Liu, H.-S.; Lin, Y.-S.; Yeh, T.-M.; Liu, C.-C.; Lei, H.-Y. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J. Gen. Virol. 2000, 81 Pt 9, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Shresta, S.; Kyle, J.L.; Snider, H.M.; Basavapatna, M.; Beatty, P.R.; Harris, E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 2004, 78, 2701–2710. [Google Scholar] [CrossRef]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Beatty, P.R.; Harris, E. Murine Model for Dengue Virus-Induced Lethal Disease with IncreasedVascular Permeability. J. Virol. 2006, 80, 10208–10217. [Google Scholar] [CrossRef]
- Schul, W.; Liu, W.; Xu, H.; Flamand, M.; Vasudevan, S. A Dengue Fever Viremia Model in Mice Shows Reduction in Viral Replication and Suppression of the Inflammatory Response after Treatment with Antiviral Drugs. J. Infect. Dis. 2007, 195, 665–674. [Google Scholar] [CrossRef]
- Orozco, S.; Schmid, M.A.; Parameswaran, P.; Lachica, R.; Henn, M.R.; Beatty, R.; Harris, E. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 2012, 93 Pt 10, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.T.; Prestwood, T.R.; Lada, S.M.; Benedict, C.A.; Shresta, S. Cardif-mediated signaling controls the initial innate response to dengue virus in vivo. J. Virol. 2009, 83, 8276–8281. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.T.; Buck, M.; Lada, S.M.; Schindler, C.; Shresta, S. STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor. PLoS Pathog. 2011, 7, e1001297. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Pugachev, K.; Zhang, Z.; Myers, G.; Levenbook, I.; Draper, K.; Lang, J.; Ocran, S.; Mitchell, F.; Parsons, M.; et al. Safety and Efficacy of Chimeric Yellow Fever-Dengue Virus Tetravalent Vaccine Formulations in Nonhuman Primates. J. Virol. 2004, 78, 4761–4775. [Google Scholar] [CrossRef] [PubMed]
- Guirakhoo, F.; Zhang, Z.; Myers, G.; Johnson, B.W.; Pugachev, K.; Nichols, R.; Brown, N.; Levenbook, I.; Draper, K.; Cyrek, S.; et al. A single amino acid substitution in the envelope protein of chimeric yellow fever-dengue 1 vaccine virus reduces neurovirulence for suckling mice and viremia/viscerotropism for monkeys. J. Virol. 2004, 78, 9998–10008. [Google Scholar] [CrossRef] [PubMed]


| Flavivirus | Cleavage/Substrate Sites | Reference | ||||
|---|---|---|---|---|---|---|
| Capsid C | NS2A/NS2B | NS2B/NS3 | NS3/NS4A | NS4B/NS5 | ||
| JEV | VNKRGRKQNKRJ ↓GGNEGS | NPNKKR ↓GWPATE | LKTTKR ↓GGVFWD | FAAGKR ↓SAISFI | KPSLKR ↓GRPGGR | [151] |
| NKRGRKQNKR ↓GGNEGSIMWL | GLMVCNPNKKR ↓GWPAT EFLSA | GYWLTLKTTKR ↓GGVFWDTPSP | WFKDFAAGKR ↓SAVSFIEVLG | - | [32] | |
| YFV | LSSRKRR ↓SHDVLT | RIFGRR ↓SIPVNE | VRGARR ↓SGDVLM | FAEGRR ↓GAAEVL | MKTGRR ↓GSANGK | [152] |
| WNV | INBBSTKQKKS ↓GGTAGF | OPNRKR ↓GWPATE | LQYTKR ↓GGVLWD | FASGKR ↓SQIGLV | KPGLKR ↓GGAKGR | [153,154,155,156] |
| - | DPNRKR ↓GW | LQYTKR ↓GG | FASGKR ↓SQ | KPGLKR ↓GG | [122] | |
| ZIKV | KERKRR ↓GADTSIGI | TRSGKR ↓SWPPSEVL | VKTGKR ↓SGALWDVP | FAAGKR ↓GAALGVME | GLVKRR ↓GGGTGETL | [127,157] |
| DENV1 | MNRRKR ↓SVTMLL | - | - | - | - | [158] |
| DENV2 | LNRRRR ↓TAGMII | RTSKKR ↓SWPLNE | EVKKQR ↓AGVLWD | FAAGRK ↓SLTLNL | - | [159] |
| DENV3 | INKRKK ↓TSLCLM | - | - | - | - | [160] |
| DENV4 | LNGRKR ↓STITLL | KGASRR ↓SWPLNE | QVKTQR ↓SGALWD | FASGRK ↓SITLDI | AQTPRR ↓GTGTTG | [161,162] |
| Flavivirus | Optimum Buffers and Reaction Conditions | Reference | ||||
|---|---|---|---|---|---|---|
| Tris-HCl | NaCl | Glycerol | Temp | pH | ||
| DENV | 50 mM | 50 mM | 35% | 37 °C | 8.5 | [67] |
| JEV | 50 mM | 25 mM | 30% | 37 °C | 9.5 | [75] |
| WNV | 200 mM | 13.5 mM | 30% | 37 °C | 9.5 | [163] |
| ZIKV | 20 or 50 mM | 150 mM | 10 or 20% | 37 °C | 8.5 | [50,164,165] |
| Tris-HCl | Acetic Acid | Glycine | Temp | pH | ||
| YFV | 75 mM | 25 mM | 25 mM | 37 °C | 7.0 | [166] |
| Sr No | Flavivirus | Antivirals Screened by Targeting NS2B/NS3 Proteases | Mechanism | Reference |
|---|---|---|---|---|
| 1 | WNV (West Nile Virus) | Benzoyl-norleucine-lysine-arginine-arginine (Bz-nKRR) tetrapeptide aldehyde | C-terminal electrophile incorporation | [177] |
| Cationic tripeptides (along with nonpeptide cap) | [176] | |||
| Peptide–boronic acid inhibitors | [173] | |||
| Benzyl ethers of 4-hydroxyphenylglycine | N-terminal capping moiety optimization | [172] | ||
| Bz-Arg-Lys-X-NH | [178] | |||
| Peptide-hybrids based on 2,4-thiazolidinedione scaffolds containing nonpolar groups | [179] | |||
| Benzyl ethers of 4-hydroxyphenylglycine | P1 and P2 basic residue modulation | [172] | ||
| Aprotinin | Noncompetitive inhibitors | [117] | ||
| Palmatine (Coptis chinensis) | [180] | |||
| Derivatives of Guanidinylated 2,5-dideoxystreptamine | Competitive inhibitors | [181] | ||
| Benzoyl-norleucine-lysine-arginine- arginine (Bz-nKRR) tetrapeptide aldehyde | Aldehydic inhibitors | [177] | ||
| Cationic tripeptides (along with nonpeptide cap) | [176] | |||
| Aprotinin | Stearic hindrance of active site | [175] | ||
| D-arginine-based 9–12-mer peptides | Mechanism yet to be determined | [175] | ||
| Furin | [182] | |||
| C-Terminal Electrophile incorporation | Peptide–boronic acid inhibitors | [173] | ||
| 2 | DENV (Dengue Virus) | Tetrapeptide: Bz-Nle-Lys-Arg-Arg-B(OH)2 (boronic acid analogue) | C-Terminal electrophile incorporation N-terminal capping moiety optimization | [170] |
| Benzyl ethers of 4-hydroxyphenylglycine | [172] | |||
| Bz-Arg-Lys-X-NH | N-terminal capping moiety optimization P1 and P2 basic residue modulation | [178] | ||
| Rhodanines and Thiazolidinediones | [183] | |||
| Benzyl ethers of 4-hydroxyphenylglycine | [172] | |||
| Plectasin | Noncompetitive inhibition | [184] | ||
| Substitution of Arg with unnatural Arg motifs in the P2 | P1 and P2 basic residue modulation Aldehydic inhibitors(against DENV 2) | [185] | ||
| Benzoyl-norleucine-lysine-arginine- arginine (Bz-nKRR) tetrapeptide aldehyde | [177] | |||
| Cationic tripeptides (along with nonpeptide cap) | Aldehydic inhibitors (against DENV 2) | [176] | ||
| Cyclopentapeptide (CKRKC) | Mechanism yet to be determined | [186] | ||
| BP-2109 | [187] | |||
| BP13944 | [188] | |||
| BT 24 (quinoline compound) | [189] | |||
| Aminobenzamide | [190] | |||
| 2,5,6-trisubstituted pyrazine compounds | [191] | |||
| Furin | [182] | |||
| Protegrin-1 | [192] | |||
| Retrocyclin-1 | [193] | |||
| Chalcone derivatives (DENV-2) | [194] | |||
| Flavonoids (fingerroot) (DENV-2) | [194] | |||
| Tyrothricin | Competitive inhibition | [195] | ||
| Derivatives of Guanidinylated 2,5-dideoxystreptamine | [181] | |||
| Retrotripeptides: R-Arg-Lys-Nle-NH2 Ivermectin Selamectin Benezethonium chloride | Mixed inhibition | [196] [195] | ||
| Peptide-boronic acid | C-terminal electrophile incorporation | [173] | ||
| 3 | ZIKV (Zika Virus) | Peptidomimetic boronic acid | Formation of salt bridge with Asp83 of NS2B | [95] |
| Bromocriptine | Mechanism yet to be determined | [197] | ||
| Novobiocin | [198] | |||
| Hydroxychloroquine | [199] | |||
| Erythrosin B | [200] | |||
| Theaflavin-3,3′-digallate | [201] | |||
| 9b (HIV protease inhibitor) | [202] | |||
| 2,5,6-trisubstituted pyrazine compounds | [191] | |||
| Aprotinin | [75] | |||
| 4 | JEV (Japanese Encephalitis Virus) | NSC135618 | Inhibits the conformational change of NS2B (allosteric inhibitor) | [203] |
| 5 | YFV (Yellow fever Virus) | Erythrosin B | Mechanism yet to be determined | [200] |
| Animal Models for Studying Dengue Virus (DENV) | |||||
|---|---|---|---|---|---|
| Animal Type | Model | Study Conducted/Findings | Reference | ||
| Nonhuman Primates | Rhesus macaquesa | Inactivated vaccine (DENV-II). | [232] | ||
| Expression of G protein in Vaccinia virus (DENV-2). | [233] | ||||
| DNA vaccine (encoding Pr-M and E) of DENV-2. | [234] | ||||
| DENV-I vaccine. | [235] | ||||
| Tetravalent vaccine expressed in Adenovirus. | [236] | ||||
| Tetravalent DNA vaccine (chimeric). | [237] | ||||
| Mutant DENV (live attenuated) vaccine. | [238] | ||||
| Inactivated DENV (tetravalent). | [239] | ||||
| DNA vaccine. | [240] | ||||
| Cynomolgous macaques | Live attenuated and recombinant vaccine comparison. | [241] | |||
| Chimeric DENV1/2 vaccine. | [242] | ||||
| Recombinant DENV. | [243] | ||||
| Recombinant protein (DENV 1–4). | [244] | ||||
| Tetravalent DENV vaccine (chimeric). | [245] | ||||
| Tetravalent DENV vaccine (live attenuated). | [246] | ||||
| DENV-2 virus-like particles. | [247] | ||||
| Mice | A/J | DENV-2 caused thrombocytopenia. | [248] | ||
| AG129 (do not have type I and II Interferon receptors) | DENV caused neurological manifestations leading to death. | [249] | |||
| DENV infection caused systemic infection and vascular leakage, leading to death. | [250] | ||||
| DENV infection resulted in splenomegaly. | [251] | ||||
| IFNAR−/− (Lack of IFN type I receptors; background of C57BL/6 mice) | DENV-2 infection resulted in viral growth in small intestine, liver, and bone marrow, resulting in death. | [252] | |||
| Cardif −/− | DENV infection in mice resulted in viral growth in lymph nodes, bone marrow, and spleen. | [253] | |||
| STAT 1 −/− | DENV infection resulted in viral growth in kidney, liver, and small intestine; however, the mice survived. | [254] | |||
| STAT 2 −/− | DENV infection resulted in viral growth in kidney, liver, and small intestine; however, the mice survived. | ||||
| STAT 1 −/− STAT 2 −/− (Lack STAT 1 and 2 proteins) | DENV infection resulted in higher viral titers in serum, kidney, liver, small intestine, and spleen, and mice death occurred. | ||||
| STAT1−/−/ IFNAR−/− (Lack of STAT1 and type I IFN receptor) | DENV infection resulted in higher viral titers in serum, kidney, liver, small intestine, and spleen, and mice death occurred. | ||||
| STAT1−/−/ IFNGR−/− (Lack of STAT1 and type II IFN receptor) | Mice survived | ||||
| Animal Models for Studying Yellow Fever Virus (YFV) | |||||
| Animal Type | Model | Study Conducted/Findings | Reference | ||
| Nonhuman Primates | Cynomolgous macaques | YFV-DENV(1–4) vaccine | [255] | ||
| YFV-DENV Chimeric vaccine | [256] | ||||
| Models for Studying Flavivirus NS2B-NS3 Proteases | |||||
| Virus Type | Cells | Animal Spp. | Outcome | Reference | |
| DENV ZIKV JEV WNV | Dermal fibroblasts (DFs) | Great apes (Pan paniscus, Pan troglodytes, Pongo pygmaeus Gorilla gorilla) | Dermal fibroblasts (DFs) demonstrated increased mice susceptibility to infection by Flaviviruses. | [215] | |
| Old World monkeys (Macaca nemestrina, Papio anubis, Macaca mulatta) | Increased mice susceptibility to infection by Flaviviruses. | ||||
| New world monkeys (Saimiri sciureus) | Increased mice susceptibility to infection by Flaviviruses. | ||||
| Mice (Tmem173Gt) | STING disruption increased mice susceptibility to infection by Flaviviruses; however, they could not develop serious infection (underlines the role of redundant pathways in viral replication dynamics). | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahaab, A.; Mustafa, B.E.; Hameed, M.; Stevenson, N.J.; Anwar, M.N.; Liu, K.; Wei, J.; Qiu, Y.; Ma, Z. Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review. Viruses 2022, 14, 44. https://doi.org/10.3390/v14010044
Wahaab A, Mustafa BE, Hameed M, Stevenson NJ, Anwar MN, Liu K, Wei J, Qiu Y, Ma Z. Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review. Viruses. 2022; 14(1):44. https://doi.org/10.3390/v14010044
Chicago/Turabian StyleWahaab, Abdul, Bahar E Mustafa, Muddassar Hameed, Nigel J. Stevenson, Muhammad Naveed Anwar, Ke Liu, Jianchao Wei, Yafeng Qiu, and Zhiyong Ma. 2022. "Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review" Viruses 14, no. 1: 44. https://doi.org/10.3390/v14010044
APA StyleWahaab, A., Mustafa, B. E., Hameed, M., Stevenson, N. J., Anwar, M. N., Liu, K., Wei, J., Qiu, Y., & Ma, Z. (2022). Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review. Viruses, 14(1), 44. https://doi.org/10.3390/v14010044








