Understanding Rhinovirus Circulation and Impact on Illness
Abstract
1. Introduction
2. Rhinovirus Epidemiological Factors
2.1. RV Transmission and Associated Illnesses
2.2. Clinical Presentation and Symptom Severity
2.3. Rhinovirus Seasonality
2.4. RV Treatment Strategies
3. RV Infections in At-Risk Populations
3.1. Children
3.2. Chronic Airway Diseases: Asthma
3.3. Elderly and Immunocompromised
4. Rhinovirus and Co-Infections
4.1. Bacterial Co-Infections
4.2. Viral Co-Infections
4.3. Viral Interference—RV Protection from Viral Illnesses Caused by Other Respiratory Viruses
5. Beyond RV Species: A Focus on RV Subtype Circulation
5.1. Rhinovirus Classification System and Surveillance
5.2. Understanding RV Subtype Diversity Using a Meta-Analysis Approach
5.3. Rhinovirus Species and Subtypes in a Panel of 31 Studies
5.4. Insight of Study Design on Questions Related to RV Subtype Circulation
5.5. Challenges in Understanding RV Subtype Circulation and Link to Illness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Study Reference | Study Location | Sample Collection Year | Age | Selection | RV-Positive Samples/Total Samples | RV + Samples Attempted VP2/VP4 Amplification | Included in Meta-Analysis (A/B/C) | % Species Distribution (A/B/C) |
|---|---|---|---|---|---|---|---|---|
| Daleno et al., 2013 [167] | Italy | 2007–2012 (November–April) | Children (0–14 years) | Hospitalized CAP | 198/643 (30.7%) | 198/198 (100%) | 151 78/14/59 | 51.7/9.3/39 |
| Espinola et al. 2013 [168] | Paraguay | May 2010–December 2011 | Children (mean 8 months) | Hospitalized Acute LRTI | 34/101 (33.66%) | 34/34 (100%) | 18 (13/1/4) | 72/5.5/22 |
| Pierangeli et al. 2013 [55] | Italy | 2010 | Children | Hospitalized RV + samples | 90 * | 73/90 (81%) | 72 (40/5/27) | 55/7/38 |
| Sansone et al. 2013 [156] | Sweden | November 2006–September 2010 | Children and Adults (51% < 10 years) | Hospitalized ARI | 1840/11468 (16%) | 170/1840 (9%) | 106 (64/11/31) | 56/9.6/32.4 |
| Kiyota et al. 2014 [169] | Japan | January 2011–November 2012 | Children and Adults (median 3 years) | ARI | 96/904 (10.6%) | 96/96 (100%) | 78 (58/4/16) | 60.4/4.2/35.4 |
| Marcone et al. 2014 [48] | Argentina | June 2008–May 2010 | Children (median 1 year) | Hospitalized and non-hospitalized ARI/ LRTI/URTI | 252/620 (40.6%) | 45/45 (100%) | 41 (21/1/19) | 46.6/2/42.2 |
| Bruning et al. 2015 [170] | The Netherlands | November 2009–December 2012 | Children (median 0.7–1.5 years) | Hospitalized and outpatients RV + samples | 120/120 (100%) | 120/120 (100%) | 107 (63/4/40) | 55/9/35 |
| Jacobs et al. 2015 [171] | USA | April 2012–March 2013 | Adults (65% > 50 years) | Hematologic malignancy | 110 * | 102/110 (92%) | 66 (46/12/8) | 64/12/21 |
| L’Huillier et al. 2015 [172] | Tanzania | April–December 2008 | Children (median 13.9 months) | Outpatients fever ≥ 38 degrees | 244/1005 (24.2%) | 244/244 (100%) | 226 (119/39/68) | 52/17/31 |
| Naughtin et al. 2015 [54] | Cambodia | June 2007–December 2009 | Children and Adults (60% < 5 years) | Hospitalized ILI /SARI | 455/4170 (10.9%) | 88/455 (19.3%) | 60 (36/10/14) | 60/16.6/28.3 |
| Richter et al. 2015 [51] | Cyprus | November 2010–October 2013 | Children (median 1.25 years) | ARI | 116/485 (23.91%) | 116/116 (100%) | 68 (36/5/27) | 54/12/35 |
| Fall et al. 2016 [173] | Senegal | January 2012–December 2014 | Children and Adults (median 4 years) | Outpatient ILI | 1415/4194 | 150/1415 (10.6%) | 87 (62/2/23) | 57.9/5.3/36.8 |
| Milanoi et al. 2016 [174] | Kenya | 2008 | Children and Adults (median 1.8 years) | Outpatient ILI | 130/517 (25%) | 37/130 (28%) | 26 (14/3/9) | 54/12/35 |
| Tran et al. 2016 [158] | Vietnam | April 2010–May 2011 | Children (mean 9 months) | Hospitalized ARI | 325/1082 (30%) | 58/325 (17.8%) | 58 (44/0/14) | 75.9/0/24.1 |
| Van der Linden et al. 2016 [49] | The Netherlands | 2007–2012 | Children (median 1.6 years) | Samples sent to diagnostic | 1102/6258 (17.6%) | 745/1102 (67.9%) | 587 (309/56/222) | 52.4/11.3/36.2 |
| Saraya et al. 2017 [109] | Japan | August 2012–May 2015 | Adults (median 56 years) | Asthma attack | 24/106 (22.6%) | 24/24 (100%) | 21 (12/1/8) | 50/4.2/45.8 |
| Andres et al. 2018 [157] | Spain | October 2014–May 2017 | Children and adults (median 5 years) | RTI suspicion | 2615/19957 (13.1%) | 1771/2615 (68%) | 1545 (948/90/507) | 63/6/31 |
| Morobe et al. 2018 [50] | Kenya | December 2015–November 2016 | Children and adults (median 2 years) | Outpatient ARI | 1057/5744 (18.4%) | 817/1057 (77.3%) | 776 (359/67/350) | 44/8.2/47.8 |
| Ng et al. 2018 [36] | Malaysia | February 2012–May 2014 | Children and Adults (median 38 years) | Outpatients ARI | 976/3935 (24.8%) | 976/976 (100%) | 111 ** (54/16/41) | 49/13/38 |
| Zhao et al. 2018 [53] | China | 2013–2015 | Children (median 1 year) | Hospitalized SARI | 280/1003 (28%) | 280/280 (100%) | 217 (140/21/56) | 50/7.5/20 |
| Baillie et al. 2019 [34] | Mali, South Africa and Zambia | August 2011–August 2013 | Children (<5 years) | Controls and Acute pneumonia (hospitalized) | 901/4404 (20.4%) | 836/901 (92.7%) | 757 (357/67/333) | 46.5/8.5/45 |
| Hung et al. 2019 [124] | Taiwan | January 2013–December 2014 | Children and Adults (median 3.8 years) | Hospitalized ARI | 76/487 | 76/76 | 47 ** (29/4/14) | 54/7.9/38 |
| Ko et al. 2019 [102] | Hong Kong | August 2016–July 2017 | Adult (median 48–77.4) | Exacerbation COPD and Asthma | 38/603 | 38/38 | 38 (21/4/13) | 55/10/34 |
| Kuypers et al. 2019 [175] | Nepal | December 2012–April 2014 | Infants (0–180 days) | Resp. symptoms not hospitalized | 547/609 | 285/647 | 265 (183/26/64) | 68/6.3/26 |
| Arden et al. 2020 [31] | Australia | 2001 | Children and Adults (median 1.5 years) | ARI | 266/1179 | 266/266 | 249 (11/108/17) | 53/4/42 |
| Linster et al. 2020 [176] | Singapore | 2007–2013 | Adults (median 31 years) | Febrile illness | 236/2950 | 163/236 | 131 (92/17/22) | 65.2/16.3/12 |
| Luka et al. 2020 [159] | Kenya | May 2017–April 2018 | 348 children and 4 adults (school) | ARI | 307/1859 | 253/307 | 241 (134/46/61) | 53/18.2/28.9 |
| Adam et al. 2021 [29] | Australia | 2006–2009 | Children (under 18 years) | Mixed previous cohorts | 91 | 43/91 | 43 (24/15/4) | 48/8/44 |
| Golke et al. 2021 [30] | Germany | 2013–2017 | Adults (mean 54.8 years) | URTI/LRTI | 506/11650 | 410/506 | 284 (173/36/75) | 60.9/12.7/26.4 |
| Haddad-Boubaker et al. 2021 [101] | Tunisia | September 2015–December 2017 | Children (median 2 months) | Hospitalized SARI, ICU | 57/271 | 49/57 | 49 (32/3/14) | 63.3/6.1/30.6 |
| Li et al. 2021 [32] | China | August 2018–December 2019 | Children (median 16–21 months) | Hospitalized ARI | 42/655 | 40/49 | 17/0/23 | 45/0/55 |
References
- Jacobs, S.E.; Lamson, D.M.; St George, K.; Walsh, T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013, 26, 135–162. [Google Scholar] [CrossRef]
- Principi, N.; Zampiero, A.; Gambino, M.; Scala, A.; Senatore, L.; Lelii, M.; Ascolese, B.; Pelucchi, C.; Esposito, S. Prospective evaluation of rhinovirus infection in healthy young children. J. Clin. Virol. 2015, 66, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Bray-Aschenbrenner, A.; Williams, H.; Buchanan, P.; Werner, J. The Resource Burden of Infections with Rhinovirus/Enterovirus, Influenza, and Respiratory Syncytial Virus in Children. Clin. Pediatr. 2019, 58, 177–184. [Google Scholar] [CrossRef]
- Asner, S.A.; Petrich, A.; Hamid, J.S.; Mertz, D.; Richardson, S.E.; Smieja, M. Clinical severity of rhinovirus/enterovirus compared to other respiratory viruses in children. Influ. Other Respir. Viruses 2014, 8, 436–442. [Google Scholar] [CrossRef]
- Drysdale, S.B.; Mejias, A.; Ramilo, O. Rhinovirus—Not just the common cold. J. Infect. 2017, 74 (Suppl. 1), S41–S46. [Google Scholar] [CrossRef]
- Greenberg, S.B. Respiratory Consequences of Rhinovirus Infection. Arch. Intern. Med. 2003, 163, 278–284. [Google Scholar] [CrossRef]
- Juvén, T.; Mertsola, J.; Waris, M.; Leinonen, M.; Meurman, O.; Roivainen, M.; Eskola, J.; Saikku, P.; Ruuskanen, O. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 2000, 19, 293–298. [Google Scholar] [CrossRef] [PubMed]
- In the Pirbright Institute, UK. Available online: https://www.picornaviridae.com/ (accessed on 20 December 2021).
- Picornaviridae Study Group. Available online: https://www.picornastudygroup.com/ (accessed on 15 December 2021).
- Gwaltney, J.M., Jr.; Moskalski, P.B.; Hendley, J.O. Hand-to-Hand Transmission of Rhinovirus Colds. Ann. Intern. Med. 1978, 88, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Winther, B.; McCue, K.; Ashe, K.; Rubino, J.; Hendley, J. Rhinovirus contamination of surfaces in homes of adults with natural colds: Transfer of virus to fingertips during normal daily activities. J. Med. Virol. 2011, 83, 906–909. [Google Scholar] [CrossRef]
- Jennings, L.C.; Dick, E.C. Transmission and control of rhinovirus colds. Eur. J. Epidemiol. 1987, 3, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Dick, E.C.; Jennings, L.C.; Mink, K.A.; Wartgow, C.D.; Inborn, S.L. Aerosol Transmission of Rhinovirus Colds. J. Infect. Dis. 1987, 156, 442–448. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, abd9149. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Nielsen, P.V.; Wei, J.; Jensen, R.L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air 2016, 27, 452–462. [Google Scholar] [CrossRef]
- Myatt, T.A.; Johnston, S.L.; Zuo, Z.; Wand, M.; Kebadze, T.; Rudnick, S.; Milton, D.K. Detection of Airborne Rhinovirus and Its Relation to Outdoor Air Supply in Office Environments. Am. J. Respir. Crit. Care Med. 2004, 169, 1187–1190. [Google Scholar] [CrossRef]
- Lessler, J.; Reich, N.G.; Brookmeyer, R.; Perl, T.M.; Nelson, K.E.; Cummings, D.A. Incubation periods of acute respiratory viral infections: A systematic review. Lancet Infect. Dis. 2009, 9, 291–300. [Google Scholar] [CrossRef]
- Chen, W.J.; Arnold, J.C.; Fairchok, M.P.; Danaher, P.J.; McDonough, E.A.; Blair, P.J.; Garcia, J.; Halsey, E.S.; Schofield, C.; Ottolini, M.; et al. Epidemiologic, clinical, and virologic characteristics of human rhinovirus infection among otherwise healthy children and adults: Rhinovirus among adults and children. J. Clin. Virol. 2015, 64, 74–82. [Google Scholar] [CrossRef]
- Heikkinen, T.; Jarvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Hayden, F.G. Rhinovirus and the lower respiratory tract. Rev. Med. Virol. 2004, 14, 17–31. [Google Scholar] [CrossRef]
- Papadopoulos, N.G. Do rhinoviruses cause pneumonia in children? Paediatr. Respir. Rev. 2004, 5 (Suppl. 1), S191–S195. [Google Scholar] [CrossRef]
- Chidlow, G.R.; Laing, I.A.; Harnett, G.B.; Greenhill, A.R.; Phuanukoonnon, S.; Siba, P.M.; Pomat, W.S.; Shellam, G.R.; Smith, D.W.; Lehmann, D. Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J. Clin. Virol. 2012, 54, 235–239. [Google Scholar] [CrossRef]
- Walker, E.; Ison, M.G. Respiratory viral infections among hospitalized adults: Experience of a single tertiary healthcare hospital. Influ. Other Respir. Viruses 2014, 8, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.-M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing Rhinovirus Illnesses in Early Life Predict Asthma Development in High-Risk Children. Am. J. Respir. Crit. Care Med. 2008, 178, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Rakes, G.P.; Arruda, E.; Ingram, J.M.; Hoover, G.E.; Zambrano, J.C.; Hayden, F.G.; Platts-Mills, T.A.E.; Heymann, P.W. Rhinovirus and Respiratory Syncytial Virus in Wheezing Children Requiring Emergency Care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999, 159, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.G.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Primers 2016, 2, 16063. [Google Scholar] [CrossRef] [PubMed]
- Chonmaitree, T.; Alvarez-Fernandez, P.; Jennings, K.; Trujillo, R.; Marom, T.; Loeffelholz, M.J.; Miller, A.L.; McCormick, D.P.; Patel, J.A.; Pyles, R.B. Symptomatic and Asymptomatic Respiratory Viral Infections in the First Year of Life: Association with Acute Otitis Media Development. Clin. Infect. Dis. 2015, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Conrad, T.; Alchikh, M.; Reiche, J.; Schweiger, B.; Rath, B. Can we distinguish respiratory viral infections based on clinical features? A prospective pediatric cohort compared to systematic literature review. Rev. Med. Virol. 2018, 28, e1997. [Google Scholar] [CrossRef] [PubMed]
- Zlateva, K.T.; de Vries, J.J.; Coenjaerts, F.E.; van Loon, A.M.; Verheij, T.; Little, P.; Butler, C.C.; Goossens, H.; Ieven, M.; Claas, E.C.; et al. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur. Respir. J. 2014, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.C.; Chen, X.; Scotch, M.; MacIntyre, C.R.; Dwyer, D.; Kok, J. The Molecular Epidemiology and Clinical Phylogenetics of Rhinoviruses Among Paediatric Cases in Sydney, Australia. Int. J. Infect. Dis. 2021, 110, 69–74. [Google Scholar] [CrossRef]
- Golke, P.; Hönemann, M.; Bergs, S.; Liebert, U.G. Human Rhinoviruses in Adult Patients in a Tertiary Care Hospital in Germany: Molecular Epidemiology and Clinical Significance. Viruses 2021, 13, 2027. [Google Scholar] [CrossRef]
- Arden, K.E.; Greer, R.M.; Wang, C.; Mackay, I.M. Genotypic diversity, circulation patterns and co-detections among rhinoviruses in Queensland, 2001. Access Microbiol. 2020, 2, e000075. [Google Scholar] [CrossRef]
- Li, W.; Yu, B.; Zhou, J.; Wang, Y.; Xue, B.; Pan, J.; Ran, Y.; Yang, X.; Yang, F.; Li, H. Genetic diversity and epidemiology of human rhinovirus among children with severe acute respiratory tract infection in Guangzhou, China. Virol. J. 2021, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-M.; Lemanske, R.F., Jr.; Evans, M.; Vang, F.; Pappas, T.; Gangnon, R.; Jackson, D.J.; Gern, J.E. Human Rhinovirus Species and Season of Infection Determine Illness Severity. Am. J. Respir. Crit. Care Med. 2012, 186, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Baillie, V.L.; Moore, D.; Mathunjwa, A.; Morailane, P.; Simões, E.A.F.; Madhi, S.A. Molecular Subtyping of Human Rhinovirus in Children from Three Sub-Saharan African Countries. J. Clin. Microbiol. 2019, 57, e00723-19. [Google Scholar] [CrossRef]
- Megremis, S.; Demetriou, P.; Makrinioti, H.; Manoussaki, A.E.; Papadopoulos, N.G. The Genomic Signature of Human Rhinoviruses A, B and C. PLoS ONE 2012, 7, e44557. [Google Scholar] [CrossRef]
- Ng, K.T.; Oong, X.Y.; Lim, S.H.; Chook, J.B.; Takebe, Y.; Chan, Y.F.; Chan, K.G.; Hanafi, N.S.; Pang, Y.K.; Kamarulzaman, A.; et al. Viral Load and Sequence Analysis Reveal the Symptom Severity, Diversity, and Transmission Clusters of Rhinovirus Infections. Clin. Infect. Dis. 2018, 67, 261–268. [Google Scholar] [CrossRef]
- Wildenbeest, J.; van der Schee, M.; Hashimoto, S.; Benschop, K.; Minnaar, R.; Sprikkelman, A.; Haarman, E.; van Aalderen, W.; Sterk, P.; Pajkrt, D.; et al. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin. Microbiol. Infect. 2016, 22, 736.e9–736.e15. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.; Devries, M.; Bacharier, L.B.; Busse, W.; Camargo, C.A., Jr.; Cohen, R.; Demuri, G.P.; Evans, M.D.; Fitzpatrick, A.M.; Gergen, P.J.; et al. Enhanced Neutralizing Antibody Responses to Rhinovirus C and Age-Dependent Patterns of Infection. Am. J. Respir. Crit. Care Med. 2021, 203, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Khoo, S.-K.; Zhang, G.; Lindsay, K.; Keil, A.D.; Knight, G.; Gern, J.E.; Laing, I.; Bizzintino, J.; Le Souef, P. Rhinovirus is the most common virus and rhinovirus-C is the most common species in paediatric intensive care respiratory admissions. Eur. Respir. J. 2018, 52, 1800207. [Google Scholar] [CrossRef] [PubMed]
- Piralla, A.; Baldanti, F.; Gerna, G. Phylogenetic Patterns of Human Respiratory Picornavirus Species, Including the Newly Identified Group C Rhinoviruses, during a 1-Year Surveillance of a Hospitalized Patient Population in Italy. J. Clin. Microbiol. 2011, 49, 373–376. [Google Scholar] [CrossRef]
- Clark, T.W.; Ewings, S.; Medina, M.-J.; Batham, S.; Curran, M.D.; Parmar, S.; Nicholson, K.G. Viral load is strongly associated with length of stay in adults hospitalised with viral acute respiratory illness. J. Infect. 2016, 73, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, A.; Hashimoto, K.; Sato, M.; Sato, T.; Kanno, S.; Takano, K.; Ito, M.; Katayose, M.; Nishimura, H.; Kawasaki, Y.; et al. Rhinovirus load and disease severity in children with lower respiratory tract infections. J. Med. Virol. 2012, 84, 1135–1142. [Google Scholar] [CrossRef]
- Xiao, Q.; Zheng, S.; Zhou, L.; Ren, L.; Xie, X.; Deng, Y.; Tian, D.; Zhao, Y.; Fu, Z.; Li, T.; et al. Impact of Human Rhinovirus Types and Viral Load on the Severity of Illness in Hospitalized Children with Lower Respiratory Tract Infections. Pediatr. Infect. Dis. J. 2015, 34, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Granados, A.; Peci, A.; McGeer, A.; Gubbay, J.B. Influenza and rhinovirus viral load and disease severity in upper respiratory tract infections. J. Clin. Virol. 2017, 86, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bizzintino, J.; Lee, W.-M.; Laing, I.; Vang, F.; Pappas, T.; Zhang, G.; Martin, A.C.; Khoo, S.-K.; Cox, D.; Geelhoed, G.C.; et al. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 2010, 37, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Gwaltney, J.M.; Hendley, J.O.; Simon, G.; Jordan, W.S. Rhinovirus Infections in an Industrial Population. I. The occurrence of illness. N. Engl. J. Med. 1966, 275, 1261–1268. [Google Scholar] [CrossRef]
- Xiang, Z.; Gonzalez, R.; Xie, Z.; Xiao, Y.; Chen, L.; Li, Y.; Liu, C.; Hu, Y.; Yao, Y.; Qian, S.; et al. Human Rhinovirus Group C Infection in Children with Lower Respiratory Tract Infection. Emerg. Infect. Dis. 2008, 14, 1665–1667. [Google Scholar] [CrossRef]
- Marcone, D.N.; Culasso, A.; Carballal, G.; Campos, R.; Echavarría, M. Genetic diversity and clinical impact of human rhinoviruses in hospitalized and outpatient children with acute respiratory infection, Argentina. J. Clin. Virol. 2014, 61, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, L.; Bruning, A.H.; Thomas, X.V.; Minnaar, R.P.; Rebers, S.P.; Schinkel, J.; de Jong, M.D.; Pajkrt, D.; Wolthers, K.C. A molecular epidemiological perspective of rhinovirus types circulating in Amsterdam from 2007 to 2012. Clin. Microbiol. Infect. 2016, 22, 1002.e9–1002.e14. [Google Scholar] [CrossRef]
- Morobe, J.M.; Nyiro, J.U.; Brand, S.; Kamau, E.; Gicheru, E.; Eyase, F.; Otieno, G.P.; Munywoki, P.K.; Agoti, C.; Nokes, J. Human rhinovirus spatial-temporal epidemiology in rural coastal Kenya, 2015-2016, observed through outpatient surveillance. Wellcome Open Res. 2019, 3, 128. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Nikolaou, E.; Panayiotou, C.; Tryfonos, C.; Koliou, M.; Christodoulou, C. Molecular epidemiology of rhinoviruses in Cyprus over three consecutive seasons. Epidemiol. Infect. 2015, 143, 1876–1883. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Yip, C.C.Y.; Lin, A.W.C.; Lee, R.A.; So, L.-Y.; Lau, Y.-L.; Chan, K.-H.; Woo, P.C.Y.; Yuen, K.-Y. Clinical and Molecular Epidemiology of Human Rhinovirus C in Children and Adults in Hong Kong Reveals a Possible Distinct Human Rhinovirus C Subgroup. J. Infect. Dis. 2009, 200, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, J.; Wu, B.; Liu, G.; Lu, R.; Tan, W. Genotypic Diversity and Epidemiology of Human Rhinovirus Among Children with Severe Acute Respiratory Tract Infection in Shanghai, 2013–2015. Front. Microbiol. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Naughtin, M.; Sareth, R.; Sentilhes, A.-C.; Vong, S.; Joffret, M.-L.; Cornillot, E.; Deubel, V.; Delpeyroux, F.; Frutos, R.; Buchy, P. Genetic diversity of human rhinoviruses in Cambodia during a three-year period reveals novel genetic types. Infect. Genet. Evol. 2015, 35, 42–49. [Google Scholar] [CrossRef]
- Pierangeli, A.; Ciccozzi, M.; Chiavelli, S.; Concato, C.; Giovanetti, M.; Cella, E.; Spano, L.; Scagnolari, C.; Moretti, C.; Papoff, P.; et al. Molecular epidemiology and genetic diversity of human rhinovirus affecting hospitalized children in Rome. Med. Microbiol. Immunol. 2013, 202, 303–311. [Google Scholar] [CrossRef]
- Cuevas, M.T.; Molinero, M.; Pozo, F.; Calvo, C.; García-García, M.L.; Reyes, N.; Ledesma, J.; Casas, I. Spread of different rhinovirus B genotypes in hospitalized children in Spain. Influ. Other Respir. Viruses 2012, 7, 623–628. [Google Scholar] [CrossRef]
- Lopes, G.P.; Amorim, P.S.; de Melo, B.D.O.; Maramaldo, C.E.C.; Bomfim, M.R.Q.; Neto, L.G.L.; Alves, M.S.; Silva, F.B.; Soeiro-Pereira, P.V.; Falcai, A. Identification and seasonality of rhinovirus and respiratory syncytial virus in asthmatic children in tropical climate. Biosci. Rep. 2020, 40, BSR20200634. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; McIntyre, C.; Savolainen-Kopra, C.; Tapparel, C.; Mackay, I.M.; Hovi, T. Proposals for the classification of human rhinovirus species C into genotypically assigned types. J. Gen. Virol. 2010, 91 Pt 10, 2409–2419. [Google Scholar] [CrossRef]
- Pitkaranta, A.; Hayden, F.G. Rhinoviruses: Important respiratory pathogens. Ann. Med. 1998, 30, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Altan, E.; Dib, J.C.; Gulloso, A.R.; Juandigua, D.E.; Deng, X.; Bruhn, R.; Hildebrand, K.; Freiden, P.; Yamamoto, J.; Schultz-Cherry, S.; et al. Effect of Geographic Isolation on the Nasal Virome of Indigenous Children. J. Virol. 2019, 93, e00681-19. [Google Scholar] [CrossRef] [PubMed]
- Vittucci, A.C.; Piccioni, L.; Coltella, L.; Ciarlitto, C.; Antilici, L.; Bozzola, E.; Midulla, F.; Palma, P.; Perno, C.F.; Villani, A. The Disappearance of Respiratory Viruses in Children during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 9550. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.; Bourcier, M.; Trémeaux, P.; Dimeglio, C.; Izopet, J. COVID-19 pandemic period, where are the seasonal viruses? J. Med. Virol. 2021, 93, 4097–4098. [Google Scholar] [CrossRef] [PubMed]
- Redlberger-Fritz, M.; Kundi, M.; Aberle, S.W.; Puchhammer-Stockl, E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J. Clin. Virol. 2021, 137, 104795. [Google Scholar] [CrossRef]
- Doggett, J.E.; Bynoe, M.L.; Tyrrell, D.A.J. Some Attempts to Produce an Experimental Vaccine with Rhinoviruses. BMJ 1963, 1, 34–36. [Google Scholar] [CrossRef]
- Mitchison, D.A. Prevention of Colds by Vaccination against a Rhinovirus: A Report by the Scientific Committee on Common Cold Vaccines. BMJ 1965, 1, 1344–1349. [Google Scholar] [PubMed]
- Girkin, J.; Loo, S.-L.; Esneau, C.; Maltby, S.; Mercuri, F.; Chua, B.; Reid, A.T.; Veerati, P.C.; Grainge, C.L.; Wark, P.A.; et al. TLR2-mediated innate immune priming boosts lung anti-viral immunity. Eur. Respir. J. 2021, 58, 2001584. [Google Scholar] [CrossRef]
- Hamory, B.H.; Hamparian, V.V.; Conant, R.M.; Gwaltney, J.M., Jr. Human responses to two decavalent rhinovirus vaccines. J. Infect. Dis. 1975, 132, 623–629. [Google Scholar] [CrossRef] [PubMed]
- McCray, J.; Werner, G. Different rhinovirus serotypes neutralized by antipeptide antibodies. Nature 1987, 329, 736–738. [Google Scholar] [CrossRef]
- Glanville, N.; Johnston, S.L. Challenges in developing a cross-serotype rhinovirus vaccine. Curr. Opin. Virol. 2015, 11, 83–88. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, M.T.; Currier, M.G.; Jenkins, J.B.; Strobert, E.A.; Kajon, A.E.; Madan-Lala, R.; Bochkov, Y.A.; Gern, J.E.; Roy, K.; et al. A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques. Nat. Commun. 2016, 7, 12838. [Google Scholar] [CrossRef]
- Thibaut, H.J.; Lacroix, C.; De Palma, A.M.; Franco, D.; Decramer, M.; Neyts, J. Toward antiviral therapy/prophylaxis for rhinovirus-induced exacerbations of chronic obstructive pulmonary disease: Challenges, opportunities, and strategies. Rev. Med. Virol. 2016, 26, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Coultas, J.; Cafferkey, J.; Mallia, P.; Johnston, S.L. Experimental Antiviral Therapeutic Studies for Human Rhinovirus Infections. J. Exp. Pharmacol. 2021, 13, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Groaz, E.; De Clercq, E.; Herdewijn, P. Anno 2021: Which antivirals for the coming decade? Annu. Rep. Med. Chem. 2021, 57, 49–107. [Google Scholar] [PubMed]
- Casanova, V.; Sousa, F.H.; Stevens, C.; Barlow, P.G. Antiviral therapeutic approaches for human rhinovirus infections. Future Virol. 2018, 13, 505–518. [Google Scholar] [CrossRef]
- Bauer, L.; Lyoo, H.; van der Schaar, H.M.; Starting, J.R.; van Kuppeveld, F.J. Direct-acting antivirals and host-targeting strategies to combat enterovirus infections. Curr. Opin. Virol. 2017, 24, 1–8. [Google Scholar] [CrossRef]
- McLean, G.G.J.; Solari, R. Emerging therapeutic approaches. In Rhinovirus Infections, Rethinking the Impact on Human Health and Disease; Bartlett, N.W., Wark, P., Knight, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 239–263. [Google Scholar]
- Hayden, F.G.; Herrington, D.T.; Coats, T.L.; Kim, K.; Cooper, E.C.; Villano, S.A.; Liu, S.; Hudson, S.; Pevear, D.C.; Collett, M.; et al. Efficacy and Safety of Oral Pleconaril for Treatment of Colds Due to Picornaviruses in Adults: Results of 2 Double-Blind, Randomized, Placebo-Controlled Trials. Clin. Infect. Dis. 2003, 36, 1523–1532. [Google Scholar] [CrossRef]
- Hayden, F.G.; Andries, K.; Janssen, P.A. Safety and efficacy of intranasal pirodavir (R77975) in experimental rhinovirus infection. Antimicrob. Agents Chemother. 1992, 36, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Senior, K. FDA panel rejects common cold treatment. Lancet Infect. Dis. 2002, 2, 264. [Google Scholar] [CrossRef]
- Lanko, K.; Sun, L.; Froeyen, M.; Leyssen, P.; Delang, L.; Mirabelli, C.; Neyts, J. Comparative analysis of the molecular mechanism of resistance to vapendavir across a panel of picornavirus species. Antivir. Res. 2021, 195, 105177. [Google Scholar] [CrossRef]
- Pevear, D.C.; Hayden, F.G.; Demenczuk, T.M.; Barone, L.R.; McKinlay, M.A.; Collett, M.S. Relationship of Pleconaril Susceptibility and Clinical Outcomes in Treatment of Common Colds Caused by Rhinoviruses. Antimicrob. Agents Chemother. 2005, 49, 4492–4499. [Google Scholar] [CrossRef]
- Mello, C.; Aguayo, E.; Rodriguez, M.; Lee, G.; Jordan, R.; Cihlar, T.; Birkus, G. Multiple Classes of Antiviral Agents ExhibitIn VitroActivity against Human Rhinovirus Type C. Antimicrob. Agents Chemother. 2013, 58, 1546–1555. [Google Scholar] [CrossRef]
- Matthews, D.A.; Dragovich, P.S.; Webber, S.E.; Fuhrman, S.A.; Patick, A.K.; Zalman, L.S.; Hendrickson, T.F.; Love, R.A.; Prins, T.J.; Marakovits, J.T.; et al. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. USA 1999, 96, 11000–11007. [Google Scholar] [CrossRef]
- Sousa, F.H.; Casanova, V.; Findlay, F.; Stevens, C.; Svoboda, P.; Pohl, J.; Proudfoot, L.; Barlow, P.G. Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides 2017, 95, 76–83. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.; Chichlowski, M.; Pastor, N.; Narayanappa, A.T.; Shah, N. In the Age of Viral Pandemic, Can Ingredients Inspired by Human Milk and Infant Nutrition Be Repurposed to Support the Immune System? Nutrients 2021, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; To, K.K.W.; Sze, K.H.; Yung, T.T.; Bian, M.; Lam, H.; Yeung, M.L.; Li, C.; Chu, H.; Yuen, K.-Y. A broad-spectrum virus- and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat. Commun. 2020, 11, 4252. [Google Scholar] [CrossRef]
- Hayden, F.G.; Albrecht, J.K.; Kaiser, D.L.; Gwaltney, J.M., Jr. Prevention of Natural Colds by Contact Prophylaxis with Intranasal Alpha2-Interferon. N. Engl. J. Med. 1986, 314, 71–75. [Google Scholar] [CrossRef]
- Djukanovic, R.; Harrison, T.; Johnston, S.L.; Gabbay, F.; Wark, P.; Thomson, N.C.; Niven, R.; Singh, D.; Reddel, H.J.; Davies, D.E.; et al. The effect of inhaled IFN-beta on worsening of asthma symptoms caused by viral infections. A randomized trial. Am. J. Respir. Crit. Care Med. 2014, 190, 145–154. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.-P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
- Bentley, J.K.; Han, M.; Jaipalli, S.; Hinde, J.L.; Lei, J.; Ishikawa, T.; Goldsmith, A.M.; Rajput, C.; Hershenson, M.B. Myristoylated rhinovirus VP4 protein activates TLR2-dependent proinflammatory gene expression. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2019, 317, L57–L70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef]
- Deliyannis, G.; Wong, C.Y.; McQuilten, H.A.; Bachem, A.; Clarke, M.; Jia, X.; Horrocks, K.; Zeng, W.; Girkin, J.; Scott, N.E.; et al. TLR2-mediated activation of innate responses in the upper airways confers antiviral protection of the lungs. JCI Insight 2021, 6, e140267. [Google Scholar] [CrossRef] [PubMed]
- Proud, P.C.; Tsitoura, D.; Watson, R.J.; Chua, B.Y.; Aram, M.J.; Bewley, K.R.; Cavell, B.E.; Cobb, R.; Dowall, S.; Ho, C.M.K.; et al. Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model. EBioMedicine 2021, 63, 103153. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F., Jr.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Tovey, E.R.; Rawlinson, W.D. A modern miasma hypothesis and back-to-school asthma exacerbations. Med Hypotheses 2011, 76, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Satia, I.; Adatia, A.; Yaqoob, S.; Greene, J.M.; O’Byrne, M.; Killian, K.J.; Johnston, N. Emergency department visits and hospitalisations for asthma, COPD and respiratory tract infections: What is the role of respiratory viruses, and return to school in September, January and March? ERJ Open Res. 2020, 6, 00593. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.W.; Johnston, S.; Norman, G.R.; Dai, J.; Sears, M.R. The September epidemic of asthma hospitalization: School children as disease vectors. J. Allergy Clin. Immunol. 2006, 117, 557–562. [Google Scholar] [CrossRef]
- Haddad-Boubaker, S.; Mefteh, K.; Mejri, C.; Bouaffsoun, A.; El Moussi, A.; Boutiba, I.; Mnif, K.; Slim, A.; Kechrid, A.; Smaoui, H. High genotypic diversity of Rhinoviruses obtained from Tunisian children with severe acute respiratory infection. J. Infect. Dev. Ctries. 2021, 15, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.-S.; Chan, P.K.-S.; Chan, R.W.Y.; Chan, K.-P.; Ip, A.; Kwok, A.; Ngai, J.C.-L.; Ng, S.-S.; On, C.T.; Hui, D.S.-C. Molecular detection of respiratory pathogens and typing of human rhinovirus of adults hospitalized for exacerbation of asthma and chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 210. [Google Scholar] [CrossRef]
- Gern, J.E. How rhinovirus infections cause exacerbations of asthma. Clin. Exp. Allergy 2014, 45, 32–42. [Google Scholar] [CrossRef]
- Gern, J.E. Rhinovirus and the initiation of asthma. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 73–78. [Google Scholar] [CrossRef]
- Ortega, H.; Nickle, D.; Carter, L. Rhinovirus and asthma: Challenges and opportunities. Rev. Med. Virol. 2021, 31, e2193. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Aguado, I.; García-García, M.L.; Ruiz-Chercoles, E.; Díaz-Martinez, E.; Albañil, R.M.; Campelo, O.; Olivas, A.; Muñóz-Gonzalez, L.; Pozo, F.; et al. Respiratory viral infections in a cohort of children during the first year of life and their role in the development of wheezing. An. Pediatr. Eng. Ed. 2017, 87, 104–110. [Google Scholar] [CrossRef]
- Mak, R.K.Y.; Tse, L.Y.; Lam, W.Y.; Wong, G.W.K.; Chan, P.K.S.; Leung, T.F. Clinical Spectrum of Human Rhinovirus Infections in Hospitalized Hong Kong Children. Pediatr. Infect. Dis. J. 2011, 30, 749–753. [Google Scholar] [CrossRef] [PubMed]
- De Winter, J.J.; Bont, L.; Wilbrink, B.; van der Ent, C.K.; Smit, H.A.; Houben, M.L. Rhinovirus wheezing illness in infancy is associated with medically attended third year wheezing in low risk infants: Results of a healthy birth cohort study. Immun. Inflamm. Dis. 2015, 3, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Saraya, T.; Kimura, H.; Kurai, D.; Ishii, H.; Takizawa, H. The molecular epidemiology of respiratory viruses associated with asthma attacks: A single-center observational study in Japan. Medicine 2017, 96, e8204. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.E.; Faux, C.E.; O’Neill, T.; McErlean, P.; Nitsche, A.; Lambert, S.B.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. Molecular characterization and distinguishing features of a novel human rhinovirus (HRV) C, HRVC-QCE, detected in children with fever, cough and wheeze during 2003. J. Clin. Virol. 2010, 47, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.E.; Chang, A.B.; Lambert, S.B.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. J. Med. Virol. 2010, 82, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Basnet, S.; Bochkov, Y.A.; Brockman-Schneider, R.A.; Kuipers, I.; Aesif, S.W.; Jackson, D.J.; Ober, C.; Palmenberg, A.C.; Gern, J.E. CDHR3 Asthma-Risk Genotype Affects Susceptibility of Airway Epithelium to Rhinovirus C Infections. Am. J. Respir. Cell Mol. Biol. 2019, 61, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Palmenberg, A.C. Rhinovirus C, Asthma, and Cell Surface Expression of Virus Receptor CDHR3. J. Virol. 2017, 91, 00072-17. [Google Scholar] [CrossRef]
- Pierangeli, A.; Scagnolari, C.; Selvaggi, C.; Verzaro, S.; Spina, M.T.; Bresciani, E.; Antonelli, G.; Bertazzoni, G. Rhinovirus frequently detected in elderly adults attending an emergency department. J. Med. Virol. 2011, 83, 2043–2047. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Zhang, A.J.; Esposito, S.; Chan, J.F.W.; Zhu, S.H.S.; Zhang, R.; Chan, T.-C.; Chan, K.-H.; Yuen, K.-Y. Unexpectedly Higher Morbidity and Mortality of Hospitalized Elderly Patients Associated with Rhinovirus Compared with Influenza Virus Respiratory Tract Infection. Int. J. Mol. Sci. 2017, 18, 259. [Google Scholar] [CrossRef]
- Lee, N.; Smith, S.; Zelyas, N.; Klarenbach, S.; Zapernick, L.; Bekking, C.; So, H.; Yip, L.; Tipples, G.; Taylor, G.; et al. Burden of noninfluenza respiratory viral infections in adults admitted to hospital: Analysis of a multiyear Canadian surveillance cohort from 2 centres. Can. Med. Assoc. J. 2021, 193, E439–E446. [Google Scholar] [CrossRef] [PubMed]
- Tapparel, C.; Cordey, S.; Junier, T.; Farinelli, L.; Van Belle, S.; Soccal, P.M.; Aubert, J.-D.; Zdobnov, E.; Kaiser, L. Rhinovirus Genome Variation during Chronic Upper and Lower Respiratory Tract Infections. PLoS ONE 2011, 6, e21163. [Google Scholar] [CrossRef]
- Han, T.H.; Chung, J.-Y.; Hwang, E.S.; Koo, J.-W. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch. Virol. 2009, 154, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, W.; Bessaud, M.; Ren, P.; Sheng, J.; Yan, H.; Zhang, J.; Lin, X.; Wang, Y.; Delpeyroux, F.; et al. Evidence of Recombination and Genetic Diversity in Human Rhinoviruses in Children with Acute Respiratory Infection. PLoS ONE 2009, 4, e6355. [Google Scholar] [CrossRef]
- Leotte, J.; Trombetta, H.; Faggion, H.Z.; Almeida, B.M.; Nogueira, M.B.; Vidal, L.R.; Raboni, S.M. Impact and seasonality of human rhinovirus infection in hospitalized patients for two consecutive years. J. Pediatr. Rio J. 2017, 93, 294–300. [Google Scholar] [CrossRef]
- Honkinen, M.; Lahti, E.; Österback, R.; Ruuskanen, O.; Waris, M. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin. Microbiol. Infect. 2012, 18, 300–307. [Google Scholar] [CrossRef]
- Linder, J.E.; Kraft, D.C.; Mohamed, Y.; Lu, Z.; Heil, L.; Tollefson, S.; Saville, B.R.; Wright, P.F.; Williams, J.; Miller, E.K. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades. J. Allergy Clin. Immunol. 2013, 131, 69–77.e6. [Google Scholar] [CrossRef] [PubMed]
- Mallia, P.; Footitt, J.; Sotero, R.; Jepson, A.; Contoli, M.; Trujillo-Torralbo, M.-B.; Kebadze, T.; Aniscenko, J.; Oleszkiewicz, G.; Gray, K.; et al. Rhinovirus Infection Induces Degradation of Antimicrobial Peptides and Secondary Bacterial Infection in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 1117–1124. [Google Scholar] [CrossRef]
- Hung, H.-M.; Yang, S.-L.; Chen, C.-J.; Chiu, C.-H.; Kuo, C.-Y.; Huang, K.-Y.A.; Lin, T.-Y.; Hsieh, Y.-C.; Gong, Y.-N.; Tsao, K.-C.; et al. Molecular epidemiology and clinical features of rhinovirus infections among hospitalized patients in a medical center in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 233–241. [Google Scholar] [CrossRef]
- Kong, D.; Zheng, Y.; Hu, L.; Chen, J.; Wu, H.; Teng, Z.; Zhou, Y.; Qiu, Q.; Lu, Y.; Pan, H. Epidemiological and co-infection characteristics of common human coronaviruses in Shanghai, 2015–2020: A retrospective observational study. Emerg. Microbes Infect. 2021, 10, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Pradel, F.K.; Javouhey, E.; Perret, M.; Rajoharison, A.; Bagnaud, A.; Billaud, G.; Vernet, G.; Lina, B.; Floret, D.; et al. The Impact of Dual Viral Infection in Infants Admitted to a Pediatric Intensive Care Unit Associated with Severe Bronchiolitis. Pediatr. Infect. Dis. J. 2008, 27, 213–217. [Google Scholar] [CrossRef]
- Harada, Y.; Kinoshita, F.; Yoshida, L.M.; le Minh, N.; Suzuki, M.; Morimoto, K.; Yuichirou, T. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr. Infect. Dis. J. 2013, 32, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.F.; Queiróz, D.A.O.; Da Silveira, H.L.; Neto, M.B.; de Paula, N.; Oliveira, T.F.M.S.; Tolardo, A.L.; Yokosawa, J. Human Rhinovirus and Disease Severity in Children. Pediatrics 2014, 133, e312–e321. [Google Scholar] [CrossRef] [PubMed]
- Amat, F.; Plantard, C.; Mulliez, A.; Petit, I.; Rochette, E.; Verdan, M.; Henquell, C.; Labbe, G. RSV-hRV co-infection is a risk factor for recurrent bronchial obstruction and early sensitization 3 years after bronchiolitis. J. Med. Virol. 2018, 90, 867–872. [Google Scholar] [CrossRef]
- Yoshida, L.-M.; Suzuki, M.; Nguyen, H.A.; Le, M.N.; Vu, T.D.; Yoshino, H.; Schmidt, W.-P.; Nguyen, T.T.A.; Le, H.T.; Morimoto, K.; et al. Respiratory syncytial virus: Co-infection and paediatric lower respiratory tract infections. Eur. Respir. J. 2013, 42, 461–469. [Google Scholar] [CrossRef]
- Smith, A.M. Host-pathogen kinetics during influenza infection and coinfection: Insights from predictive modeling. Immunol. Rev. 2018, 285, 97–112. [Google Scholar] [CrossRef]
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between rhinovirus and influenza A virus: A clinical data analysis and experimental infection study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Casalegno, J.S.; Ottmann, M.; Duchamp, M.B.; Escuret, V.; Billaud, G.; Frobert, E.; Morfin, F.; Lina, B. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin. Microbiol. Infect. 2010, 16, 326–329. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. SARS-CoV-2 coinfections: Could influenza and the common cold be beneficial? J. Med. Virol. 2020, 92, 2623–2630. [Google Scholar] [CrossRef]
- Uncapher, C.R.; Dewitt, C.M.; Colonno, R.J. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991, 180, 814–817. [Google Scholar] [CrossRef]
- Abraham, G.; Colonno, R.J. Many rhinovirus serotypes share the same cellular receptor. J. Virol. 1984, 51, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hofer, F.; Gruenberger, M.; Kowalski, H.; Machat, H.; Huettinger, M.; Kuechler, E.; Blass, D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 1994, 91, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef]
- Cheemarla, N.R.; Watkins, T.A.; Mihaylova, V.T.; Wang, B.; Zhao, D.; Wang, G.; Zhao, D.; Wang, G.; Landry, M.L.; Foxman, E.F. Dynamic innate immune response determines susceptibility to SARS-CoV-2 infection and early replication kinetics. J. Exp. Med. 2021, 218, e202010583. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.; Goldfarb, D.M.; Haney, J.; Amat, J.A.R.; Herder, V.; Stewart, M.; Szemiel, A.M.; Baguelin, M.; Murcia, P.R. Human Rhinovirus Infection Blocks Severe Acute Respiratory Syndrome Coronavirus 2 Replication Within the Respiratory Epithelium: Implications for COVID-19 Epidemiology. J. Infect. Dis. 2021, 224, 31–38. [Google Scholar] [CrossRef]
- Murphy, R.C.; Lai, Y.; Barrow, K.A.; Hamerman, J.A.; Lacy-Hulbert, A.; Piliponsky, A.M.; Zieger, S.F.; Altemeier, W.A.; Debley, J.S.; Gharib, S.A.; et al. Effects of Asthma and Human Rhinovirus A16 on the Expression of SARS-CoV-2 Entry Factors in Human Airway Epithelium. Am. J. Respir. Cell Mol. Biol. 2020, 63, 859–863. [Google Scholar] [CrossRef]
- Onabajo, O.O.; Banday, A.R.; Stanifer, M.L.; Yan, W.; Obajemu, A.; Santer, D.M.; Piontkivska, H.; Vargas, J.M.; Ting, T.J.; Kee, C.; et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat. Genet. 2020, 52, 1283–1293. [Google Scholar] [CrossRef]
- Blume, C.; Jackson, C.L.; Spalluto, C.M.; Legebeke, J.; Nazlamova, L.; Conforti, F.; Perotin, J.-M.; Frank, M.; Butler, J.; Crispin, M.; et al. A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection. Nat. Genet. 2021, 53, 205–214. [Google Scholar] [CrossRef]
- Van Leuven, J.T.; Gonzalez, A.J.; Ijezie, E.C.; Wixom, A.Q.; Clary, J.L.; Naranjo, M.N.; Ridenhour, N.J.; Miller, C.R.; Miura, T.A. Rhinovirus Reduces the Severity of Subsequent Respiratory Viral Infections by Interferon-Dependent and-Independent Mechanisms. mSphere 2021, 6, e0047921. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Ijezie, E.C.; Balemba, O.B.; Miura, T.A. Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model. J. Virol. 2018, 92, 00881-18. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Geiser, J.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C. Author Correction: Interferon-Dependent and Respiratory Virus-Specific Interference in Dual Infections of Airway Epithelia. Sci. Rep. 2020, 10, 12523. [Google Scholar] [CrossRef]
- Gama, R.E.; Horsnell, P.R.; Hughes, P.J.; North, C.; Stanway, G.; Bruce, C.B.; Al-Nakib, W. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J. Med. Virol. 1989, 28, 73–77. [Google Scholar] [CrossRef]
- Ireland, D.C.; Kent, J.; Nicholson, K.G. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J. Med. Virol. 1993, 40, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, Y.A.; Grindle, K.; Vang, F.; Evans, M.; Gern, J.E. Improved Molecular Typing Assay for Rhinovirus Species A, B, and C. J. Clin. Microbiol. 2014, 52, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Faux, C.E.; Arden, K.; Lambert, S.; Nissen, M.; Nolan, T.M.; Chang, A.B.; Sloots, T.P.; Mackay, I.M. Usefulness of Published PCR Primers in Detecting Human Rhinovirus Infection. Emerg. Infect. Dis. 2011, 17, 296–298. [Google Scholar] [CrossRef]
- McIntyre, C.L.; Leitch, E.C.M.; Savolainen-Kopra, C.; Hovi, T.; Simmonds, P. Analysis of Genetic Diversity and Sites of Recombination in Human Rhinovirus Species C. J. Virol. 2010, 84, 10297–10310. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.H.; Rampersaud, H.; Houck, H.J. Comparison of Two Multiplex Methods for Detection of Respiratory Viruses: FilmArray RP and xTAG RVP. J. Clin. Microbiol. 2011, 49, 2449–2453. [Google Scholar] [CrossRef] [PubMed]
- Palmenberg, A.C.; Gern, J.E. Classification and Evolution of Human Rhinoviruses. Methods Mol. Biol 2015, 1221, 1–10. [Google Scholar]
- Esneau, C.; Bartlett, N.; Bochkov, Y.A. Rhinovirus structure, replication, and classification. In Rhinovirus Infections; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 1–23. [Google Scholar]
- Esneau, C.; Croft, S.; Loo, S.-L.; Ghildyal, R. Rhinovirus diversity and virulence factors. In Rhinovirus Infections, Rethinking the Impact on Human Health and Disease; Bartlett, N.W., Wark, P., Knight, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–59. [Google Scholar]
- Sansone, M.; Andersson, M.; Brittain-Long, R.; Andersson, L.-M.; Olofsson, S.; Westin, J.; Lindh, M. Rhinovirus infections in western Sweden: A four-year molecular epidemiology study comparing local and globally appearing types. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.; Peremiquel-Trillas, P.; Gimferrer, L.; Isern, A.; Piñana, M.; Rodrigo-Pendás, J. Ángel; Codina, M.G.; Martín, M.D.C.; Fuentes, F.; Rubio, S.; et al. Genetic diversity of rhinoviruses detected at a tertiary hospital in Catalonia (Spain) during the 2014–2017 seasons. Future Microbiol. 2018, 13, 1565–1573. [Google Scholar] [CrossRef]
- Tran, D.N.; Trinh, Q.D.; Pham, N.T.K.; Pham, T.M.H.; Ha, M.T.; Nguyen, T.Q.N.; Okitsu, S.; Shimizu, H.; Hayakawa, S.; Mizuguchi, M.; et al. Human rhinovirus infections in hospitalized children: Clinical, epidemiological and virological features. Epidemiol. Infect. 2016, 144, 346–354. [Google Scholar] [CrossRef]
- Luka, M.M.; Kamau, E.; Adema, I.; Munywoki, P.K.; Otieno, G.P.; Gicheru, E.; Gichuki, A.; Kibinge, N.; Agoti, C.N.; Nokes, D.J. Molecular Epidemiology of Human Rhinovirus From 1-Year Surveillance Within a School Setting in Rural Coastal Kenya. Open Forum Infect. Dis. 2020, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.-B.; Wo, Y.; Wang, L.-Y.; Wang, H.-Y.; Huang, D.-D.; Zhang, X.-A.; Liu, W.; Cao, W.-C. Molecular Epidemiology of Human Rhinovirus in Children with Acute Respiratory Diseases in Chongqing, China. Sci. Rep. 2014, 4, 6686. [Google Scholar] [CrossRef]
- Onyango, C.O.; Welch, S.R.; Munywoki, P.K.; Agoti, C.N.; Bett, A.; Ngama, M.; Myers, R.; Cane, P.A.; Nokes, D.J. Molecular epidemiology of human rhinovirus infections in Kilifi, coastal Kenya. J. Med. Virol. 2012, 84, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, A.; Leitch, C.; Gaunt, E.; Harvala, H.; Simmonds, P. Screening Respiratory Samples for Detection of Human Rhinoviruses (HRVs) and Enteroviruses: Comprehensive VP4-VP2 Typing Reveals High Incidence and Genetic Diversity of HRV Species C. J. Clin. Microbiol. 2009, 47, 3958–3967. [Google Scholar] [CrossRef]
- Ratnamohan, V.M.; Zeng, F.; Donovan, L.; MacIntyre, C.R.; Kok, J.; Dwyer, D.E. Phylogenetic analysis of human rhinoviruses collected over four successive years in Sydney, Australia. Influ. Other Respir. Viruses 2016, 10, 493–503. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Kim, D.W.; Jung, H.D.; Min Cheong, H.; Kim, K.H.; Kim, D.S.; Kim, Y.-J. Identification of Recombinant Human Rhinovirus A and C in Circulating Strains from Upper and Lower Respiratory Infections. PLoS ONE 2013, 8, e68081. [Google Scholar] [CrossRef]
- Rathe, J.A.; Liu, X.; Tallon, L.J.; Gern, J.E.; Liggett, S.B. Full-genome sequence and analysis of a novel human rhinovirus strain within a divergent HRV-A clade. Arch. Virol. 2009, 155, 83–87. [Google Scholar] [CrossRef][Green Version]
- Louie, J.K.; Yagi, S.; Nelson, F.A.; Kiang, D.; Glaser, C.A.; Rosenberg, J.; Cahill, C.K.; Schnurr, D.P. Rhinovirus Outbreak in a Long Term Care Facility for Elderly Persons Associated with Unusually High Mortality. Clin. Infect. Dis. 2005, 41, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Daleno, C.; Piralla, A.; Scala, A.; Senatore, L.; Principi, N.; Esposito, S. Phylogenetic Analysis of Human Rhinovirus Isolates Collected from Otherwise Healthy Children with Community-Acquired Pneumonia during Five Successive Years. PLoS ONE 2013, 8, e80614. [Google Scholar] [CrossRef]
- Espínola, E.E.; Russomando, G.; Aquino, C.; Basualdo, W. Phylogeny-based classification of human rhinoviruses detected in hospitalized children with acute lower respiratory infection in Paraguay, 2010–2011. J. Med. Virol. 2013, 85, 1645–1651. [Google Scholar] [CrossRef]
- Kiyota, N.; Kobayashi, M.; Tsukagoshi, H.; Ryo, A.; Harada, S.; Kusaka, T.; Obuchi, M.; Shimojo, N.; Noda, M.; Kimura, H. Genetic analysis of human rhinovirus species A to C detected in patients with acute respiratory infection in Kumamoto prefecture, Japan 2011–2012. Infect. Genet. Evol. 2013, 21, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A.H.L.; Thomas, X.V.; van der Linden, L.; Wildenbeest, J.G.; Minnaar, R.P.; Jansen, R.R.; de Jong, M.D.; Sterk, P.J. Clinical, virological and epidemiological characteristics of rhinovirus infections in early childhood: A comparison between non-hospitalised and hospitalised children. J. Clin. Virol. 2015, 73, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Lamson, D.M.; Soave, R.; Guzman, B.H.; Shore, T.B.; Ritchie, E.K.; Zappetti, D.; Satlin, M.J.; Leonard, J.P.; van Besien, K.; et al. Clinical and molecular epidemiology of human rhinovirus infections in patients with hematologic malignancy. J. Clin. Virol. 2015, 71, 51–58. [Google Scholar] [CrossRef] [PubMed]
- L’Huillier, A.G.; Kaiser, L.; Petty, T.J.; Kilowoko, M.; Kyungu, E.; Hongoa, P.; Vieille, G.; Turin, L.; Genton, B.; D’Acremont, V.; et al. Molecular Epidemiology of Human Rhinoviruses and Enteroviruses Highlights Their Diversity in Sub-Saharan Africa. Viruses 2015, 7, 6412–6423. [Google Scholar] [CrossRef]
- Fall, A.; Dia, N.; Kébé, O.; Sarr, F.D.; Kiori, D.E.; Cissé, E.H.A.K.; Sy, S.; Goudiaby, D.; Richard, V.; Diop, O.M.; et al. Enteroviruses and Rhinoviruses: Molecular Epidemiology of the Most Influenza-Like Illness Associated Viruses in Senegal. Am. J. Trop. Med. Hyg. 2016, 95, 339–347. [Google Scholar] [CrossRef]
- Milanoi, S.; Ongus, J.R.; Gachara, G.; Coldren, R.; Bulimo, W. Serotype and genetic diversity of human rhinovirus strains that circulated in Kenya in 2008. Influ. Other Respir. Viruses 2016, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, J.; Perchetti, G.A.; Chu, H.Y.; Newman, K.L.; Katz, J.; Khatry, S.K.; LeClerq, S.C.; Jerome, K.R.; Tielsch, J.M.; Englund, J.A. Phylogenetic characterization of rhinoviruses from infants in Sarlahi, Nepal. J. Med. Virol. 2019, 91, 2108–2116. [Google Scholar] [CrossRef]
- Linster, M.; Donato, C.; Mah, M.G.; Grau, M.L.; Low, J.G.; Ooi, E.E.; Su, Y.C.; Smith, G.J.; Vijaykrishna, D. Genetic diversity of respiratory enteroviruses and rhinoviruses in febrile adults, Singapore, 2007–2013. Influ. Other Respir. Viruses 2019, 14, 67–71. [Google Scholar] [CrossRef] [PubMed]
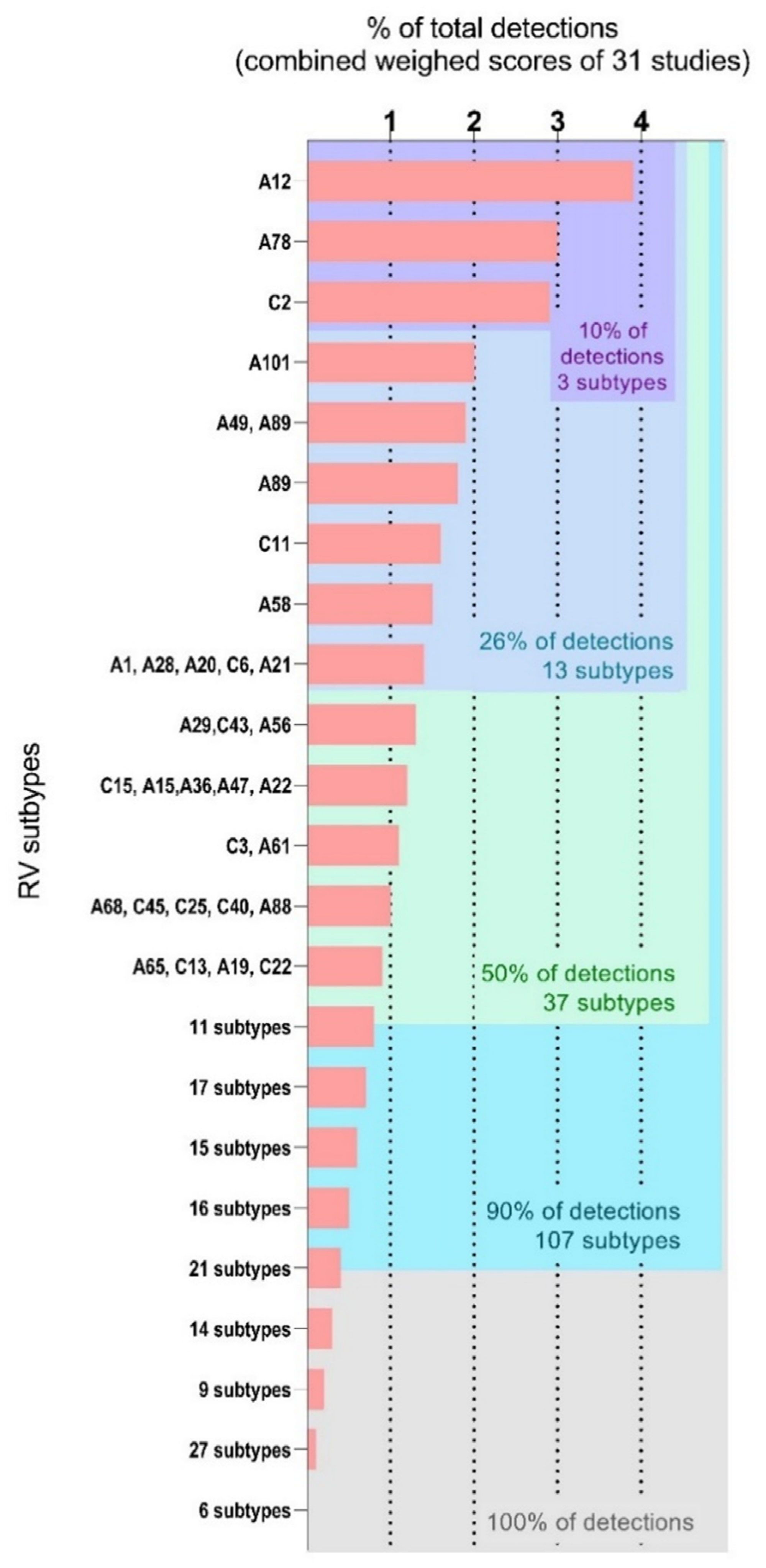
| Studies from Asia (11) | Studies from Europe (8) | Studies from Africa (7) | ||||||
|---|---|---|---|---|---|---|---|---|
| Top 25 Subtypes | % Detections | Rank Compared to General | Top 25 Subtypes | % Detections | Rank Compared to General | Top 25 Subtypes | % Detections | Rank Compared to General |
| A12 | 4.1 | 0 | A78 | 4.8 | 0 | A12 | 4.7 | 0 |
| C2 | 3.0 | 1 | A12 | 4.3 | 1 | A101 | 3.6 | 2 |
| A78 | 2.5 | −1 | C43 | 2.8 | 12 | C2 | 2.9 | 0 |
| A49 | 2.4 | 1 | C2 | 2.8 | 1 | A78 | 2.8 | −2 |
| A89 | 2.2 | 1 | A49 | 2.2 | 1 | C13 | 2.4 | 25 |
| A36 | 1.9 | 13 | C25 | 2.1 | 13 | A65 | 2.2 | 23 |
| C6 | 1.9 | 5 | A22 | 1.9 | 5 | A20 | 2.1 | 4 |
| A56 | 1.9 | 8 | A28 | 1.8 | 8 | A15 | 2.1 | 10 |
| A21 | 1.9 | 4 | C5 | 1.8 | 4 | C45 | 2.1 | 16 |
| A47 | 1.7 | 10 | A59 | 1.7 | 10 | A28 | 1.9 | 0 |
| A29 | 1.6 | 3 | A58 | 1.7 | 3 | A58 | 1.9 | −3 |
| A61 | 1.6 | 11 | A89 | 1.7 | 11 | C11 | 1.8 | −5 |
| A68 | 1.5 | 11 | A101 | 1.7 | 11 | A47 | 1.7 | 7 |
| C3 | 1.4 | 8 | A56 | 1.7 | 8 | A1 | 1.7 | −5 |
| A38 | 1.4 | 47 | C6 | 1.6 | 47 | A2 | 1.7 | 32 |
| C1 | 1.3 | 21 | C15 | 1.6 | 21 | C43 | 1.6 | −1 |
| A20 | 1.3 | −6 | C22 | 1.4 | −6 | C22 | 1.6 | 15 |
| C11 | 1.3 | −11 | A21 | 1.2 | −11 | B69 | 1.5 | 62 |
| A34 | 1.3 | 20 | A53 | 1.2 | 20 | B70 | 1.5 | 52 |
| A1 | 1.3 | −11 | C12 | 1.1 | −11 | A81 | 1.5 | 37 |
| C15 | 1.3 | −4 | C3 | 1.1 | −4 | B48 | 1.4 | 39 |
| A7 | 1.3 | 27 | A10 | 1.0 | 27 | C36 | 1.3 | 54 |
| A33 | 1.2 | 18 | C7 | 1.0 | 18 | C3 | 1.2 | −1 |
| C35 | 1.2 | 34 | C23 | 1.0 | 34 | B84 | 1.1 | 59 |
| A101 | 1.2 | −21 | C27 | 1.0 | −21 | A29 | 1.1 | −11 |
| Hospitalized (12) | Hospitalized vs. Outpatients | ||||
|---|---|---|---|---|---|
| Top 25 Subtypes | % Detections | Rank Compared to General (31) | Top 25 Subtypes | % Detections | Rank Compared to Outpatients (9) |
| A12 | 6.0 | 0 | A12 | 5.4 | 0 |
| A78 | 5.0 | 0 | A78 | 4.8 | 1 |
| C2 | 3.1 | 0 | C2 | 4.0 | −1 |
| A101 | 2.8 | 0 | A101 | 2.9 | 12 |
| A89 | 2.2 | 1 | A89 | 2.6 | 87 |
| C6 | 2.1 | 6 | C6 | 2.1 | 13 |
| A61 | 2.1 | 16 | A61 | 2.1 | 92 |
| A22 | 2.0 | 13 | A22 | 1.8 | 118 |
| C43 | 2.0 | 6 | C43 | 1.8 | 71 |
| A49 | 1.9 | −5 | A49 | 1.7 | 17 |
| A56 | 1.7 | 5 | A56 | 1.7 | 21 |
| A88 | 1.7 | 16 | A88 | 1.5 | 51 |
| A68 | 1.6 | 11 | A68 | 1.5 | 67 |
| A36 | 1.6 | 5 | A36 | 1.4 | 15 |
| C25 | 1.5 | 11 | C25 | 1.4 | 21 |
| A20 | 1.5 | −5 | A20 | 1.4 | −9 |
| A15 | 1.5 | 1 | A15 | 1.3 | −2 |
| C45 | 1.4 | 7 | C45 | 1.3 | 95 |
| A59 | 1.4 | 16 | A59 | 1.3 | 50 |
| C9 | 1.3 | 85 | C9 | 1.3 | 28 |
| A28 | 1.3 | −11 | A28 | 1.3 | −15 |
| A80 | 1.3 | 32 | A80 | 1.2 | 42 |
| A16 | 1.2 | 30 | A16 | 1.2 | 38 |
| A58 | 1.2 | −16 | A58 | 1.2 | −19 |
| C22 | 1.2 | 13 | C22 | 1.2 | −14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding Rhinovirus Circulation and Impact on Illness. Viruses 2022, 14, 141. https://doi.org/10.3390/v14010141
Esneau C, Duff AC, Bartlett NW. Understanding Rhinovirus Circulation and Impact on Illness. Viruses. 2022; 14(1):141. https://doi.org/10.3390/v14010141
Chicago/Turabian StyleEsneau, Camille, Alexandra Cate Duff, and Nathan W. Bartlett. 2022. "Understanding Rhinovirus Circulation and Impact on Illness" Viruses 14, no. 1: 141. https://doi.org/10.3390/v14010141
APA StyleEsneau, C., Duff, A. C., & Bartlett, N. W. (2022). Understanding Rhinovirus Circulation and Impact on Illness. Viruses, 14(1), 141. https://doi.org/10.3390/v14010141






