Does HTLV-1 Infection Show Phenotypes Found in Sjögren’s Syndrome?
Abstract
1. Introduction
2. Animal Models
2.1. Tax Transgenic Mice
2.2. Env-pX Transgenic Rat
2.3. HBZ Transgenic Mice
3. The Epidemiology of HTLV-1 Infection in SS
4. The Expression of HTLV-1 in SS
4.1. The Expression of HTLV-1 Gene in Labial Salivary Glands (LSGs)
| Target Gene | SS | Control | Author, Journal, Year | Ref. |
|---|---|---|---|---|
| tax | tax gene detected in 2/9 (22%) SS LSGs by ISH or PCR | tax gene detected in 0/9 (0%) control LSGs | Mariette, Arthritis Rheum, 1993 | [75] |
| tax | tax gene only was detected in 4/14 (29%) LSGs by PCR | Not performed | Sumida, Arthritis Rheum, 1994 | [76] |
| pX | gag, pol, env and pX gene were detected by PCR and pX by in situ PCR hybridization of 5 HTLV-1+SS patients | Not performed | Ohyama, Arthritis Rheum, 1998 | [77] |
| tax | tax gene was detected in 3/6 (50%) of SS patients from endemic area | tax gene was detected in 2/10 (20%) of dryness syndrome from non-endemic area | Tangy, J Infect Dis, 1999 | [78] |
| tax | Only 3/17 (18%) of HTLV-1-seronegative SS patients had tax gene detected by nested PCR | Not performed | Mizokami, Scand J Rheumatol, 1998 | [79] |
| tax | tax gene was detected in LSG from 30% of SS patients by PCR | tax gene was detected in 28% of inflammatory diseases and 4% of normal subjects | Mariette, Clin Exp Rheumatol, 2000 | [80] |
| tax, HBZ | tax was detected in MNCs and ductal epithelia cells by ISH in 3 HAM-SS patients. HBZ was observed in ATL and HTLV-1 carrier with SS | Neither tax nor HBZ was found in LSGs from 3 non-SS subjects | Nakamura, Clin Exp Rheumatol, 2018 | [82] |
4.2. The Expression of HTLV-1 Protein in LSGs
5. The Transmission of HTLV-1 to Salivary Gland Epithelial Cells
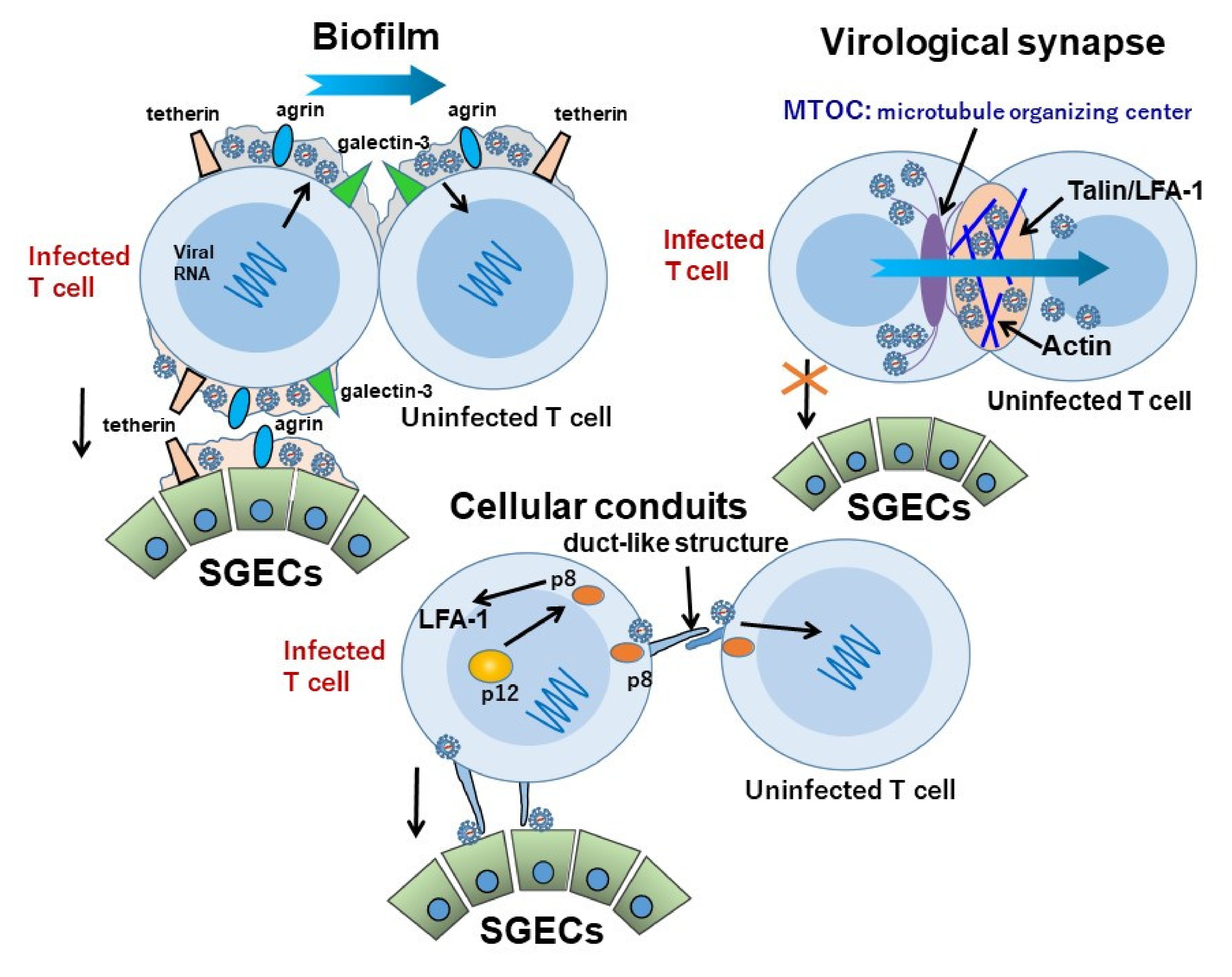
5.1. The Transmission of HTLV-1 between Lymphocytes
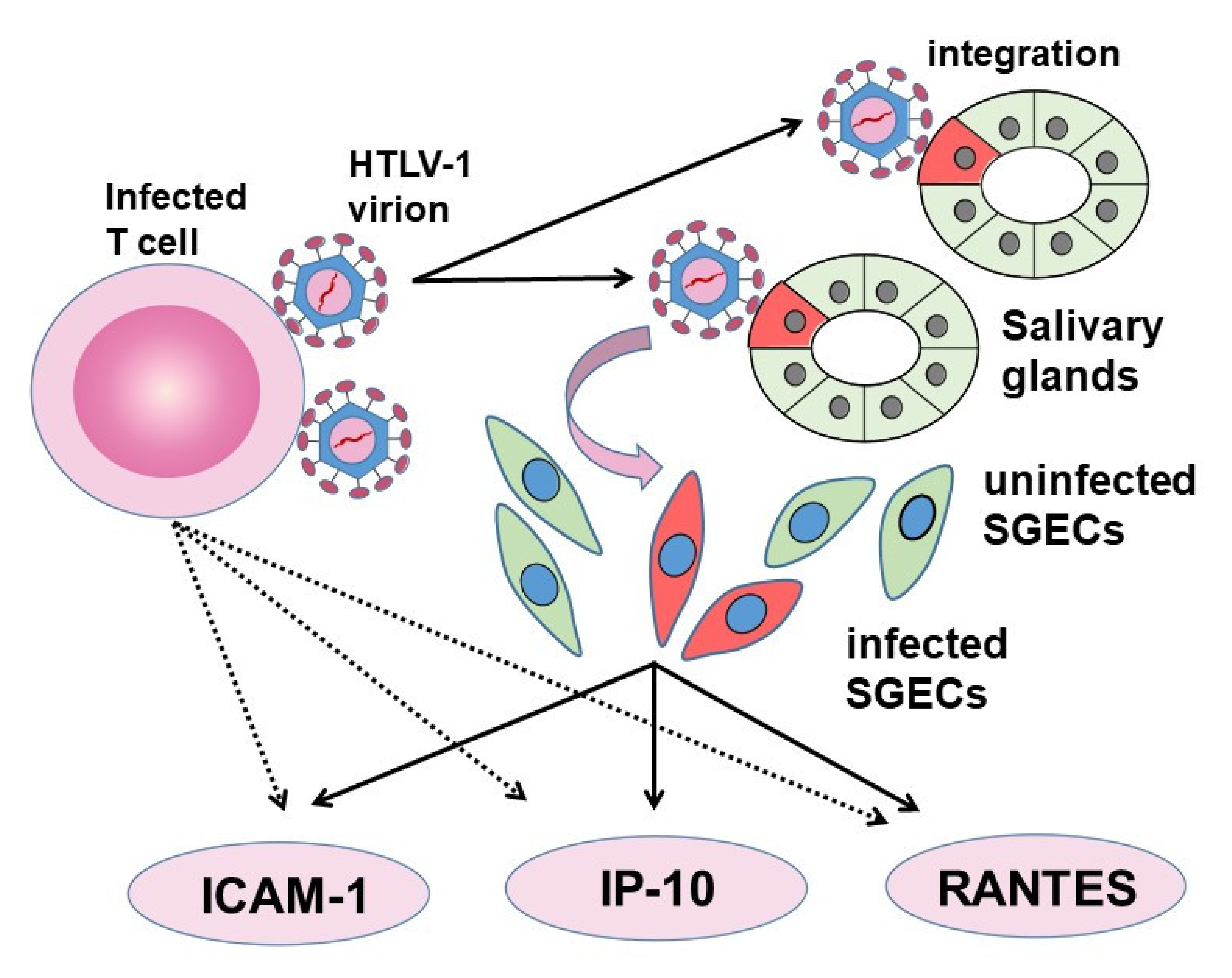
5.2. The Transmission of HTLV-1 between Lymphocytes and SGECs
5.3. The Role of SGECs in HTLV-1 Infection in SS
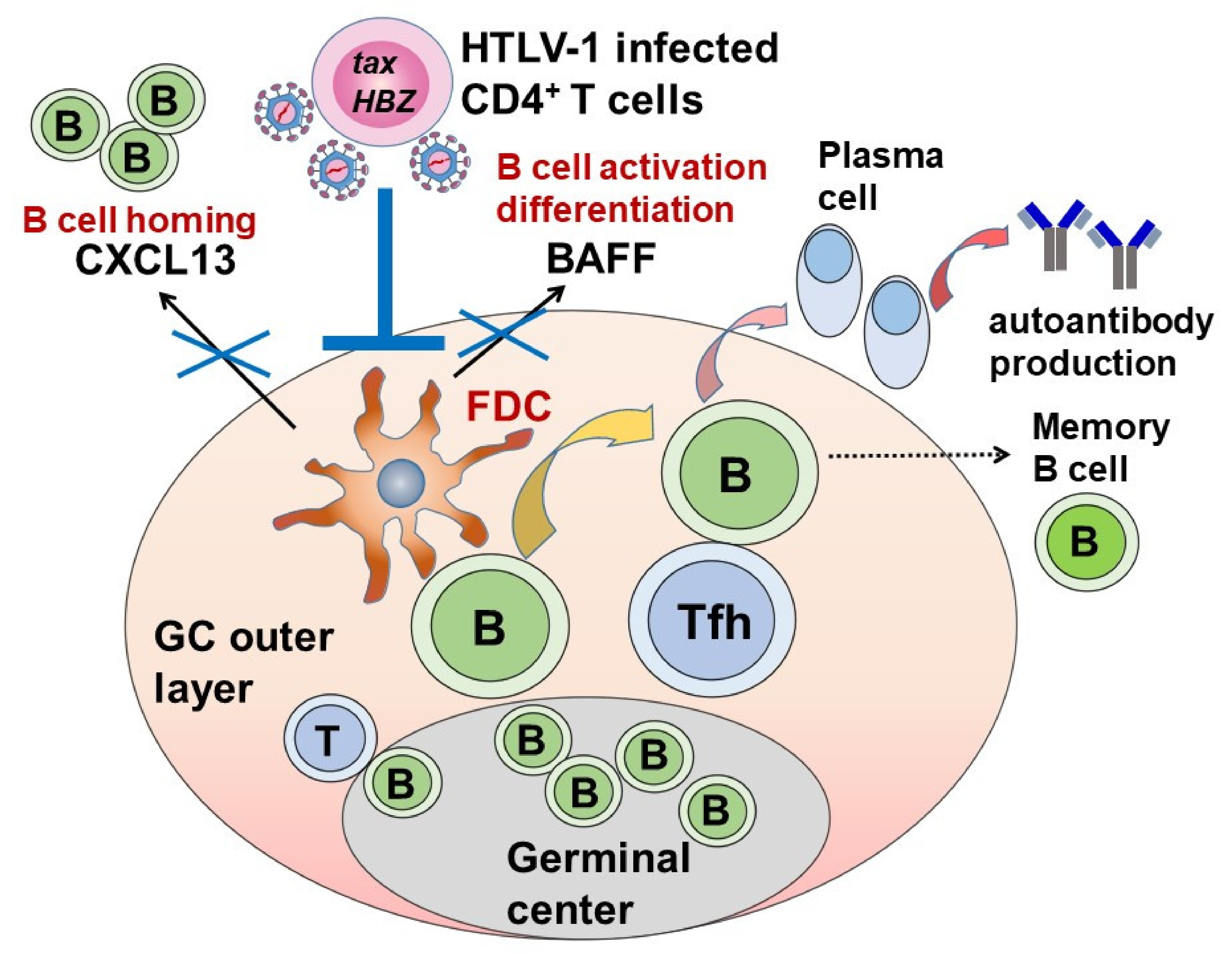
5.4. The Effect of HTLV-1 on Autoantibody Production in SS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Eguchi, K.; Matsuoka, N.; Ida, H.; Nakashima, M.; Sakai, M.; Sakito, S.; Kawakami, A.; Terada, K.; Shimada, H.; Kawabe, Y.; et al. Antibodies to HTLV-I in Sjogren’s syndrome. Ann. Rheum. Dis. 1992, 51, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Coulderc, L.J.; Desgranges, C.; Coste, J.; Caubarrere, I.; Clauvel, J.P. Antibodies to HTLV-I in Sjögren’s Syndrome. Lancet 1995, 345, 72. [Google Scholar] [PubMed]
- Vogetseder, W. Antibodies to HTLV-I in Sjögren’s Syndrome. Lancet 1995, 345, 72. [Google Scholar] [PubMed]
- Mariette, X.; Cherot, P.; Cazals, D.; Brocheriou, C.; Brouet, J.C.; Agbalika, F. Antibodies to HTLV-I in Sjögren’s Syndrome. Lancet 1995, 345, 71. [Google Scholar] [CrossRef] [PubMed]
- Ulirsch, R.C.; Jaffe, E.S. Sjögren’s syndrome-like illness associated with the acquired immunodeficiency syndrome-related complex. Hum. Pathol. 1987, 18, 1063–1068. [Google Scholar] [CrossRef]
- Talal, N. AIDS and Sjögren’s syndrome. Bull. Rheum. Dis. 1991, 40, 6–8. [Google Scholar] [PubMed]
- Coll, J.; Palazon, J.; Yazbeck, H.; Gutierrez, J.; Aubo, C.; Benito, P.; Jagiello, P.; Maldyk, H.; Marrugat, J.; Anglada, J.; et al. Antibodies to human immunodeficiency virus (HIV-1) in autoimmune diseases: Primary Sjögren’s syndrome, systemic lupus erythematosus, rheumatoid arthritis and autoimmune thyroid diseases. Clin. Rheumatol. 1995, 14, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Chilton, T.; Scott, S.; Benton, L.; Howell, F.V.; Vaughan, J.H. Potential role of Epstein-Barr virus in Sjogren’s syndrome. Rheum. Dis. Clin. N. Am. 1987, 13, 275–292. [Google Scholar] [CrossRef]
- Croia, C.; Astorri, E.; Murray-Brown, W.; Willis, A.; Brokstad, K.A.; Sutcliffe, N.; Piper, K.; Jonsson, R.; Tappuni, A.R.; Pitzalis, C.; et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjogren’s syndrome. Arthritis Rheumatol. 2014, 66, 2545–2557. [Google Scholar] [CrossRef]
- Haddad, J.; Deny, P.; Munz-Gotheil, C.; Mal, F.; Beaugrand, M.; Munz-Gotheil, C.; Callard, P.; Ambrosini, J.-C. Lymphocytic sialadenitis of Sjögren’s syndrome associated with chronic hepatitis C virus liver disease. Lancet 1992, 339, 321–323. [Google Scholar] [CrossRef]
- Aceti, A.; Taliani, G.; Sorice, M.; Amendolea, M. HCV and Sjögren’s syndrome. Lancet 1992, 339, 1425–1426. [Google Scholar] [CrossRef]
- Rodriguez-Cuartero, A.; Garcia-Vera, E.; Gomez-Cerro, A. Hepatitis C virus and Sjögren’s syndrome. Infection 1994, 22, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chu, A. Sjogren’s syndrome and viral infections. Rheumatol. Ther. 2021, 8, 1051–1059. [Google Scholar] [CrossRef]
- Sorgato, C.C.; Lins-E-Silva, M.; Leão, J.C.; Vasconcelos, L.R.; Romão, T.P.; Duarte, A.L.; Gueiros, L.A. EBV and CMV viral load in rheumatoid arthritis and their role in associated Sjogren’s syndrome. J. Oral Pathol. Med. 2020, 49, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Nocturne, G.; Mariette, X. Current and future therapies for primary Sjögren syndrome. Nat. Rev. Rheumatol. 2021, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Parisis, D.; Chivasso, C.; Perret, J.; Soyfoo, M.S.; Delporte, C. Current state of knowledge on primary Sjögren’s syndrome, an autoimmune exocrinopathy. J. Clin. Med. 2020, 9, 2299. [Google Scholar] [CrossRef] [PubMed]
- Garry, R.F.; Fermin, C.D.; Hart, D.J.; Alexander, S.S.; Donehower, L.A.; Luo-Zhang, H. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science 1990, 250, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Talal, N.; Flescher, E.; Dang, H. Are endogenous retroviruses involved in human autoimmune disease? J. Autoimmun. 1992, 5 (Suppl. A), 61–66. [Google Scholar] [CrossRef]
- Koike, K.; Moriya, K.; Ishibashi, K.; Yotsuyanagi, H.; Shintani, Y.; Fujie, H.; Kurokawa, K.; Matsuura, Y.; Miyamura, T. Sialadenitis histologically resembling Sjogren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc. Natl. Acad. Sci. USA 1997, 94, 233–236. [Google Scholar] [CrossRef]
- Shillitoe, E.J.; Daniels, T.E.; Whitcher, J.P.; Strand, C.V.; Talal, N.; Greenspan, J.S. Antibody to cytomegalovirus in patients with Sjögren’s syndrome. As determined by an enzyme-linked immunosorbent assay. Arthritis Rheum. 1982, 25, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Origgi, L.; Hu, C.; Bertetti, E.; Asero, R.; D’Agostino, P.; Radelli, L.; Riboldi, P. Antibodies to Epstein-Barr virus and cytomegalovirus in primary Sjogren’s syndrome. Boll. Ist. Sieroter. Milan. 1988, 67, 265–274. [Google Scholar] [PubMed]
- Scully, C. Sjögren’s syndrome: No demonstrable association by serology of secondary Sjögren’s syndrome with cytomegalovirus. J. Oral Pathol. Med. 1990, 19, 43–44. [Google Scholar] [CrossRef]
- Fleck, M.; Kern, E.R.; Zhou, T.; Lang, B.; Mountz, J.D. Murine cytomegalovirus induces a Sjögren’s syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis Rheum. 1998, 41, 2175–2184. [Google Scholar] [CrossRef]
- Hidaka, M.; Inoue, J.; Yoshida, M.; Seiki, M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988, 7, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Kobayashi, N. The pX region of HTLV-I. Princess Takamatsu Symp. 1984, 15, 205–217. [Google Scholar]
- Ruben, S.M.; Perkins, A.; Rosen, C.A. Activation of NF-kappa B by the HTLV-I trans-activator protein Tax requires an additional factor present in lymphoid cells. New Biol. 1989, 1, 275–284. [Google Scholar] [PubMed]
- Gaudray, G.; Gachon, F.; Basbous, J.; Biard-Piechaczyk, M.; Devaux, C.; Mesnard, J.-M. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 2002, 76, 12813–12822. [Google Scholar] [CrossRef] [PubMed]
- Basbous, J.; Arpin, C.; Gaudray, G.; Piechaczyk, M.; Devaux, C.; Mesnard, J.-M. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J. Biol. Chem. 2003, 278, 43620–43627. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, K.; Uchiyama, T.; Sagawa, K.; Yodoi, J. Adult T Cell Leukemia in Japan. In Topics in Hematology; Excerpta Medica: Amsterdam, The Netherlands, 1977; pp. 73–77. [Google Scholar]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 1981, 78, 6476–6480. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. A retrovirus from human leukemia cell lines: Its isolation, characterization, and implication in human adult T-cell leukemia (ATL). Princess Takamatsu Symp. 1982, 12, 285–294. [Google Scholar]
- Osame, M.; Matsumoto, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Tara, M.; Igata, A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann. Neurol. 1987, 21, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mochizuki, M.; Yamaguchi, K. HTLV-1 uveitis (HU). Leukemia 1997, 11 (Suppl. S3), 582–584. [Google Scholar]
- Satake, M.; Yamaguchi, K.; Tadokoro, K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J. Med. Virol. 2012, 84, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Iwanaga, M.; Sagara, Y.; Watanabe, T.; Okuma, K. Incidence of human T-lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: A nationwide retrospective cohort analysis. Lancet Infect. Dis. 2016, 16, 1246–1254. [Google Scholar] [CrossRef]
- Green, J.E.; Hinrichs, S.H.; Vogel, J.; Jay, G. Exocrinopathy resembling Sjögren’s syndrome in HTLV-1 tax transgenic mice. Nature 1989, 341, 72–74. [Google Scholar] [CrossRef]
- Green, J.E. Trans activation of nerve growth factor in transgenic mice containing the human T-cell lymphotropic virus type I tax gene. Mol. Cell Biol. 1991, 11, 4635–4641. [Google Scholar]
- Nerenberg, M.; Hinrichs, S.H.; Reynolds, R.K.; Khoury, G.; Jay, G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987, 237, 1324–1329. [Google Scholar] [CrossRef]
- Hinrichs, S.H.; Nerenberg, M.; Reynolds, R.K.; Khoury, G.; Jay, G. A transgenic mouse model for human neurofibromatosis. Science 1987, 237, 1340–1343. [Google Scholar] [CrossRef]
- Peebles, R.S.; Maliszewski, C.R.; Sato, T.A.; Hanley-Hyde, J.; Maroulakou, I.G.; Hunziker, R.; Schneck, J.P.; E Green, J. Abnormal B-cell function in HTLV-I-tax transgenic mice. Oncogene 1995, 10, 1045–1051. [Google Scholar] [PubMed]
- Iwakura, Y.; Tosu, M.; Yoshida, E.; Takiguchi, M.; Sato, K.; Kitajima, I.; Nishioka, K.; Yamamoto, K.; Takeda, T.; Hatanaka, M.; et al. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science 1991, 253, 1026–1028. [Google Scholar] [CrossRef]
- Habu, K.; Nakayama-Yamada, J.; Asano, M.; Saijo, S.; Itagaki, K.; Horai, R.; Yamamoto, H.; Sekiguchi, T.; Nosaka, T.; Hatanaka, M.; et al. The human T cell leukemia virus type I-tax gene is responsible for the development of both inflammatory polyarthropathy resembling rheumatoid arthritis and noninflammatory ankylotic arthropathy in transgenic mice. J. Immunol. 1999, 162, 2956–2963. [Google Scholar]
- Yoshiki, T.; Kondo, N.; Chubachi, T.; Tateno, M.; Togashi, T.; Itoh, T. Rat lymphoid cell lines with HTLV-I production. III. Transmission of HTLV-I into rats and analysis of cell surface antigens associated with HTLV-I. Arch. Virol. 1987, 97, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, N.; Abe, M.; Seto, K.; Sakurai, H.; Ikeda, H.; Wakisaka, A.; Togashi, T.; Tateno, M.; Yoshiki, T. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J. Exp. Med. 1992, 176, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, T. Chronic progressive myeloneuropathy in WKAH rats induced by HTLV-I infection as an animal model for HAM/TSP in humans. Intervirology 1995, 38, 229–237. [Google Scholar] [CrossRef]
- Yamada, T.; Yamaoka, S.; Goto, T.; Nakai, M.; Tsujimoto, Y.; Hatanaka, M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J. Virol. 1994, 68, 3374–3379. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kannagi, M.; Ohashi, T.; Kato, H.; Arai, M.; Nunez, G.; Iwanaga, Y.; Yamamoto, N.; Ohtani, K.; Nakamura, M.; et al. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 1999, 73, 7981–7987. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ikeda, H.; Ishizu, A.; Nakamaru, Y.; Sugaya, T.; Kikuchi, K.; Yamada, S.; Wakisaka, A.; Kasai, N.; Koike, T.; et al. A wide spectrum of collagen vascular and autoimmune diseases in transgenic rats carrying the env-pX gene of human T lymphocyte virus type I. Int. Immunol. 1997, 9, 339–346. [Google Scholar] [CrossRef]
- Nakamaru, Y.; Ishizu, A.; Ikeda, H.; Sugaya, T.; Fugo, K.; Higuchi, M.; Yamazaki, H.; Yoshiki, T. Immunological hyperresponsiveness in HTLV-I LTR-env-pX transgenic rats: A prototype animal model for collagen vascular and HTLV-I-related inflammatory diseases. Pathobiology 2001, 69, 11–18. [Google Scholar] [CrossRef]
- Owen, S.M.; Rudolph, D.L.; Dezzutti, C.S.; Shibata, N.; Naik, S.; Caughman, S.W.; Lal, R.B. Transcriptional activation of the intercellular adhesion molecule 1 (CD54) gene by human T lymphotropic virus types I and II Tax is mediated through a palindromic response element. AIDS Res. Hum. Retrovir. 1997, 13, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Macaire, H.; Riquet, A.; Moncollin, V.; Biémont-Trescol, M.C.; Dodon, M.D.; Hermine, O.; Debaud, A.L.; Mahieux, R.; Mesnard, J.M.; Pierre, M.; et al. Tax protein-induced expression of antiapoptotic Bfl-1 protein contributes to survival of human T-cell leukemia virus type 1 (HTLV-1)-infected T-cells. J. Biol. Chem. 2012, 287, 21357–21370. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, T.; Mitani, K.; Ueno, H.; Kanda, Y.; Yazaki, Y.; Hirai, H. Triple synergism of human T-lymphotropic virus type 1-encoded tax, GATA-binding protein, and AP-1 is required for constitutive expression of the interleukin-5 gene in adult T-cell leukemia cells. Mol. Cell Biol. 1997, 17, 4272–42781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexandre, C.; Verrier, B. Four regulatory elements in the human c-fos promoter mediate transactivation by HTLV-1 Tax protein. Oncogene 1991, 6, 543–551. [Google Scholar] [PubMed]
- Sakamoto, K.M.; Nimer, S.D.; Rosenblatt, J.D.; Gasson, J.C. HTLV-I and HTLV-II tax trans-activate the human EGR-1 promoter through different cis-acting sequences. Oncogene 1992, 7, 2125–2130. [Google Scholar]
- Fujii, M.; Tsuchiya, H.; Chuhjo, T.; Akizawa, T.; Seiki, M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992, 6, 2066–20676. [Google Scholar] [CrossRef]
- Janknecht, R.; Wells, N.J.; Hunter, T. TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998, 12, 2114–2119. [Google Scholar] [CrossRef]
- Zhao, T.; Satou, Y.; Sugata, K.; Miyazato, P.; Green, P.L.; Imamura, T.; Matsuoka, M. HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood 2011, 118, 1865–1876. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Taguchi, N.; Satou, Y.; Miyazato, P.; Ohshima, K.; Nakagawa, M.; Katagiri, K.; Kinashi, T.; Matsuoka, M. HTLV-1 bZIP factor induces inflammation through labile Foxp3 expression. PLoS Pathog. 2013, 9, e1003630. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Guimarães de Souza, V.; Lobato Martins, M.; Carneiro-Proietti, A.B.; Januário, J.N.; Ladeira, R.V.P.; Silva, C.M.S.; Pires, C.; Gomes, S.C.; Martins, C.D.S.; Mochel, E.G. High prevalence of HTLV-1 and 2 viruses in pregnant women in São Luis, state of Maranhão, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 159–162. [Google Scholar] [CrossRef][Green Version]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Gruber, K. Australia tackles HTLV-1. Lancet Infect. Dis. 2018, 18, 1073–1074. [Google Scholar] [CrossRef]
- Kakugawa, T.; Sakamoto, N.; Ishimoto, H.; Shimizu, T.; Nakamura, H.; Nawata, A.; Ito, C.; Sato, S.; Hanaka, T.; Oda, K.; et al. Lymphocytic focus score is positively related to airway and interstitial lung diseases in primary Sjögren’s syndrome. Respir. Med. 2018, 137, 95–102. [Google Scholar] [CrossRef]
- Lima, C.M.; Santos, S.; Dourado, A.; Carvalho, N.; Bittencourt, V.; Lessa, M.M.; de Siqueira, I.; Carvalho, E.M. Association of Sicca syndrome with proviral load and proinflammatory cytokines in HTLV-1 Infection. J. Immunol. Res. 2016, 2016, 8402059. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Katamine, S.; Eguchi, K.; Miyamoto, T.; Eguchi, K.; Kita, M.; Shimada, H.; Yamashita, I.; Nagataki, S.; Iwata, K.; et al. Prevalence of serum and salivary antibodies to HTLV-1 in Sjögren’s syndrome. Lancet 1994, 344, 1116–1119. [Google Scholar] [CrossRef]
- Nakamura, H.; Eguchi, K.; Nakamura, T.; Mizokami, A.; Shirabe, S.; Kawakami, A.; Matsuoka, N.; Migita, K.; Kawabe, Y.; Nagataki, S. High prevalence of Sjögren’s syndrome in patients with HTLV-I associated myelopathy. Ann. Rheum. Dis. 1997, 56, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Shimizu, T.; Takagi, Y.; Takahashi, Y.; Horai, Y.; Nakashima, Y.; Sato, S.; Shiraishi, H.; Nakamura, T.; Fukuoka, J.; et al. Reevaluation for clinical manifestations of HTLV-I-seropositive patients with Sjögren’s syndrome. BMC Musculoskelet. Disord. 2015, 16, 335. [Google Scholar] [CrossRef]
- Merle, H.; Cabre, P.; Smadja, D.; Josset, P.; Landau, M.; Vernant, J.C. Sicca syndrome and HTLV-I-associated myelopathy/tropical spastic paraparesis. Jpn. J. Ophthalmol. 1999, 43, 509–512. [Google Scholar] [CrossRef]
- Mariette, X.; Agbalika, F.; Daniel, M.T.; Bisson, M.; Lagrange, P.; Brouet, J.C.; Morinet, F. Detection of human T lymphotropic virus type I tax gene in salivary gland epithelium from two patients with Sjögren’s syndrome. Arthritis Rheum. 1993, 36, 1423–1428. [Google Scholar] [CrossRef]
- Sumida, T.; Yonaha, F.; Maeda, T.; Kita, Y.; Iwamoto, I.; Koike, T.; Yoshida, S. Expression of sequences homologous to HTLV-I tax gene in the labial salivary glands of Japanese patients with Sjögren’s syndrome. Arthritis Rheum. 1994, 37, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Nakamura, S.; Hara, H.; Shinohara, M.; Sasaki, M.; Ikebe-Hiroki, A.; Mouri, T.; Tsunawaki, S.; Abe, K.; Shirasuna, K.; et al. Accumulation of human T lymphotropic virus type I-infected T cells in the salivary glands of patients with human T lymphotropic virus type I-associated Sjogren’s syndrome. Arthritis Rheum. 1998, 41, 1972–1978. [Google Scholar] [CrossRef]
- Tangy, F.; Ossondo, M.; Vernant, J.C.; Smadja, D.; Blétry, O.; Baglin, A.C.; Ozden, S. Human T cell leukemia virus type I expression in salivary glands of infected patients. J. Infect. Dis. 1999, 179, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Eguchi, K.; Moriuchi, R.; Futsuki, Y.; Terada, K.; Nakamura, H.; Miyamoto, T.; Katamine, A.S. Low copy numbers of human T-cell lymphotropic virus type I (HTLV-I) tax-like DNA detected in the salivary gland of seronegative patients with Sjogren’s syndrome in an HTLV-I endemic area. Scand. J. Rheumatol. 1998, 27, 435–440. [Google Scholar] [CrossRef]
- Mariette, X.; Agbalika, F.; Zucker-Franklin, D.; Clerc, D.; Janin, A.; Cherot, P.; Brouet, J.C. Detection of the tax gene of HTLV-I in labial salivary glands from patients with Sjogren’s syndrome and other diseases of the oral cavity. Clin. Exp. Rheumatol. 2000, 18, 341–347. [Google Scholar] [PubMed]
- Lee, S.J.; Lee, J.S.; Shin, M.G.; Tanaka, Y.; Park, D.-J.; Kim, T.-J.; Park, Y.-W.; Lee, S.-S. Detection of HTLV-1 in the labial salivary glands of patients with Sjogren’s syndrome: A distinct clinical subgroup? J. Rheumatol. 2012, 39, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Hasegawa, H.; Sasaki, D.; Takatani, A.; Shimizu, T.; Kurushima, S.; Horai, Y.; Nakashima, Y.; Nakamura, T.; Fukuoka, J.; et al. Detection of human T lymphotropic virus type-I bZIP factor and tax in the salivary glands of Sjogren’s syndrome patients. Clin. Exp. Rheumatol. 2018, 112, 51–60. [Google Scholar]
- Shattles, W.G.; Brookes, S.M.; Venables, P.J.; Clark, D.A.; Maini, R.N. Expression of antigen reactive with a monoclonal antibody to HTLV-1 P19 in salivary glands in Sjogren’s syndrome. Clin. Exp. Immunol. 1992, 89, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Renard, J.N.; Mizuno, F.; Narita, Y.; Uchida, Y.; Higashiyama, H.; Sakurai, H.; Saito, I. Retrovirus in salivary glands from patients with Sjogren’s syndrome. J. Clin. Pathol. 1997, 50, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Qian, L.; Wang, G.; Zhang, H.; Wang, X.; Chen, K.; Zhai, Z.; Li, Q.; Wang, Y.; et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren’s syndrome. J. Rheumatol. 2007, 34, 2438–2445. [Google Scholar]
- Satou, Y.; Utsunomiya, A.; Tanabe, J.; Nakagawa, M.; Nosaka, K.; Matsuoka, M. HTLV-1 modulates the frequency and phenotype of FoxP3+CD4+ T cells in virus-infected individuals. Retrovirology 2012, 30, 46. [Google Scholar] [CrossRef]
- Igakura, T.; Stinchcombe, J.C.; Goon, P.K.; Taylor, G.P.; Weber, J.N.; Griffiths, G.M.; Tanaka, Y.; Osame, M.; Bangham, C.R.M. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 2003, 299, 1713–1716. [Google Scholar] [CrossRef]
- Barnard, A.L.; Igakura, T.; Tanaka, Y.; Taylor, G.P.; Bangham, C. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood 2005, 106, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Pais-Correia, A.M.; Sachse, M.; Guadagnini, S.; Robbiati, V.; Lasserre, R.; Gessain, A.; Gout, O.; Alcover, A.; Thoulouze, M.-I. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 2010, 16, 83–89. [Google Scholar] [CrossRef]
- Thoulouze, M.I.; Alcover, A. Can viruses form biofilms? Trends Microbiol. 2011, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Maali, Y.; Journo, C.; Mahieux, R.; Dutartre, H. Microbial biofilms: Human T-cell leukemia virus type 1 first in line for viral biofilm but far behind bacterial biofilms. Front. Microbiol. 2020, 11, 2041. [Google Scholar] [CrossRef]
- Van Prooyen, N.; Gold, H.; Andresen, V.; Schwartz, O.; Jones, K.; Ruscetti, F.; Lockett, S.; Gudla, P.; Venzon, D.; Franchini, G. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. USA 2010, 107, 20738–20743. [Google Scholar] [CrossRef]
- Malbec, M.; Roesch, F.; Schwartz, O. A new role for the HTLV-1 p8 protein: Increasing intercellular conduits and viral cell-to-cell transmission. Viruses 2011, 3, 254–259. [Google Scholar] [CrossRef]
- Donhauser, N.; Socher, E.; Millen, S.; Heym, S.; Sticht, H.; Thoma-Kress, A.K. Transfer of HTLV-1 p8 and Gag to target T-cells depends on VASP, a novel interaction partner of p8. PLoS Pathog. 2020, 16, e1008879. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Huang, Y.K.; Bertolette, D.C.; Ruscetti, F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008, 14, 429–436. [Google Scholar] [CrossRef]
- Jain, P.; Manuel, S.L.; Khan, Z.K.; Ahuja, J.; Quann, K.; Wigdahl, B. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J. Virol. 2009, 83, 10908–10921. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.-L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.-M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.; Heveker, N.; Pique, C.; et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef]
- Jin, Q.; Alkhatib, B.; Cornetta, K.; Alkhatib, G. Alternate receptor usage of neuropilin-1 and glucose transporter protein 1 by the human T cell leukemia virus type 1. Virology 2010, 396, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Martin-Latil, S.; Gnädig, N.F.; Mallet, A.; Desdouits, M.; Guivel-Benhassine, F.; Jeannin, P.; Prevost, M.-C.; Schwartz, O.; Gessain, A.; Ozden, S.; et al. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood 2012, 120, 572–580. [Google Scholar] [CrossRef]
- Nakamura, H.; Shimizu, T.; Takatani, A.; Suematsu, T.; Nakamura, T.; Kawakami, A. Initial human T-cell leukemia virus type 1 infection of the salivary gland epithelial cells requires a biofilm-like structure. Virus Res. 2019, 269, 197643. [Google Scholar] [CrossRef]
- Nakamura, H.; Shimizu, T.; Kawakami, A. Role of viral infections in the pathogenesis of Sjogren’s syndrome: Different characteristics of Epstein-Barr Virus and HTLV-1. J. Clin. Med. 2020, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Barros, L.R.; Linhares-Lacerda, L.; Moreira-Ramos, K.; Ribeiro-Alves, M.; Motta, M.C.M.; Bou-Habib, D.C.; Savino, W. HTLV-1-infected thymic epithelial cells convey the virus to CD4(+) T lymphocytes. Immunobiology 2017, 222, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Z.; Mahesh, S.P.; Kurup, S.K.; Giam, C.-Z.; Nussenblatt, R.B.; Kurup, S.K. HTLV-1 infection of human retinal pigment epithelial cells and inhibition of viral infection by an antibody to ICAM-1. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Eguchi, K.; Terada, K.; Nakashima, M.; Yamashita, I.; Ida, H.; Kawabe, Y.; Aoyagi, T.; Takino, H.; Nakamura, T. Infection of human synovial cells by human T cell lymphotropic virus type I. Proliferation and granulocyte/macrophage colony-stimulating factor production by synovial cells. J. Clin. Investig. 1993, 92, 1957–1966. [Google Scholar] [CrossRef]
- Nakamura, H.; Takahashi, Y.; Yamamoto-Fukuda, T.; Horai, Y.; Nakashima, Y.; Arima, K.; Nakamura, T.; Koji, T.; Kawakami, A. Direct infection of primary salivary gland epithelial cells by human T lymphotropic virus type I in patients with Sjogren’s syndrome. Arthritis Rheumatol. 2015, 67, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Błochowiak, K.J.; Olewicz-Gawlik, A.; Trzybulska, D.; Nowak-Gabryel, M.; Kocięcki, J.; Witmanowski, H.; Sokalski, J. Serum ICAM-1, VCAM-1 and E-selectin levels in patients with primary and secondary Sjogren’s syndrome. Adv. Clin. Exp. Med. 2017, 26, 835–842. [Google Scholar] [CrossRef]
- Ogawa, N.; Ping, L.; Zhenjun, L.; Takada, Y.; Sugai, S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis Rheum. 2002, 46, 2730–2741. [Google Scholar] [CrossRef]
- Petrek, M.; Cermáková, Z.; Hutyrová, B.; Micekova, D.; Drabek, J.; Rovensky, J.; Bosak, V. CC chemokine receptor 5 and interleukin-1 receptor antagonist gene polymorphisms in patients with primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2002, 20, 701–703. [Google Scholar]
- Shi, K.; Hayashida, K.; Kaneko, M.; Hashimoto, J.; Tomita, T.; Lipsky, P.E.; Yoshikawa, H.; Ochi, T. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J. Immunol. 2001, 166, 650–655. [Google Scholar] [CrossRef]
- Ansel, K.M.; Ngo, V.N.; Hyman, P.L.; Luther, S.; Forster, R.; Sedgwick, J.D.; Browning, J.; Lipp, M.; Cyster, J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000, 406, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vissers, J.L.; Hartgers, F.C.; Lindhout, E.; Figdor, C.G.; Adema, G.J. BLC (CXCL13) is expressed by different dendritic cell subsets in vitro and in vivo. Eur. J. Immunol. 2001, 31, 1544–1549. [Google Scholar] [CrossRef]
- Salomonsson, S.; Jonsson, M.V.; Skarstein, K.; Brokstad, K.A.; Hjelmstrom, P.; Wahren-Herlenius, M.; Jonsson, R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003, 48, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Hayashi, T.; Nakamura, T.; Iwamoto, N.; Yamasaki, S.; Ida, H.; Eguchi, K. Low prevalence of ectopic germinal centre formation in patients with HTLV-I-associated Sjogren’s syndrome. Rheumatology 2009, 48, 854–855. [Google Scholar] [CrossRef][Green Version]
- Nakamura, H.; Takagi, Y.; Kawakami, A.; Ida, H.; Nakamura, T.; Eguchi, K. HTLV-I infection results in resistance toward salivary gland destruction of Sjogren’s syndrome. Clin. Exp. Rheumatol. 2008, 26, 653–655. [Google Scholar] [PubMed]
- Takatani, A.; Nakamura, H.; Furukawa, K.; Endo, Y.; Umeda, M.; Shimizu, T.; Nishihata, S.; Kitaoka, K.; Nakamura, T.; Kawakami, A. Inhibitory effect of HTLV-1 infection on the production of B-cell activating factors in established follicular dendritic cell-like cells. Immun. Inflamm. Dis. 2021, 9, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; MacKay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.-L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999, 189, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.D.; Sarosi, I.; Xia, X.Z.; McCabe, S.; Miner, K.; Solovyev, I.; Hawkins, N.; Kelley, M.; Chang, D.; Van, G.; et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sato, T.; Abe, M.; Nagai, S.; Onai, N.; Yoneyama, H.; Zhang, Y.-Y.; Suzuki, T.; Hashimoto, S.-I.; Shirai, T.; et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J. Exp. Med. 2001, 193, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
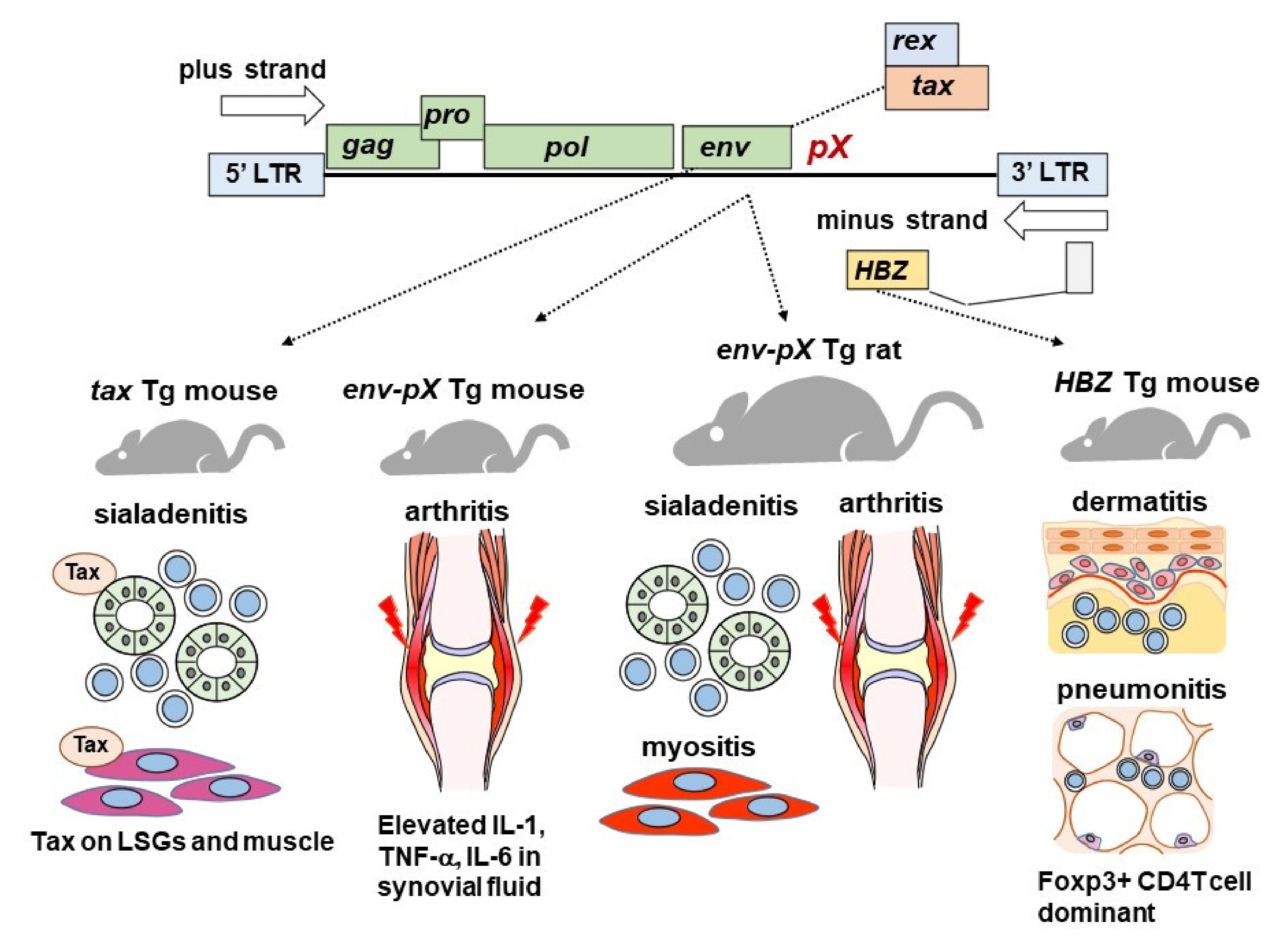
| Tg animals with HTLV-1 genes including tax and env-pX showed sialadenitis that resembled SS. Tax protein was found in tax Tg mice. |
| Epidemiologically, anti-HTLV-1 antibody was highly detected in SS. HAM was frequently complicated with SS, but a low frequency of autoantibodies was observed. |
| HTLV-1-related genes were detected by PCR or ISH in SS LSGs. A low frequency of tax was detectable in LSGs of patients with anti-HTLV-1-seronegative SS or other conditions |
| HTLV-1 virus particles were transmitted to salivary gland epithelial cells (SGECs) derived from SS at least via biofilm. |
| HTLV-1 was able to infect SGECs from SS patients, and SGECs after viral integration were able to induce chronic inflammation. |
| HTLV-1 had an inhibitory function against follicular dendritic cells, and this function is a key component against autoantibody production system. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, H.; Tsukamoto, M.; Nagasawa, Y.; Kitamura, N.; Shimizu, T.; Kawakami, A.; Nagata, K.; Takei, M. Does HTLV-1 Infection Show Phenotypes Found in Sjögren’s Syndrome? Viruses 2022, 14, 100. https://doi.org/10.3390/v14010100
Nakamura H, Tsukamoto M, Nagasawa Y, Kitamura N, Shimizu T, Kawakami A, Nagata K, Takei M. Does HTLV-1 Infection Show Phenotypes Found in Sjögren’s Syndrome? Viruses. 2022; 14(1):100. https://doi.org/10.3390/v14010100
Chicago/Turabian StyleNakamura, Hideki, Masako Tsukamoto, Yosuke Nagasawa, Noboru Kitamura, Toshimasa Shimizu, Atsushi Kawakami, Kinya Nagata, and Masami Takei. 2022. "Does HTLV-1 Infection Show Phenotypes Found in Sjögren’s Syndrome?" Viruses 14, no. 1: 100. https://doi.org/10.3390/v14010100
APA StyleNakamura, H., Tsukamoto, M., Nagasawa, Y., Kitamura, N., Shimizu, T., Kawakami, A., Nagata, K., & Takei, M. (2022). Does HTLV-1 Infection Show Phenotypes Found in Sjögren’s Syndrome? Viruses, 14(1), 100. https://doi.org/10.3390/v14010100






