Compassionate Use of GC5131 (Hyperimmunoglobulin) Therapy in Critically Ill Patients Diagnosed with COVID-19: A Case Series and Review of Literature
Abstract
:1. Introduction
2. Materials and Methods
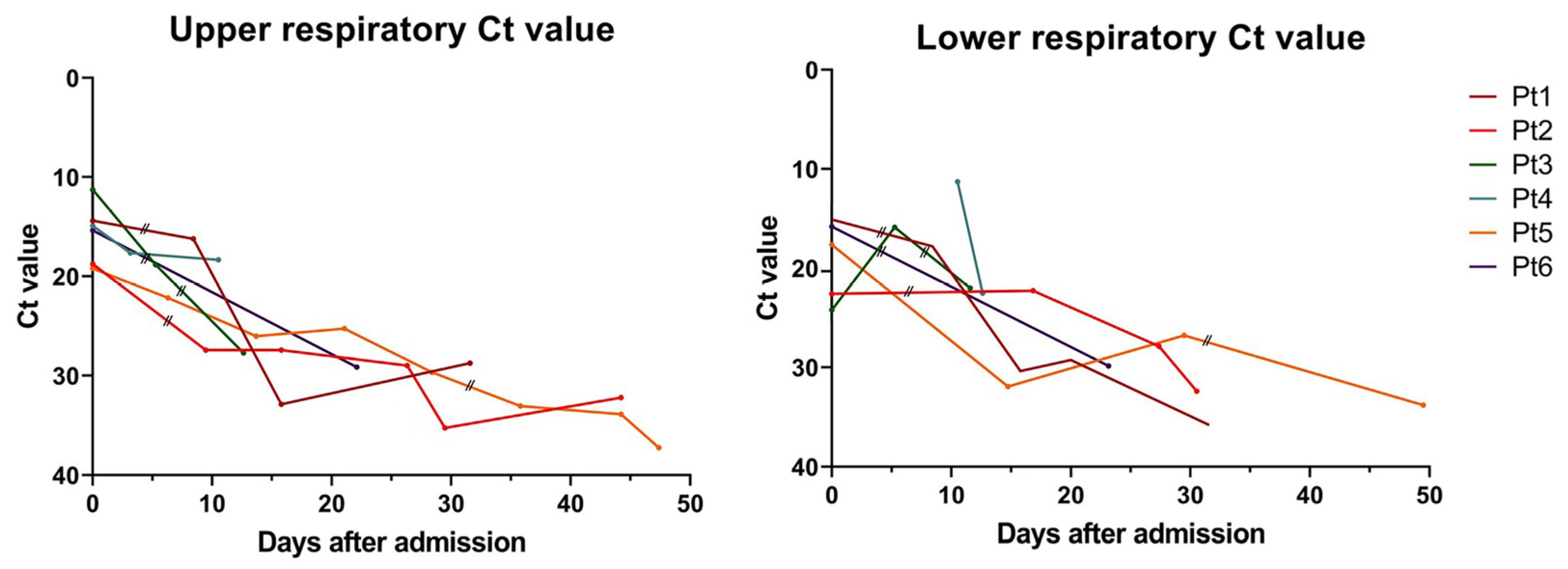
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartoletti, M.; Marconi, L.; Scudeller, L.; Pancaldi, L.; Tedeschi, S.; Giannella, M.; Rinaldi, M.; Bussini, L.; Valentini, I.; Ferravante, A.F. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: A multicentre study. Clin. Microbiol. Infect. 2021, 27, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Scarabel, L.; Guardascione, M.; Dal Bo, M.; Toffoli, G. Pharmacological strategies to prevent SARS-CoV-2 infection and to treat the early phases of COVID-19 disease. Int. J. Infect. Dis. 2021, 104, 441–451. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.A.; Habiballah, S.B.; Platt, C.D.; Geha, R.S.; Chou, J.S.; McDonald, D.R. Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution! Clin. Immunol. 2020, 216, 108459. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Monsalve, D.M.; Acosta-Ampudia, Y.; Camacho, B.; Gallo, J.E.; Rojas-Villarraga, A.; Ramírez-Santana, C.; Díaz-Coronado, J.C.; Manrique, R. Convalescent plasma in COVID-19: Possible mechanisms of action. Autoimmun. Rev. 2020, 19, 102554. [Google Scholar] [CrossRef] [PubMed]
- Cagdas, D. Convalescent plasma and hyperimmune globulin therapy in COVID-19. Expert. Rev. Clin. Immunol. 2021, 17, 309–316. [Google Scholar] [CrossRef]
- Im, J.H.; Nahm, C.H.; Baek, J.H.; Kwon, H.Y.; Lee, J.-S. Convalescent Plasma Therapy in Coronavirus Disease 2019: A Case Report and Suggestions to Overcome Obstacles. J. Korean. Med. Sci. 2020, 35, 1146085. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, M.; Rahbari, N.N. Compassionate use of medicinal products in Europe: Current status and perspectives. Bull. World. Health Organ. 2011, 89, 163. [Google Scholar] [CrossRef]
- Valk, S.J.; Piechotta, V.; Chai, K.L.; Doree, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.; Kimber, C.; McQuilten, Z.; So-Osman, C. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020, 5, CD013600. [Google Scholar]
- Klasse, P. Neutralization of virus infectivity by antibodies: Old problems in new perspectives. Adv. Biol. 2014, 2014, 157895. [Google Scholar] [CrossRef] [Green Version]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y. The feasibility of convalescent plasma therapy in severe COVID-19 patients: A pilot study. medRxiv 2020. [Google Scholar] [CrossRef]
- Gharbharan, A.; Jordans, C.C.; GeurtsvanKessel, C.; den Hollander, J.G.; Karim, F.; Mollema, F.P.; Stalenhoef-Schukken, J.E.; Dofferhoff, A.; Ludwig, I.; Koster, A. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M. Convalescent plasma antibody levels and the risk of death from COVID-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, G.; Pastori, C.; Lopalco, L. Humoral immune responses in COVID-19 patients: A window on the state of the art. Front. Immunol. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Patel, S.; Saxena, B.; Mehta, P. Recent updates in the clinical trials of therapeutic monoclonal antibodies targeting cytokine storm for the management of COVID-19. Heliyon 2021, 7, e06158. [Google Scholar] [CrossRef]
- Liu, L.; To, K.K.-W.; Chan, K.-H.; Wong, Y.-C.; Zhou, R.; Kwan, K.-Y.; Fong, C.H.-Y.; Chen, L.-L.; Choi, C.Y.-K.; Lu, L. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg. Microbes Infect. 2020, 9, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Okba, N.M.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D. Severe acute respiratory syndrome coronavirus 2− specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020, 26, 1478. [Google Scholar] [CrossRef]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.-M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Group, C.P.S.; Nguyen-Van-Tam, J.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.T.; Lin, H.-M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J. Convalescent plasma treatment of severe COVID-19: A propensity score–matched control study. Nat. Med. 2020, 26, 1708–1713. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy. Clin. Immunol. 2020, 146, 119–127.e4. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, B.; Xia, H.; Fan, H.; Zhu, M.; Zhu, L.; Zhang, H.; Tao, X.; Cheng, S.; Chen, J. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiol. Infect. 2020, 148, E199. [Google Scholar] [CrossRef]
- Korea Biomedical Review. Available online: https://www.koreabiomed.com/news/articleView.html?idxno=11315 (accessed on 6 September 2021).
- Ali, S.; Uddin, S.M.; Shalim, E.; Sayeed, M.A.; Anjum, F.; Saleem, F.; Muhaymin, S.M.; Ali, A.; Ali, M.R.; Ahmed, I. Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: A phase I/II randomized control trial. EClinicalMedicine 2021, 36, 100926. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy. Immunol. 2020, 38, 10–18. [Google Scholar]
- Webb, B.J.; Buckel, W.; Vento, T.; Butler, A.M.; Grisel, N.; Brown, S.M.; Peltan, I.D.; Spivak, E.S.; Shah, M.; Sakata, T. Real-World Effectiveness and Tolerability of Monoclonal Antibodies for Ambulatory Patients with Early COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Dölken, L.; Stich, A.; Spinner, C.D. Remdesivir for Early COVID-19 Treatment of High-Risk Individuals Prior to or at Early Disease Onset—Lessons Learned. Viruses 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age | 78 | 78 | 71 | 68 | 45 | 68 |
| Sex | Male | Female | Male | Male | Male | Female |
| Smoking history | Ex-smoker | No | Ex-smoker | No | No | No |
| Presenting symptoms | Fever, cough, myalgia | Fever, cough, dyspnea, diarrhea, sore throat | Fever, chills, general weakness | Dyspnea | Fever, diarrhea, general weakness | Cough, dyspnea |
| Days from symptoms onset to diagnosis | 6 | 1 | 5 | 5 | 8 | 3 |
| Date the GC5131 was administered | 2020.10.23 | 2020.11.18 | 2020.11.18 | 2020.12.12 | 2020.12.16 | 2020.12.17 |
| Days from application to administration of GC5131 | 9 | 6 | 6 | 5 | 2 | 3 |
| Days from application to approval for the compassionate use of GC5131 | 5 | 5 | 5 | 4 | 1 | 2 |
| Days from diagnosis to administration of GC5131 | 31 | 20 | 10 | 8 | 4 | 6 |
| Days from symptom onset to administration of GC5131 | 37 | 21 | 15 | 13 | 12 | 9 |
| Severity on admission day | Moderate | Severe | Severe | Severe | Critical | Severe |
| Severity on day of administration of GC5131 | Critical | Critical | Critical | Critical | Critical | Critical |
| Comorbidities | HTN Old stroke HCMP Asthma Dyslipidemia | HTN Dyslipidemia | Old TB COPD BPH Dyslipidemia | BPH | HTN Colon cancer | Obesity |
| HbA1c | 5.7 | 6.5 | 6.5 | 6.1 | 7.7 | 6.4 |
| Treatment (Before) a | ||||||
| Antivirals | Remdesivir | Remdesivir | None | Remdesivir | None | Remdesivir |
| Antibiotics or antifungal agents | Ceftriaxone, piperacillin-tazobactam, zithromax, meropenem, teicoplanin, doripenem, levofloxacin, fluconazole, colistin | Meropenem, moxifloxacin, piperacillin-tazobactam, teicoplanin, metronidazole, amikacin, fluconazole | Ceftriaxone, zithromax | Piperacillin-tazobactam, zithromax | Meropenem | Ceftriaxone, zithromax, meropenem, vancomycin |
| Steroid | Dexam | Dexam | Dexam | Dexam | Dexam | Dexam |
| Oxygen delivery devices | Ventilator | HFNC | Ventilator | Ventilator | Ventilator | Ventilator |
| Treatment (After) b | ||||||
| Antivirals | None | None | None | None | None | None |
| Antibiotics or antifungal agents | Meropenem, doripenem, colistin | Piperacillin-tazobactam, amikacin, teicoplanin, fluconazole | Piperacillin-tazobactam, zithromax, meropenem, vancomycin | Meropenem, levofloxacin, vancomycin, piperacillin-tazobactam, gentamicin, colistin, fluconazole | Meropenem, vancomycin | Meropenem, vancomycin, trimethoprim/sulfamethoxazole |
| Steroid | None | Dexam | Dexam | Dexam | Dexam | Dexam |
| Oxygen delivery devices | Ventilator | HFNC | Ventilator | Ventilator | Ventilator | Ventilator, ECMO |
| Outcome | Recovery, discharge 20 days after GC5131 administration | Death, 2 days after GC5131 administration | Death, 6 days after GC5131 administration | Death, 45 days after GC5131 administration | Death, 8 days after GC5131 administration | Death, 30 days after GC5131 administration |
| Cause of death | Respiratory failure | Respiratory failure | Respiratory failure | Respiratory failure | Respiratory failure, catheter related sepsis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Hwang, S.; Kwon, K. Compassionate Use of GC5131 (Hyperimmunoglobulin) Therapy in Critically Ill Patients Diagnosed with COVID-19: A Case Series and Review of Literature. Viruses 2021, 13, 1826. https://doi.org/10.3390/v13091826
Choi S, Hwang S, Kwon K. Compassionate Use of GC5131 (Hyperimmunoglobulin) Therapy in Critically Ill Patients Diagnosed with COVID-19: A Case Series and Review of Literature. Viruses. 2021; 13(9):1826. https://doi.org/10.3390/v13091826
Chicago/Turabian StyleChoi, Sunha, Soyoon Hwang, and Kitae Kwon. 2021. "Compassionate Use of GC5131 (Hyperimmunoglobulin) Therapy in Critically Ill Patients Diagnosed with COVID-19: A Case Series and Review of Literature" Viruses 13, no. 9: 1826. https://doi.org/10.3390/v13091826
APA StyleChoi, S., Hwang, S., & Kwon, K. (2021). Compassionate Use of GC5131 (Hyperimmunoglobulin) Therapy in Critically Ill Patients Diagnosed with COVID-19: A Case Series and Review of Literature. Viruses, 13(9), 1826. https://doi.org/10.3390/v13091826






