RNA Structures and Their Role in Selective Genome Packaging
Abstract
1. Introduction
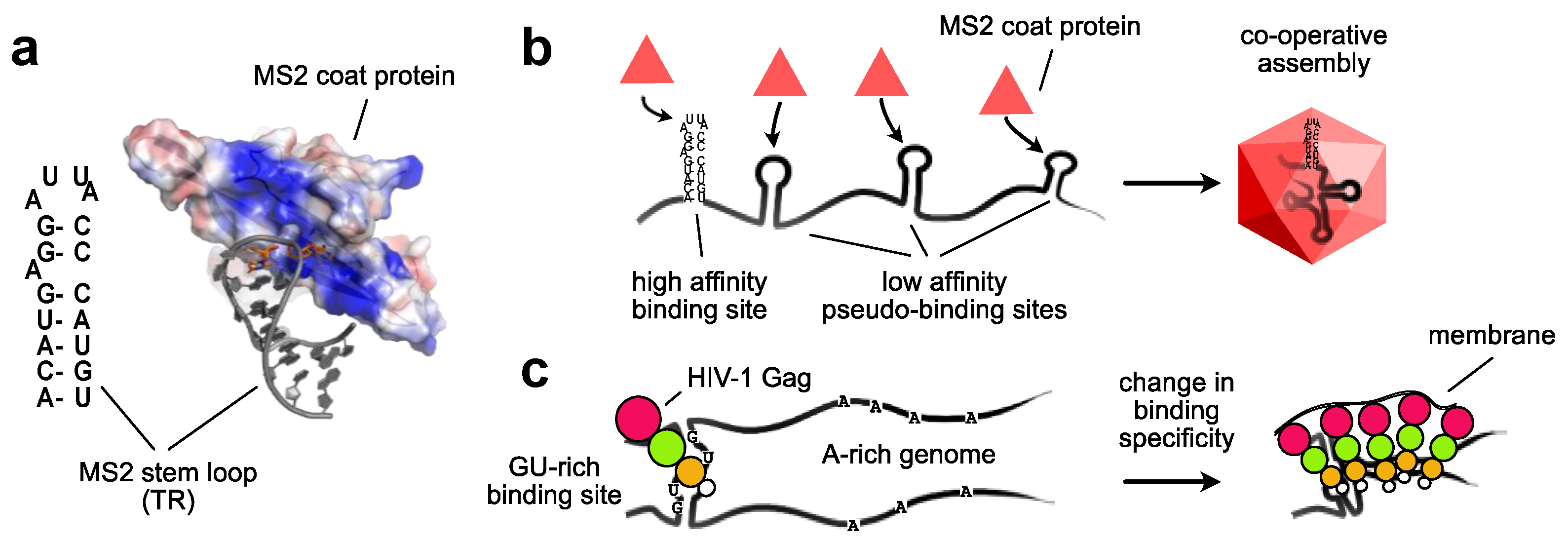
2. Packaging Signals in RNA Viruses
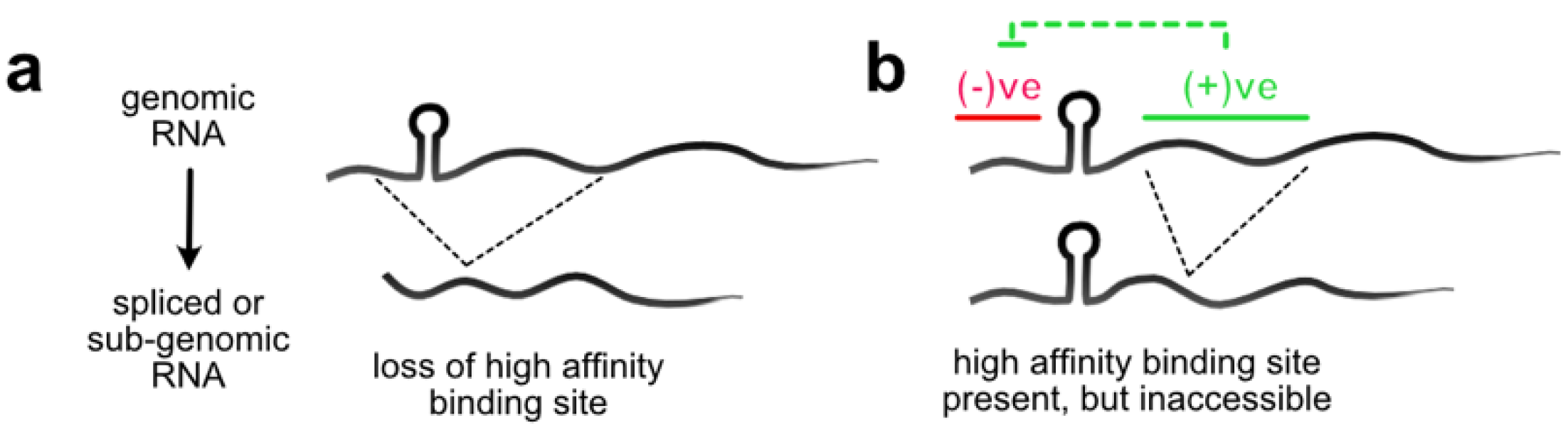
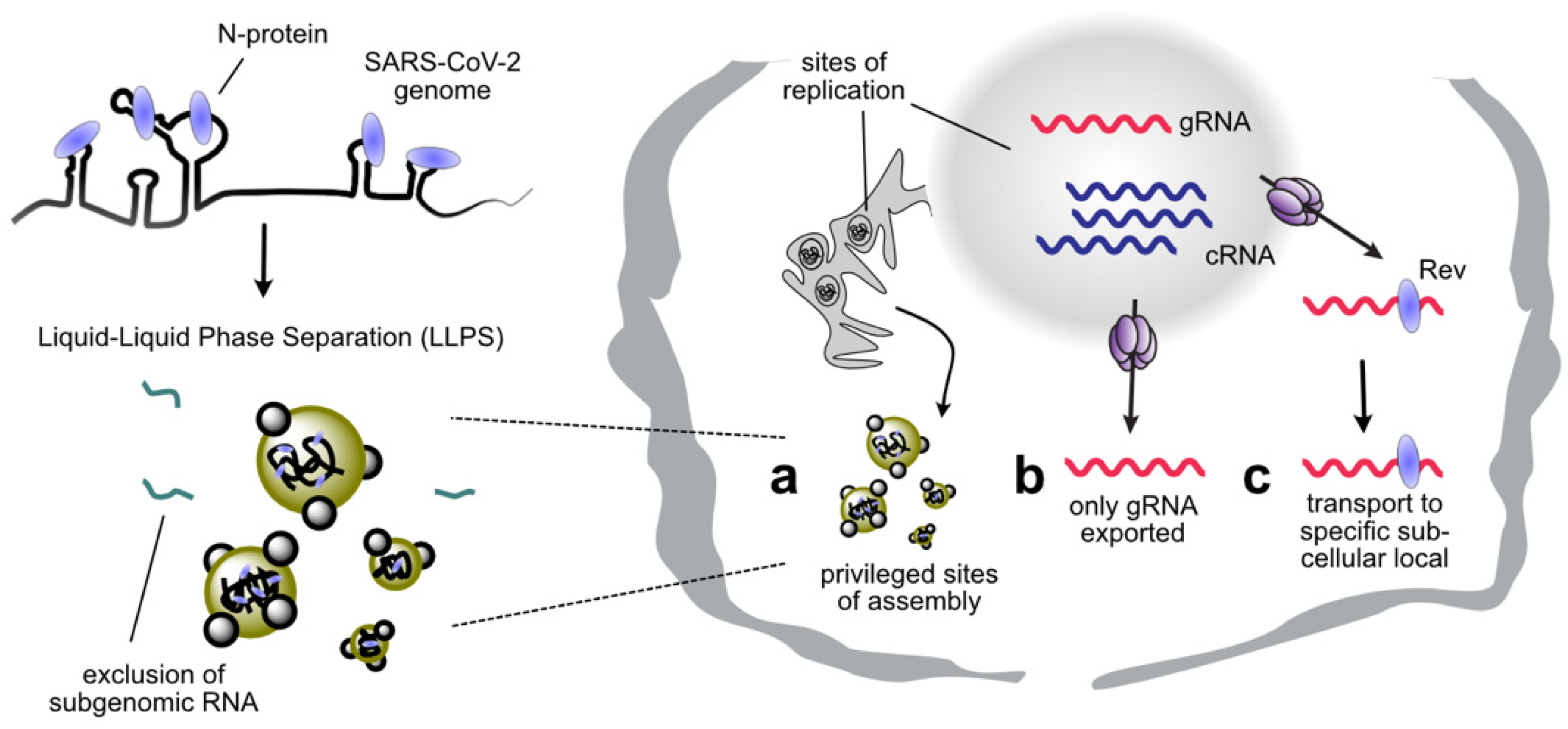
3. RNA Structure as a Regulator of Genome Packaging
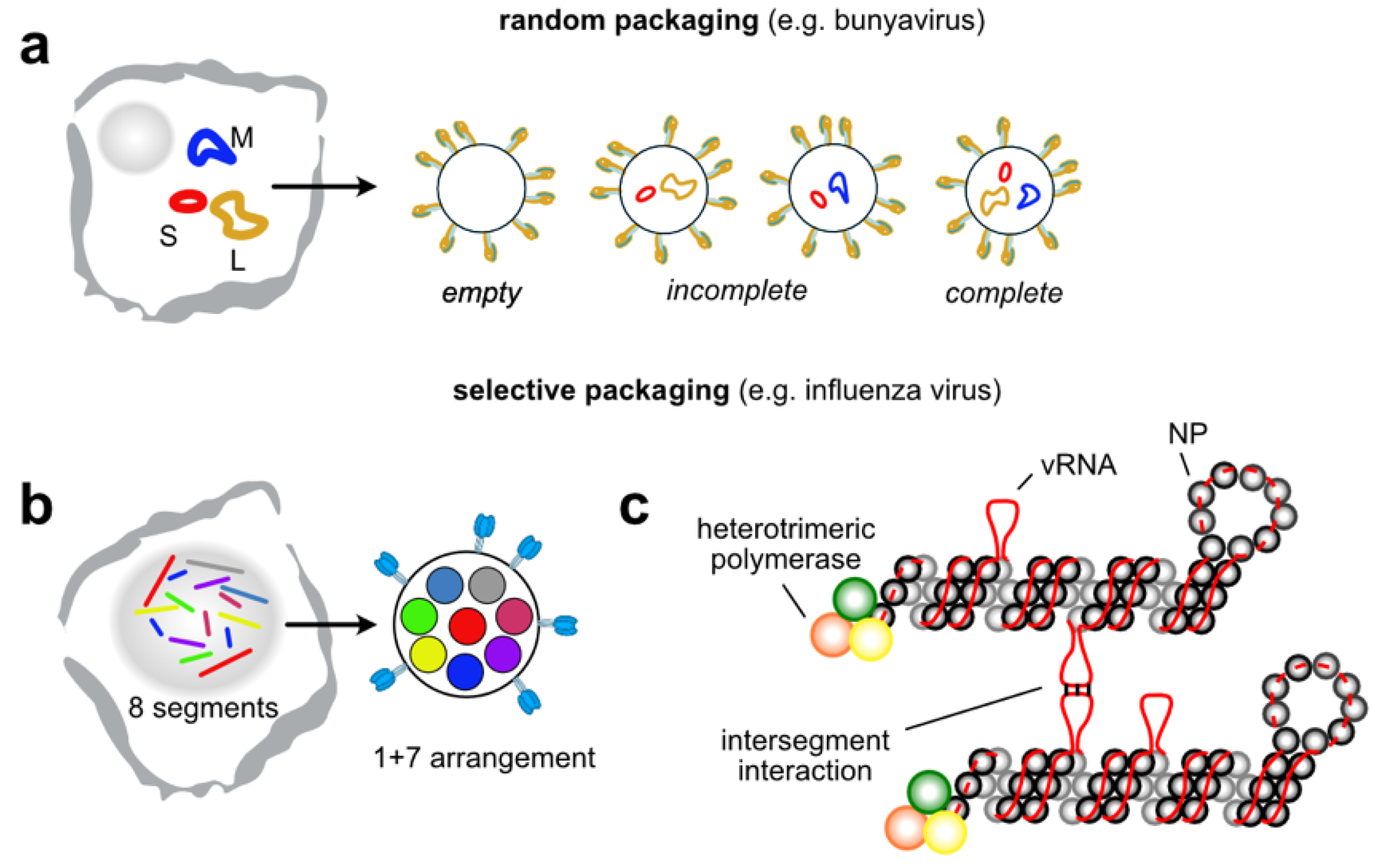
4. Intermolecular RNA-RNA Interactions in Segmented Viruses
5. RNA Packaging and Evolution
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sun, S.; Rao, V.B.; Rossmann, M.G. Genome Packaging in Viruses. Curr. Opin. Struct. Biol. 2010, 20, 114–120. [Google Scholar] [CrossRef]
- Adams, R.L.; Pirakitikulr, N.; Pyle, A.M. Functional RNA Structures throughout the Hepatitis C Virus Genome. Curr. Opin. Virol. 2017, 24, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Gorelick, R.J.; Vasa, S.M.; Guex, N.; Rein, A.; Mathews, D.H.; Giddings, M.C.; Weeks, K.M. High-Throughput SHAPE Analysis Reveals Structures in HIV-1 Genomic RNA Strongly Conserved across Distinct Biological States. PLoS Biol. 2008, 6, e96. [Google Scholar] [CrossRef]
- Rangan, R.; Zheludev, I.N.; Hagey, R.J.; Pham, E.A.; Wayment-Steele, H.K.; Glenn, J.S.; Das, R. RNA Genome Conservation and Secondary Structure in SARS-CoV-2 and SARS-Related Viruses: A First Look. RNA 2020, 26, 937–959. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.S. Coronavirus Genomic RNA Packaging. Virology 2019, 537, 198–207. [Google Scholar] [CrossRef]
- Mailler, E.; Bernacchi, S.; Marquet, R.; Paillart, J.-C.; Vivet-Boudou, V.; Smyth, R.P.; Mailler, E.; Bernacchi, S.; Marquet, R.; Paillart, J.-C.; et al. The Life-Cycle of the HIV-1 Gag-RNA Complex. Viruses 2016, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Borodavka, A.; Desselberger, U.; Patton, J.T. Genome Packaging in Multi-Segmented DsRNA Viruses: Distinct Mechanisms with Similar Outcomes. Curr. Opin. Virol. 2018, 33, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Isel, C.; Moules, V.; Marquet, R. Selective Packaging of the Influenza A Genome and Consequences for Genetic Reassortment. Trends Microbiol. 2014, 22, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.A.; Westhof, E. The Dynamic Landscapes of RNA Architecture. Cell 2009, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Newburn, L.R.; White, K.A. Trans-Acting RNA-RNA Interactions in Segmented RNA Viruses. Viruses 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.P.; Davenport, M.P.; Mak, J. The Origin of Genetic Diversity in HIV-1. Virus Res. 2012, 169, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Comas-Garcia, M. Packaging of Genomic RNA in Positive-Sense Single-Stranded RNA Viruses: A Complex Story. Viruses 2019, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Sanchez de Groot, N.; Armaos, A.; Graña-Montes, R.; Alriquet, M.; Calloni, G.; Vabulas, R.M.; Tartaglia, G.G. RNA Structure Drives Interaction with Proteins. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peabody, D.S. The RNA Binding Site of Bacteriophage MS2 Coat Protein. EMBO J. 1993, 12, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.Z.; Syed, R.; Kodandapani, R.; Wickersham, J.; Peabody, D.S.; Ely, K.R. Crystal Structure of the MS2 Coat Protein Dimer: Implications for RNA Binding and Virus Assembly. Structure 1995, 3, 255–263. [Google Scholar] [CrossRef]
- Dai, X.; Li, Z.; Lai, M.; Shu, S.; Du, Y.; Zhou, Z.H.; Sun, R. In Situ Structures of the Genome and Genome-Delivery Apparatus in a Single-Stranded RNA Virus. Nature 2017, 541, 112–116. [Google Scholar] [CrossRef]
- Halstead, J.M.; Lionnet, T.; Wilbertz, J.H.; Wippich, F.; Ephrussi, A.; Singer, R.H.; Chao, J.A. Translation. An RNA Biosensor for Imaging the First Round of Translation from Single Cells to Living Animals. Science 2015, 347, 1367–1671. [Google Scholar] [CrossRef]
- Bertrand, E.; Chartrand, P.; Schaefer, M.; Shenoy, S.M.; Singer, R.H.; Long, R.M. Localization of ASH1 MRNA Particles in Living Yeast. Mol. Cell 1998, 2, 437–445. [Google Scholar] [CrossRef]
- Horn, W.T.; Convery, M.A.; Stonehouse, N.J.; Adams, C.J.; Liljas, L.; Phillips, S.E.V.; Stockley, P.G. The Crystal Structure of a High Affinity RNA Stem-Loop Complexed with the Bacteriophage MS2 Capsid: Further Challenges in the Modeling of Ligand-RNA Interactions. RNA 2004, 10, 1776–1782. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Araya, C.L.; Chircus, L.M.; Layton, C.J.; Chang, H.Y.; Snyder, M.P.; Greenleaf, W.J. Quantitative Analysis of RNA-Protein Interactions on a Massively Parallel Array Reveals Biophysical and Evolutionary Landscapes. Nat. Biotechnol. 2014, 32, 562–568. [Google Scholar] [CrossRef]
- Smyth, R.P.; Despons, L.; Huili, G.; Bernacchi, S.; Hijnen, M.; Mak, J.; Jossinet, F.; Weixi, L.; Paillart, J.C.; Von Kleist, M.; et al. Mutational Interference Mapping Experiment (MIME) for Studying RNA Structure and Function. Nat. Methods 2015, 12, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Masliah, G.; Barraud, P.; Allain, F.H.-T. RNA Recognition by Double-Stranded RNA Binding Domains: A Matter of Shape and Sequence. Cell. Mol. Life Sci. 2013, 70, 1875–1895. [Google Scholar] [CrossRef]
- Treger, M.; Westhof, E. Statistical Analysis of Atomic Contacts at RNA-Protein Interfaces. J. Mol. Recognit. 2001, 14, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Rolfsson, Ó.; Middleton, S.; Manfield, I.W.; White, S.J.; Fan, B.; Vaughan, R.; Ranson, N.A.; Dykeman, E.; Twarock, R.; Ford, J.; et al. Direct Evidence for Packaging Signal-Mediated Assembly of Bacteriophage MS2. J. Mol. Biol. 2016, 428, 431–448. [Google Scholar] [CrossRef]
- Twarock, R.; Stockley, P.G. RNA-Mediated Virus Assembly: Mechanisms and Consequences for Viral Evolution and Therapy. Annu. Rev. Biophys. 2019, 48, 495–514. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Wroblewski, E.; Leonov, G.; Phillips, S.E.V.; Tuma, R.; Twarock, R.; Stockley, P.G. Rewriting Nature’s Assembly Manual for a SsRNA Virus. Proc. Natl. Acad. Sci. USA 2017, 114, 12255–12260. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; White, S.J.; Thompson, R.F.; Bingham, R.; Weiß, E.U.; Maskell, D.P.; Zlotnick, A.; Dykeman, E.; Tuma, R.; Twarock, R.; et al. HBV RNA Pre-Genome Encodes Specific Motifs That Mediate Interactions with the Viral Core Protein That Promote Nucleocapsid Assembly. Nat. Microbiol. 2017, 2, 17098. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Anastasakis, D.G.; Hafner, M.; Kielian, M. Multiple Capsid Protein Binding Sites Mediate Selective Packaging of the Alphavirus Genomic RNA. Nat. Commun. 2020, 11, 4693. [Google Scholar] [CrossRef] [PubMed]
- Tetter, S.; Terasaka, N.; Steinauer, A.; Bingham, R.J.; Clark, S.; Scott, A.J.P.; Patel, N.; Leibundgut, M.; Wroblewski, E.; Ban, N.; et al. Evolution of a Virus-like Architecture and Packaging Mechanism in a Repurposed Bacterial Protein. Science 2021, 372, 1220–1224. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Zang, T.; Blanco-Melo, D.; Powell, C.; Jannain, D.; Errando, M.; Bieniasz, P.D. Global Changes in the RNA Binding Specificity of HIV-1 Gag Regulate Virion Genesis. Cell 2014, 159, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Keating, C.P.; Hill, M.K.; Hawkes, D.J.; Smyth, R.P.; Isel, C.; Le, S.-Y.Y.; Palmenberg, A.C.; Marshall, J.A.; Marquet, R.; Nabel, G.J.; et al. The A-Rich RNA Sequences of HIV-1 Pol Are Important for the Synthesis of Viral CDNA. Nucleic Acids Res. 2009, 37, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Zandi, R.; van der Schoot, P.; Reguera, D.; Kegel, W.; Reiss, H. Classical Nucleation Theory of Virus Capsids. Biophys. J. 2006, 90, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, P.; Bruinsma, R. Electrostatics and the Assembly of an RNA Virus. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 71, 061928. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, H.S.; Khoo, K.K.; Garvey, M.; Waddington, L.; Leis, A.; Hijnen, M.; Velkov, T.; Dumsday, G.J.; McKinstry, W.J.; Mak, J. The Thermodynamics of Pr55Gag-RNA Interaction Regulate the Assembly of HIV. PLoS Pathog. 2017, 13, e1006221. [Google Scholar] [CrossRef] [PubMed]
- Muriaux, D.; Mirro, J.; Harvin, D.; Rein, A. RNA Is a Structural Element in Retrovirus Particles. Proc. Natl. Acad. Sci. USA 2001, 98, 5246–5251. [Google Scholar] [CrossRef] [PubMed]
- Bond, K.; Tsvetkova, I.B.; Wang, J.C.-Y.; Jarrold, M.F.; Dragnea, B. Virus Assembly Pathways: Straying Away but Not Too Far. Small 2020, 16, e2004475. [Google Scholar] [CrossRef]
- Perlmutter, J.D.; Qiao, C.; Hagan, M.F. Viral Genome Structures Are Optimal for Capsid Assembly. Elife 2013, 2, e00632. [Google Scholar] [CrossRef]
- Erdemci-Tandogan, G.; Wagner, J.; van der Schoot, P.; Podgornik, R.; Zandi, R. Effects of RNA Branching on the Electrostatic Stabilization of Viruses. Phys. Rev. E 2016, 94, 022408. [Google Scholar] [CrossRef] [PubMed]
- Mougel, M.; Zhang, Y.; Barklis, E. Cis-Active Structural Motifs Involved in Specific Encapsidation of Moloney Murine Leukemia Virus RNA. J. Virol. 1996, 70, 5043–5050. [Google Scholar] [CrossRef]
- Murphy, J.E.; Goff, S.P. Construction and Analysis of Deletion Mutations in the U5 Region of Moloney Murine Leukemia Virus: Effects on RNA Packaging and Reverse Transcription. J. Virol. 1989, 63, 319–327. [Google Scholar] [CrossRef]
- Ding, P.; Kharytonchyk, S.; Waller, A.; Mbaekwe, U.; Basappa, S.; Kuo, N.; Frank, H.M.; Quasney, C.; Kidane, A.; Swanson, C.; et al. Identification of the Initial Nucleocapsid Recognition Element in the HIV-1 RNA Packaging Signal. Proc. Natl. Acad. Sci. USA 2020, 117, 17737–17746. [Google Scholar] [CrossRef]
- Lu, K.; Heng, X.; Summers, M.F. Structural Determinants and Mechanism of HIV-1 Genome Packaging. J. Mol. Biol. 2011, 410, 609–633. [Google Scholar] [CrossRef]
- Luban, J.; Goff, S.P. Binding of Human Immunodeficiency Virus Type 1 (HIV-1) RNA to Recombinant HIV-1 Gag Polyprotein. J. Virol. 1991, 65, 3203–3212. [Google Scholar] [CrossRef]
- Luban, J.; Goff, S.P. Mutational Analysis of Cis-Acting Packaging Signals in Human Immunodeficiency Virus Type 1 RNA. J. Virol. 1994, 68, 3784–3793. [Google Scholar] [CrossRef]
- McBride, M.S.; Panganiban, A.T. The Human Immunodeficiency Virus Type 1 Encapsidation Site Is a Multipartite RNA Element Composed of Functional Hairpin Structures. J. Virol. 1996, 70, 2963–2973. [Google Scholar] [CrossRef]
- Lever, A.; Gottlinger, H.; Haseltine, W.; Sodroski, J. Identification of a Sequence Required for Efficient Packaging of Human Immunodeficiency Virus Type 1 RNA into Virions. J. Virol. 1989, 63, 4085–4087. [Google Scholar] [CrossRef]
- Aldovini, A.; Young, R.A. Mutations of RNA and Protein Sequences Involved in Human Immunodeficiency Virus Type 1 Packaging Result in Production of Noninfectious Virus. J. Virol. 1990, 64, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Clavel, F.; Orenstein, J.M. A Mutant of Human Immunodeficiency Virus with Reduced RNA Packaging and Abnormal Particle Morphology. J. Virol. 1990, 64, 5230–5234. [Google Scholar] [CrossRef]
- De Guzman, R.N.; Wu, Z.R.; Stalling, C.C.; Pappalardo, L.; Borer, P.N.; Summers, M.F. Structure of the HIV-1 Nucleocapsid Protein Bound to the SL3 Psi-RNA Recognition Element. Science 1998, 279, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; De Guzman, R.N.; Turner, R.B.; Chancellor, K.J.; Wu, Z.R.; Summers, M.F. NMR Structure of the HIV-1 Nucleocapsid Protein Bound to Stem-Loop SL2 of the Psi-RNA Packaging Signal. Implications for Genome Recognition. J. Mol. Biol. 2000, 301, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahab, E.W.; Smyth, R.P.; Mailler, E.; Bernacchi, S.; Vivet-Boudou, V.; Hijnen, M.; Jossinet, F.; Mak, J.; Paillart, J.-C.; Marquet, R. Specific Recognition of the HIV-1 Genomic RNA by the Gag Precursor. Nat. Commun. 2014, 5, 4304. [Google Scholar] [CrossRef]
- Houzet, L.; Paillart, J.C.; Smagulova, F.; Maurel, S.; Morichaud, Z.; Marquet, R.; Mougel, M. HIV Controls the Selective Packaging of Genomic, Spliced Viral and Cellular RNAs into Virions through Different Mechanisms. Nucleic Acids Res. 2007, 35, 2695–2704. [Google Scholar] [CrossRef]
- Smyth, R.P.; Smith, M.R.; Jousset, A.-C.; Despons, L.; Laumond, G.; Decoville, T.; Cattenoz, P.; Moog, C.; Jossinet, F.; Mougel, M.; et al. In Cell Mutational Interference Mapping Experiment (in Cell MIME) Identifies the 5′ Polyadenylation Signal as a Dual Regulator of HIV-1 Genomic RNA Production and Packaging. Nucleic Acids Res. 2018, 46, e57. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, S.; Abd El-Wahab, E.W.; Dubois, N.; Hijnen, M.; Smyth, R.P.; Mak, J.; Marquet, R.; Paillart, J.-C. HIV-1 Pr55Gag Binds Genomic and Spliced RNAs with Different Affinity and Stoichiometry. RNA Biol. 2017, 14, 90–103. [Google Scholar] [CrossRef]
- Comas-Garcia, M.; Datta, S.A.; Baker, L.; Varma, R.; Gudla, P.R.; Rein, A. Dissection of Specific Binding of HIV-1 Gag to the “packaging Signal” in Viral RNA. Elife 2017, 6, e27055. [Google Scholar] [CrossRef] [PubMed]
- Rein, A. RNA Packaging in HIV. Trends Microbiol. 2019, 27, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Jones, C.P.; Parent, L.J.; Rouzina, I.; Musier-Forsyth, K. Distinct Binding Interactions of HIV-1 Gag to Psi and Non-Psi RNAs: Implications for Viral Genomic RNA Packaging. RNA 2013, 19, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Comas-Garcia, M.; Kroupa, T.; Datta, S.A.; Harvin, D.P.; Hu, W.-S.; Rein, A. Efficient Support of Virus-like Particle Assembly by the HIV-1 Packaging Signal. Elife 2018, 7, e38438. [Google Scholar] [CrossRef]
- Hsin, W.-C.; Chang, C.-H.; Chang, C.-Y.; Peng, W.-H.; Chien, C.-L.; Chang, M.-F.; Chang, S.C. Nucleocapsid Protein-Dependent Assembly of the RNA Packaging Signal of Middle East Respiratory Syndrome Coronavirus. J. Biomed. Sci. 2018, 25, 47. [Google Scholar] [CrossRef]
- Hsieh, P.-K.; Chang, S.C.; Huang, C.-C.; Lee, T.-T.; Hsiao, C.-W.; Kou, Y.-H.; Chen, I.-Y.; Chang, C.-K.; Huang, T.-H.; Chang, M.-F. Assembly of Severe Acute Respiratory Syndrome Coronavirus RNA Packaging Signal into Virus-like Particles Is Nucleocapsid Dependent. J. Virol. 2005, 79, 13848–13855. [Google Scholar] [CrossRef]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 Structure and Replication Characterized by in Situ Cryo-Electron Tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef]
- Iserman, C.; Roden, C.A.; Boerneke, M.A.; Sealfon, R.S.G.; McLaughlin, G.A.; Jungreis, I.; Fritch, E.J.; Hou, Y.J.; Ekena, J.; Weidmann, C.A.; et al. Genomic RNA Elements Drive Phase Separation of the SARS-CoV-2 Nucleocapsid. Mol. Cell 2020, 80, 1078–1091.e6. [Google Scholar] [CrossRef]
- Wang, J.; Shi, C.; Xu, Q.; Yin, H. SARS-CoV-2 Nucleocapsid Protein Undergoes Liquid-Liquid Phase Separation into Stress Granules through Its N-Terminal Intrinsically Disordered Region. Cell Discov. 2021, 7, 5. [Google Scholar] [CrossRef]
- Savastano, A.; Ibáñez de Opakua, A.; Rankovic, M.; Zweckstetter, M. Nucleocapsid Protein of SARS-CoV-2 Phase Separates into RNA-Rich Polymerase-Containing Condensates. Nat. Commun. 2020, 11, 6041. [Google Scholar] [CrossRef]
- Chen, H.; Cui, Y.; Han, X.; Hu, W.; Sun, M.; Zhang, Y.; Wang, P.-H.; Song, G.; Chen, W.; Lou, J. Liquid-Liquid Phase Separation by SARS-CoV-2 Nucleocapsid Protein and RNA. Cell Res. 2020, 30, 1143–1145. [Google Scholar] [CrossRef]
- Carlson, C.R.; Asfaha, J.B.; Ghent, C.M.; Howard, C.J.; Hartooni, N.; Safari, M.; Frankel, A.D.; Morgan, D.O. Phosphoregulation of Phase Separation by the SARS-CoV-2 N Protein Suggests a Biophysical Basis for Its Dual Functions. Mol. Cell 2020, 80, 1092–1103.e4. [Google Scholar] [CrossRef]
- Perdikari, T.M.; Murthy, A.C.; Ryan, V.H.; Watters, S.; Naik, M.T.; Fawzi, N.L. SARS-CoV-2 Nucleocapsid Protein Phase-Separates with RNA and with Human HnRNPs. EMBO J. 2020, 39, e106478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.; Cai, S.; Zhuang, Z.; Zhao, Z.; Jin, S.; Xie, W.; Zhou, L.; Zhang, L.; Zhao, J.; et al. RNA-Induced Liquid Phase Separation of SARS-CoV-2 Nucleocapsid Protein Facilitates NF-ΚB Hyper-Activation and Inflammation. Signal Transduct. Target. Ther. 2021, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Stuchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; et al. The SARS-CoV-2 Nucleocapsid Protein Is Dynamic, Disordered, and Phase Separates with RNA. Nat. Commun. 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ye, Q.; Singh, D.; Cao, Y.; Diedrich, J.K.; Yates, J.R.; Villa, E.; Cleveland, D.W.; Corbett, K.D. The SARS-CoV-2 Nucleocapsid Phosphoprotein Forms Mutually Exclusive Condensates with RNA and the Membrane-Associated M Protein. Nat. Commun. 2021, 12, 502. [Google Scholar] [CrossRef]
- Guseva, S.; Milles, S.; Jensen, M.R.; Salvi, N.; Kleman, J.-P.; Maurin, D.; Ruigrok, R.W.H.; Blackledge, M. Measles Virus Nucleo- and Phosphoproteins Form Liquid-like Phase-Separated Compartments That Promote Nucleocapsid Assembly. Sci. Adv. 2020, 6, eaaz7095. [Google Scholar] [CrossRef] [PubMed]
- Etibor, T.A.; Yamauchi, Y.; Amorim, M.J. Liquid Biomolecular Condensates and Viral Lifecycles: Review and Perspectives. Viruses 2021, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Protter, D.S.W.; Rao, B.S.; Van Treeck, B.; Lin, Y.; Mizoue, L.; Rosen, M.K.; Parker, R. Intrinsically Disordered Regions Can Contribute Promiscuous Interactions to RNP Granule Assembly. Cell Rep. 2018, 22, 1401–1412. [Google Scholar] [CrossRef]
- Wang, S.; Dai, T.; Qin, Z.; Pan, T.; Chu, F.; Lou, L.; Zhang, L.; Yang, B.; Huang, H.; Lu, H.; et al. Targeting Liquid-Liquid Phase Separation of SARS-CoV-2 Nucleocapsid Protein Promotes Innate Antiviral Immunity by Elevating MAVS Activity. Nat. Cell Biol. 2021, 23, 718–732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yu, Y.; Sun, L.-M.; Xing, J.-Q.; Li, T.; Zhu, Y.; Wang, M.; Yu, Y.; Xue, W.; Xia, T.; et al. GCG Inhibits SARS-CoV-2 Replication by Disrupting the Liquid Phase Condensation of Its Nucleocapsid Protein. Nat. Commun. 2021, 12, 2114. [Google Scholar] [CrossRef] [PubMed]
- Pflug, A.; Guilligay, D.; Reich, S.; Cusack, S. Structure of Influenza A Polymerase Bound to the Viral RNA Promoter. Nature 2014, 516, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Tchatalbachev, S.; Flick, R.; Hobom, G. The Packaging Signal of Influenza Viral RNA Molecules. RNA 2001, 7, 979–989. [Google Scholar] [CrossRef]
- Chaimayo, C.; Hayashi, T.; Underwood, A.; Hodges, E.; Takimoto, T. Selective Incorporation of VRNP into Influenza A Virions Determined by Its Specific Interaction with M1 Protein. Virology 2017, 505, 23–32. [Google Scholar] [CrossRef]
- Brandt, S.; Blissenbach, M.; Grewe, B.; Konietzny, R.; Grunwald, T.; Uberla, K. Rev Proteins of Human and Simian Immunodeficiency Virus Enhance RNA Encapsidation. PLoS Pathog. 2007, 3, e54. [Google Scholar] [CrossRef] [PubMed]
- Blissenbach, M.; Grewe, B.; Hoffmann, B.; Brandt, S.; Uberla, K. Nuclear RNA Export and Packaging Functions of HIV-1 Rev Revisited. J. Virol. 2010, 84, 6598–6604. [Google Scholar] [CrossRef]
- Pocock, G.M.; Becker, J.T.; Swanson, C.M.; Ahlquist, P.; Sherer, N.M. HIV-1 and M-PMV RNA Nuclear Export Elements Program Viral Genomes for Distinct Cytoplasmic Trafficking Behaviors. PLoS Pathog. 2016, 12, e1005565. [Google Scholar] [CrossRef]
- Grewe, B.; Ehrhardt, K.; Hoffmann, B.; Blissenbach, M.; Brandt, S.; Uberla, K. The HIV-1 Rev Protein Enhances Encapsidation of Unspliced and Spliced, RRE-Containing Lentiviral Vector RNA. PLoS ONE 2012, 7, e48688. [Google Scholar] [CrossRef] [PubMed]
- Serganov, A.; Nudler, E. A Decade of Riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lykke-Andersen, J. Emerging Roles for Ribonucleoprotein Modification and Remodeling in Controlling RNA Fate. Trends Cell Biol. 2013, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Stefanovic, S.; Mihailescu, M.R. Hepatitis C Virus RNA: Molecular Switches Mediated by Long-Range RNA-RNA Interactions? Nucleic Acids Res. 2013, 41, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Romero-López, C.; Barroso-Deljesus, A.; García-Sacristán, A.; Briones, C.; Berzal-Herranz, A. End-to-End Crosstalk within the Hepatitis C Virus Genome Mediates the Conformational Switch of the 3′X-Tail Region. Nucleic Acids Res. 2014, 42, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Pirakitikulr, N.; Kohlway, A.; Lindenbach, B.D.; Pyle, A.M. The Coding Region of the HCV Genome Contains a Network of Regulatory RNA Structures. Mol. Cell 2016, 62, 111–120. [Google Scholar] [CrossRef]
- Friebe, P.; Boudet, J.; Simorre, J.-P.; Bartenschlager, R. Kissing-Loop Interaction in the 3′ End of the Hepatitis C Virus Genome Essential for RNA Replication. J. Virol. 2005, 79, 380–392. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Stump, D.D.; Branch, A.D.; Rice, C.M. A Cis-Acting Replication Element in the Sequence Encoding the NS5B RNA-Dependent RNA Polymerase Is Required for Hepatitis C Virus RNA Replication. J. Virol. 2004, 78, 1352–1366. [Google Scholar] [CrossRef]
- Lee, H.; Shin, H.; Wimmer, E.; Paul, A. V Cis-Acting RNA Signals in the NS5B C-Terminal Coding Sequence of the Hepatitis C Virus Genome. J. Virol. 2004, 78, 10865–10877. [Google Scholar] [CrossRef]
- Tuplin, A.; Struthers, M.; Simmonds, P.; Evans, D.J. A Twist in the Tail: SHAPE Mapping of Long-Range Interactions and Structural Rearrangements of RNA Elements Involved in HCV Replication. Nucleic Acids Res. 2012, 40, 6908–6921. [Google Scholar] [CrossRef]
- Shi, G.; Ando, T.; Suzuki, R.; Matsuda, M.; Nakashima, K.; Ito, M.; Omatsu, T.; Oba, M.; Ochiai, H.; Kato, T.; et al. Involvement of the 3′ Untranslated Region in Encapsidation of the Hepatitis C Virus. PLoS Pathog. 2016, 12, e1005441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romero-López, C.; Berzal-Herranz, A. The Functional RNA Domain 5BSL3.2 within the NS5B Coding Sequence Influences Hepatitis C Virus IRES-Mediated Translation. Cell. Mol. Life Sci. 2012, 69, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Kim, S.; Shimakami, T.; Lemon, S.M.; Mihailescu, M.-R. Hepatitis C Virus Genomic RNA Dimerization Is Mediated via a Kissing Complex Intermediate. RNA 2010, 16, 913–925. [Google Scholar] [CrossRef]
- Ivanyi-Nagy, R.; Kanevsky, I.; Gabus, C.; Lavergne, J.-P.; Ficheux, D.; Penin, F.; Fossé, P.; Darlix, J.-L. Analysis of Hepatitis C Virus RNA Dimerization and Core-RNA Interactions. Nucleic Acids Res. 2006, 34, 2618–2633. [Google Scholar] [CrossRef]
- Huthoff, H.; Berkhout, B. Two Alternating Structures of the HIV-1 Leader RNA. RNA 2001, 7, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Ooms, M.; Huthoff, H.; Russell, R.; Liang, C.; Berkhout, B. A Riboswitch Regulates RNA Dimerization and Packaging in Human Immunodeficiency Virus Type 1 Virions. J. Virol. 2004, 78, 10814–10819. [Google Scholar] [CrossRef]
- Brown, J.D.; Kharytonchyk, S.; Chaudry, I.; Iyer, A.S.; Carter, H.; Becker, G.; Desai, Y.; Glang, L.; Choi, S.H.; Singh, K.; et al. Structural Basis for Transcriptional Start Site Control of HIV-1 RNA Fate. Science 2020, 368, 413–417. [Google Scholar] [CrossRef]
- Abbink, T.E.M.; Berkhout, B. A Novel Long Distance Base-Pairing Interaction in Human Immunodeficiency Virus Type 1 RNA Occludes the Gag Start Codon. J. Biol. Chem. 2003, 278, 11601–11611. [Google Scholar] [CrossRef] [PubMed]
- Keane, S.C.; Heng, X.; Lu, K.; Kharytonchyk, S.; Ramakrishnan, V.; Carter, G.; Barton, S.; Hosic, A.; Florwick, A.; Santos, J.; et al. RNA Structure. Structure of the HIV-1 RNA Packaging Signal. Science 2015, 348, 917–921. [Google Scholar] [CrossRef]
- Lu, K.; Heng, X.; Garyu, L.; Monti, S.; Garcia, E.L.; Kharytonchyk, S.; Dorjsuren, B.; Kulandaivel, G.; Jones, S.; Hiremath, A.; et al. NMR Detection of Structures in the HIV-1 5′-Leader RNA That Regulate Genome Packaging. Science 2011, 334, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Abbink, T.E.M.; Ooms, M.; Haasnoot, P.C.J.; Berkhout, B. The HIV-1 Leader RNA Conformational Switch Regulates RNA Dimerization but Does Not Regulate MRNA Translation †. Biochemistry 2005, 44, 9058–9066. [Google Scholar] [CrossRef]
- Brigham, B.S.; Kitzrow, J.P.; Reyes, J.-P.C.; Musier-Forsyth, K.; Munro, J.B. Intrinsic Conformational Dynamics of the HIV-1 Genomic RNA 5′UTR. Proc. Natl. Acad. Sci. USA 2019, 116, 10372–10381. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.K.L.; Smyth, R.P.R.P.; Pereira, C.F.C.F.; Cameron, P.U.; Lewin, S.R.S.R.; Jaworowski, A.; Mak, J. Early Events of HIV-1 Infection: Can Signaling Be the Next Therapeutic Target? J. Neuroimmune Pharmacol. 2011, 6, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Skripkin, E.; Paillart, J.C.; Marquet, R.; Ehresmann, B.; Ehresmann, C.; Lina, B.; Moulès, V.; Marquet, R. Identification of the Primary Site of the Human Immunodeficiency Virus Type 1 RNA Dimerization in Vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 4945–4949. [Google Scholar] [CrossRef]
- Paillart, J.C.; Marquet, R.; Skripkin, E.; Ehresmann, B.; Ehresmann, C. Mutational Analysis of the Bipartite Dimer Linkage Structure of Human Immunodeficiency Virus Type 1 Genomic RNA. J. Biol. Chem. 1994, 269, 27486–27493. [Google Scholar] [CrossRef]
- Paillart, J.C.; Berthoux, L.; Ottmann, M.; Darlix, J.L.; Marquet, R.; Ehresmann, B.; Ehresmann, C. A Dual Role of the Putative RNA Dimerization Initiation Site of Human Immunodeficiency Virus Type 1 in Genomic RNA Packaging and Proviral DNA Synthesis. J. Virol. 1996, 70, 8348–8354. [Google Scholar] [CrossRef]
- Paillart, J.-C.; Shehu-Xhilaga, M.; Marquet, R.; Mak, J. Dimerization of Retroviral RNA Genomes: An Inseparable Pair. Nat. Rev. Microbiol. 2004, 2, 461–472. [Google Scholar] [CrossRef]
- Russell, R.S.; Liang, C.; Wainberg, M.A. Is HIV-1 RNA Dimerization a Prerequisite for Packaging? Yes, No, Probably? Retrovirology 2004, 1, 23. [Google Scholar] [CrossRef]
- Keane, S.C.; Van, V.; Frank, H.M.; Sciandra, C.A.; McCowin, S.; Santos, J.; Heng, X.; Summers, M.F. NMR Detection of Intermolecular Interaction Sites in the Dimeric 5′-Leader of the HIV-1 Genome. Proc. Natl. Acad. Sci. USA 2016, 113, 13033–13038. [Google Scholar] [CrossRef]
- Boyd, P.S.; Brown, J.B.; Brown, J.D.; Catazaro, J.; Chaudry, I.; Ding, P.; Dong, X.; Marchant, J.; O’Hern, C.T.; Singh, K.; et al. NMR Studies of Retroviral Genome Packaging. Viruses 2020, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kharytonchyk, S.; Monti, S.; Smaldino, P.J.; Van, V.; Bolden, N.C.; Brown, J.D.; Russo, E.; Swanson, C.; Shuey, A.; Telesnitsky, A.; et al. Transcriptional Start Site Heterogeneity Modulates the Structure and Function of the HIV-1 Genome. Proc. Natl. Acad. Sci. USA 2016, 113, 13378–13383. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of Hepatitis C Virus RNA Abundance by a Liver-Specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef]
- Luna, J.M.; Scheel, T.K.H.; Danino, T.; Shaw, K.S.; Mele, A.; Fak, J.J.; Nishiuchi, E.; Takacs, C.N.; Catanese, M.T.; de Jong, Y.P.; et al. Hepatitis C Virus RNA Functionally Sequesters MiR-122. Cell 2015, 160, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L.; Schütz, S.; Sarnow, P. Position-Dependent Function for a Tandem MicroRNA MiR-122-Binding Site Located in the Hepatitis C Virus RNA Genome. Cell Host Microbe 2008, 4, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Masaki, T.; Yamane, D.; McGivern, D.R.; Lemon, S.M. Competing and Noncompeting Activities of MiR-122 and the 5′ Exonuclease Xrn1 in Regulation of Hepatitis C Virus Replication. Proc. Natl. Acad. Sci. USA 2013, 110, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Sedano, C.D.; Sarnow, P. Hepatitis C Virus Subverts Liver-Specific MiR-122 to Protect the Viral Genome from Exoribonuclease Xrn2. Cell Host Microbe 2014, 16, 257–264. [Google Scholar] [CrossRef]
- Shimakami, T.; Yamane, D.; Jangra, R.K.; Kempf, B.J.; Spaniel, C.; Barton, D.J.; Lemon, S.M. Stabilization of Hepatitis C Virus RNA by an Ago2-MiR-122 Complex. Proc. Natl. Acad. Sci. USA 2012, 109, 941–946. [Google Scholar] [CrossRef]
- Niepmann, M. Activation of Hepatitis C Virus Translation by a Liver-Specific MicroRNA. Cell Cycle 2009, 8, 1473–1477. [Google Scholar] [CrossRef]
- Roberts, A.P.E.; Lewis, A.P.; Jopling, C.L. MiR-122 Activates Hepatitis C Virus Translation by a Specialized Mechanism Requiring Particular RNA Components. Nucleic Acids Res. 2011, 39, 7716–7729. [Google Scholar] [CrossRef]
- Schult, P.; Roth, H.; Adams, R.L.; Mas, C.; Imbert, L.; Orlik, C.; Ruggieri, A.; Pyle, A.M.; Lohmann, V. MicroRNA-122 Amplifies Hepatitis C Virus Translation by Shaping the Structure of the Internal Ribosomal Entry Site. Nat. Commun. 2018, 9, 2613. [Google Scholar] [CrossRef]
- Henke, J.I.; Goergen, D.; Zheng, J.; Song, Y.; Schüttler, C.G.; Fehr, C.; Jünemann, C.; Niepmann, M. MicroRNA-122 Stimulates Translation of Hepatitis C Virus RNA. EMBO J. 2008, 27, 3300–3310. [Google Scholar] [CrossRef]
- Kunden, R.D.; Ghezelbash, S.; Khan, J.Q.; Wilson, J.A. Location Specific Annealing of MiR-122 and Other Small RNAs Defines an Hepatitis C Virus 5′ UTR Regulatory Element with Distinct Impacts on Virus Translation and Genome Stability. Nucleic Acids Res. 2020, 48, 9235–9249. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Arend, K.C.; Li, Y.; Yamane, D.; McGivern, D.R.; Kato, T.; Wakita, T.; Moorman, N.J.; Lemon, S.M. MiR-122 Stimulates Hepatitis C Virus RNA Synthesis by Altering the Balance of Viral RNAs Engaged in Replication versus Translation. Cell Host Microbe 2015, 17, 217–228. [Google Scholar] [CrossRef]
- Fukuhara, T.; Kambara, H.; Shiokawa, M.; Ono, C.; Katoh, H.; Morita, E.; Okuzaki, D.; Maehara, Y.; Koike, K.; Matsuura, Y. Expression of MicroRNA MiR-122 Facilitates an Efficient Replication in Nonhepatic Cells upon Infection with Hepatitis C Virus. J. Virol. 2012, 86, 7918–7933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Mak, J.; Wainberg, M.A.; Parniak, M.A.; Cohen, E.; Kleiman, L. Variable TRNA Content in HIV-1IIIB. Biochem. Biophys. Res. Commun. 1992, 185, 1005–1015. [Google Scholar] [CrossRef]
- Jiang, M.; Mak, J.; Ladha, A.; Cohen, E.; Klein, M.; Rovinski, B.; Kleiman, L. Identification of TRNAs Incorporated into Wild-Type and Mutant Human Immunodeficiency Virus Type 1. J. Virol. 1993, 67, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Seif, E.; Niu, M.; Kleiman, L. Annealing to Sequences within the Primer Binding Site Loop Promotes an HIV-1 RNA Conformation Favoring RNA Dimerization and Packaging. RNA 2013, 19, 1384–1393. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Kortekaas, J. Single-Molecule FISH Reveals Non-Selective Packaging of Rift Valley Fever Virus Genome Segments. PLoS Pathog. 2016, 12, e1005800. [Google Scholar] [CrossRef]
- Bermúdez-Méndez, E.; Katrukha, E.A.; Spruit, C.M.; Kortekaas, J.; Wichgers Schreur, P.J. Visualizing the Ribonucleoprotein Content of Single Bunyavirus Virions Reveals More Efficient Genome Packaging in the Arthropod Host. Commun. Biol. 2021, 4, 345. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.C.; von Kirchbach, J.C.; Gog, J.R.; Digard, P. Genome Packaging in Influenza A Virus. J. Gen. Virol. 2010, 91, 313–328. [Google Scholar] [CrossRef]
- Chou, Y.-Y.; Vafabakhsh, R.; Doganay, S.; Gao, Q.; Ha, T.; Palese, P.; Doğanay, S.; Gao, Q.; Ha, T.; Palese, P. One Influenza Virus Particle Packages Eight Unique Viral RNAs as Shown by FISH Analysis. Proc. Natl. Acad. Sci. USA 2012, 109, 9101–9106. [Google Scholar] [CrossRef] [PubMed]
- Haralampiev, I.; Prisner, S.; Nitzan, M.; Schade, M.; Jolmes, F.; Schreiber, M.; Loidolt-Krüger, M.; Jongen, K.; Chamiolo, J.; Nilson, N.; et al. Selective Flexible Packaging Pathways of the Segmented Genome of Influenza A Virus. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Sagara, H.; Yen, A.; Takada, A.; Kida, H.; Cheng, R.H.; Kawaoka, Y. Architecture of Ribonucleoprotein Complexes in Influenza A Virus Particles. Nature 2006, 439, 490–492. [Google Scholar] [CrossRef]
- Noda, T.; Murakami, S.; Nakatsu, S.; Imai, H.; Muramoto, Y.; Shindo, K.; Sagara, H.; Kawaoka, Y. Importance of the 1 + 7 Configuration of Ribonucleoprotein Complexes for Influenza A Virus Genome Packaging. Nat. Commun. 2018, 9, 54. [Google Scholar] [CrossRef]
- Fournier, E.; Moules, V.; Essere, B.; Paillart, J.-C.; Sirbat, J.-D.; Isel, C.; Cavalier, A.; Rolland, J.-P.; Thomas, D.; Lina, B.; et al. A Supramolecular Assembly Formed by Influenza A Virus Genomic RNA Segments. Nucleic Acids Res. 2012, 40, 2197–2209. [Google Scholar] [CrossRef] [PubMed]
- Duhaut, S.D.; McCauley, J.W. Defective RNAs Inhibit the Assembly of Influenza Virus Genome Segments in a Segment-Specific Manner. Virology 1996, 216, 326–337. [Google Scholar] [CrossRef]
- Duhaut, S.D.; Dimmock, N.J. Heterologous Protection of Mice from a Lethal Human H1N1 Influenza A Virus Infection by H3N8 Equine Defective Interfering Virus: Comparison of Defective RNA Sequences Isolated from the DI Inoculum and Mouse Lung. Virology 1998, 248, 241–253. [Google Scholar] [CrossRef]
- Jennings, P.A.; Finch, J.T.; Winter, G.; Robertson, J.S. Does the Higher Order Structure of the Influenza Virus Ribonucleoprotein Guide Sequence Rearrangements in Influenza Viral RNA? Cell 1983, 34, 619–627. [Google Scholar] [CrossRef]
- Noble, S.; Dimmock, N.J. Characterization of Putative Defective Interfering (DI) A/WSN RNAs Isolated from the Lungs of Mice Protected from an Otherwise Lethal Respiratory Infection with Influenza Virus A/WSN (H1N1): A Subset of the Inoculum DI RNAs. Virology 1995, 210, 9–19. [Google Scholar] [CrossRef][Green Version]
- Dos Santos Afonso, E.; Escriou, N.; Leclercq, I.; van der Werf, S.; Naffakh, N. The Generation of Recombinant Influenza A Viruses Expressing a PB2 Fusion Protein Requires the Conservation of a Packaging Signal Overlapping the Coding and Noncoding Regions at the 5′ End of the PB2 Segment. Virology 2005, 341, 34–46. [Google Scholar] [CrossRef]
- Liang, Y.; Hong, Y.; Parslow, T.G. Cis-Acting Packaging Signals in the Influenza Virus PB1, PB2, and PA Genomic RNA Segments. J. Virol. 2005, 79, 10348–10355. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, T.; Ly, H.; Parslow, T.G.; Liang, Y. Mutational Analyses of Packaging Signals in Influenza Virus PA, PB1, and PB2 Genomic RNA Segments. J. Virol. 2008, 82, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Fujii, Y.; Noda, T.; Muramoto, Y.; Watanabe, T.; Takada, A.; Goto, H.; Horimoto, T.; Kawaoka, Y. Importance of Both the Coding and the Segment-Specific Noncoding Regions of the Influenza A Virus NS Segment for Its Efficient Incorporation into Virions. J. Virol. 2005, 79, 3766–3774. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Goto, H.; Watanabe, T.; Yoshida, T.; Kawaoka, Y. Selective Incorporation of Influenza Virus RNA Segments into Virions. Proc. Natl. Acad. Sci. USA 2003, 100, 2002–2007. [Google Scholar] [CrossRef]
- Marsh, G.A.; Hatami, R.; Palese, P. Specific Residues of the Influenza A Virus Hemagglutinin Viral RNA Are Important for Efficient Packaging into Budding Virions. J. Virol. 2007, 81, 9727–9736. [Google Scholar] [CrossRef]
- Ozawa, M.; Maeda, J.; Iwatsuki-Horimoto, K.; Watanabe, S.; Goto, H.; Horimoto, T.; Kawaoka, Y. Nucleotide Sequence Requirements at the 5′ End of the Influenza A Virus M RNA Segment for Efficient Virus Replication. J. Virol. 2009, 83, 3384–3388. [Google Scholar] [CrossRef][Green Version]
- Ozawa, M.; Fujii, K.; Muramoto, Y.; Yamada, S.; Yamayoshi, S.; Takada, A.; Goto, H.; Horimoto, T.; Kawaoka, Y. Contributions of Two Nuclear Localization Signals of Influenza A Virus Nucleoprotein to Viral Replication. J. Virol. 2007, 81, 30–41. [Google Scholar] [CrossRef]
- Hutchinson, E.C.; Wise, H.M.; Kudryavtseva, K.; Curran, M.D.; Digard, P. Characterisation of Influenza A Viruses with Mutations in Segment 5 Packaging Signals. Vaccine 2009, 27, 6270–6275. [Google Scholar] [CrossRef]
- Goto, H.; Muramoto, Y.; Noda, T.; Kawaoka, Y. The Genome-Packaging Signal of the Influenza A Virus Genome Comprises a Genome Incorporation Signal and a Genome-Bundling Signal. J. Virol. 2013, 87, 11316–11322. [Google Scholar] [CrossRef]
- Noda, T.; Sugita, Y.; Aoyama, K.; Hirase, A.; Kawakami, E.; Miyazawa, A.; Sagara, H.; Kawaoka, Y. Three-Dimensional Analysis of Ribonucleoprotein Complexes in Influenza A Virus. Nat. Commun. 2012, 3, 639. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Moules, V.; Essere, B.; Paillart, J.C.; Sirbat, J.D.; Cavalier, A.; Rolland, J.P.; Thomas, D.; Lina, B.; Isel, C.; et al. Interaction Network Linking the Human H3N2 Influenza A Virus Genomic RNA Segments. Vaccine 2012, 30, 7359–7367. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, C.; Yver, M.; Isel, C.; Smyth, R.P.R.P.; Rosa-Calatrava, M.; Lina, B.; Moulès, V.; Marquet, R. A Functional Sequence-Specific Interaction between Influenza A Virus Genomic RNA Segments. Proc. Natl. Acad. Sci. USA 2013, 110, 16604–16609. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, C.; Isel, C.; Fournier, E.; Moules, V.; Cavalier, A.; Thomas, D.; Lina, B.; Marquet, R. An in Vitro Network of Intermolecular Interactions between Viral RNA Segments of an Avian H5N2 Influenza A Virus: Comparison with a Human H3N2 Virus. Nucleic Acids Res. 2013, 41, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.C.; Curran, M.D.; Read, E.K.; Gog, J.R.; Digard, P. Mutational Analysis of Cis-Acting RNA Signals in Segment 7 of Influenza A Virus. J. Virol. 2008, 82, 11869–11879. [Google Scholar] [CrossRef]
- Gilbertson, B.; Zheng, T.; Gerber, M.; Printz-Schweigert, A.; Ong, C.; Marquet, R.; Isel, C.; Rockman, S.; Brown, L. Influenza NA and PB1 Gene Segments Interact during the Formation of Viral Progeny: Localization of the Binding Region within the PB1 Gene. Viruses 2016, 8, 238. [Google Scholar] [CrossRef]
- Dadonaite, B.; Gilbertson, B.; Knight, M.L.; Trifkovic, S.; Rockman, S.; Laederach, A.; Brown, L.E.; Fodor, E.; Bauer, D.L.V. The Structure of the Influenza A Virus Genome. Nat. Microbiol. 2019, 4, 1781–1789. [Google Scholar] [CrossRef]
- Le Sage, V.; Kanarek, J.P.; Snyder, D.J.; Cooper, V.S.; Lakdawala, S.S.; Lee, N. Mapping of Influenza Virus RNA-RNA Interactions Reveals a Flexible Network. Cell Rep. 2020, 31, 107823. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, H.Y. The RNA Base-Pairing Problem and Base-Pairing Solutions. Cold Spring Harb. Perspect. Biol. 2018, 10, a034926. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.T.; Sun, Z.; Zhuang, F.; Robb, G.B. Bias in Ligation-Based Small RNA Sequencing Library Construction Is Determined by Adaptor and RNA Structure. PLoS ONE 2015, 10, e0126049. [Google Scholar] [CrossRef]
- Bolte, H.; Rosu, M.E.; Hagelauer, E.; García-Sastre, A.; Schwemmle, M. Packaging of the Influenza A Virus Genome Is Governed by a Plastic Network of RNA/Protein Interactions. J. Virol. 2018, 93, e01861-18. [Google Scholar] [CrossRef]
- Williams, G.D.; Townsend, D.; Wylie, K.M.; Kim, P.J.; Amarasinghe, G.K.; Kutluay, S.B.; Boon, A.C.M. Nucleotide Resolution Mapping of Influenza A Virus Nucleoprotein-RNA Interactions Reveals RNA Features Required for Replication. Nat. Commun. 2018, 9, 465. [Google Scholar] [CrossRef]
- Moreira, É.A.; Weber, A.; Bolte, H.; Kolesnikova, L.; Giese, S.; Lakdawala, S.; Beer, M.; Zimmer, G.; García-Sastre, A.; Schwemmle, M.; et al. A Conserved Influenza A Virus Nucleoprotein Code Controls Specific Viral Genome Packaging. Nat. Commun. 2016, 7, 12861. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Estes, M.K.; Prasad, B.V.V. Emerging Themes in Rotavirus Cell Entry, Genome Organization, Transcription and Replication. Virus Res. 2004, 101, 67–81. [Google Scholar] [CrossRef]
- Fajardo, T.; Sung, P.-Y.; Celma, C.C.; Roy, P. Rotavirus Genomic RNA Complex Forms via Specific RNA-RNA Interactions: Disruption of RNA Complex Inhibits Virus Infectivity. Viruses 2017, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, T.; Sung, P.-Y.; Roy, P. Disruption of Specific RNA-RNA Interactions in a Double-Stranded RNA Virus Inhibits Genome Packaging and Virus Infectivity. PLoS Pathog. 2015, 11, e1005321. [Google Scholar] [CrossRef]
- Sung, P.-Y.; Roy, P. Sequential Packaging of RNA Genomic Segments during the Assembly of Bluetongue Virus. Nucleic Acids Res. 2014, 42, 13824–13838. [Google Scholar] [CrossRef]
- AlShaikhahmed, K.; Leonov, G.; Sung, P.-Y.; Bingham, R.J.; Twarock, R.; Roy, P. Dynamic Network Approach for the Modelling of Genomic Sub-Complexes in Multi-Segmented Viruses. Nucleic Acids Res. 2018, 46, 12087–12098. [Google Scholar] [CrossRef] [PubMed]
- Lakdawala, S.S.; Wu, Y.; Wawrzusin, P.; Kabat, J.; Broadbent, A.J.; Lamirande, E.W.; Fodor, E.; Altan-Bonnet, N.; Shroff, H.; Subbarao, K. Influenza a Virus Assembly Intermediates Fuse in the Cytoplasm. PLoS Pathog. 2014, 10, e1003971. [Google Scholar] [CrossRef]
- Chou, Y.; Heaton, N.S.; Gao, Q.; Palese, P.; Singer, R.H.; Singer, R.; Lionnet, T. Colocalization of Different Influenza Viral RNA Segments in the Cytoplasm before Viral Budding as Shown by Single-Molecule Sensitivity FISH Analysis. PLoS Pathog. 2013, 9, e1003358. [Google Scholar] [CrossRef]
- Payne, J.L.; Wagner, A. The Causes of Evolvability and Their Evolution. Nat. Rev. Genet. 2019, 20, 24–38. [Google Scholar] [CrossRef]
- Smyth, R.P.; Negroni, M. A Step Forward Understanding HIV-1 Diversity. Retrovirology 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.M.; Nelson, M.I.; Turner, P.E.; Patton, J.T. Reassortment in Segmented RNA Viruses: Mechanisms and Outcomes. Nat. Rev. Microbiol. 2016, 14, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.P.; Schlub, T.E.; Grimm, A.J.; Waugh, C.; Ellenberg, P.; Chopra, A.; Mallal, S.; Cromer, D.; Mak, J.; Davenport, M.P. Identifying Recombination Hot Spots in the HIV-1 Genome. J. Virol. 2014, 88, 2891–2902. [Google Scholar] [CrossRef] [PubMed]
- Schlub, T.E.; Smyth, R.P.; Grimm, A.J.; Mak, J.; Davenport, M.P. Accurately Measuring Recombination between Closely Related HIV-1 Genomes. PLoS Comput. Biol. 2010, 6, e1000766. [Google Scholar] [CrossRef]
- Cromer, D.; Schlub, T.E.; Smyth, R.P.; Grimm, A.J.; Chopra, A.; Mallal, S.; Davenport, M.P.; Mak, J. HIV-1 Mutation and Recombination Rates Are Different in Macrophages and T-Cells. Viruses 2016, 8, 118. [Google Scholar] [CrossRef]
- Schlub, T.E.; Grimm, A.J.; Smyth, R.P.; Cromer, D.; Chopra, A.; Mallal, S.; Venturi, V.; Waugh, C.; Mak, J.; Davenport, M.P. Fifteen to Twenty Percent of HIV Substitution Mutations Are Associated with Recombination. J. Virol. 2014, 88, 3837–3849. [Google Scholar] [CrossRef]
- Levy, D.N.; Aldrovandi, G.M.; Kutsch, O.; Shaw, G.M. Dynamics of HIV-1 Recombination in Its Natural Target Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 4204–4209. [Google Scholar] [CrossRef]
- Chin, M.P.S.; Rhodes, T.D.; Chen, J.; Fu, W.; Hu, W.-S. Identification of a Major Restriction in HIV-1 Intersubtype Recombination. Proc. Natl. Acad. Sci. USA 2005, 102, 9002–9007. [Google Scholar] [CrossRef]
- Paillart, J.C.; Skripkin, E.; Ehresmann, B.; Ehresmann, C.; Marquet, R. A Loop-Loop “Kissing” Complex Is the Essential Part of the Dimer Linkage of Genomic HIV-1 RNA. Proc. Natl. Acad. Sci. USA 1996, 93, 5572–5577. [Google Scholar] [CrossRef]
- Hill, M.K.; Shehu-Xhilaga, M.; Campbell, S.M.; Poumbourios, P.; Crowe, S.M.; Mak, J. The Dimer Initiation Sequence Stem-Loop of Human Immunodeficiency Virus Type 1 Is Dispensable for Viral Replication in Peripheral Blood Mononuclear Cells. J. Virol. 2003, 77, 8329–8335. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Sonza, S.; Mak, J. Primary T-Lymphocytes Rescue the Replication of HIV-1 DIS RNA Mutants in Part by Facilitating Reverse Transcription. Nucleic Acids Res. 2008, 36, 1578–1588. [Google Scholar] [CrossRef]
- Cobbin, J.C.; Ong, C.; Verity, E.; Gilbertson, B.P.; Rockman, S.P.; Brown, L.E. Influenza Virus PB1 and Neuraminidase Gene Segments Can Cosegregate during Vaccine Reassortment Driven by Interactions in the PB1 Coding Region. J. Virol. 2014, 88, 8971–8980. [Google Scholar] [CrossRef]
- Essere, B.; Yver, M.; Gavazzi, C.; Terrier, O.; Isel, C.; Fournier, E.; Giroux, F.; Textoris, J.; Julien, T.; Socratous, C.; et al. Critical Role of Segment-Specific Packaging Signals in Genetic Reassortment of Influenza A Viruses. Proc. Natl. Acad. Sci. USA 2013, 110, E3840–E3848. [Google Scholar] [CrossRef]
- White, M.C.; Tao, H.; Steel, J.; Lowen, A.C. H5N8 and H7N9 Packaging Signals Constrain HA Reassortment with a Seasonal H3N2 Influenza A Virus. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Gao, Q.; Palese, P. Rewiring the RNAs of Influenza Virus to Prevent Reassortment. Proc. Natl. Acad. Sci. USA 2009, 106, 15891–15896. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Holmes, E.C. The Evolution of Epidemic Influenza. Nat. Rev. Genet. 2007, 8, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rangan, R.; Watkins, A.M.; Chacon, J.; Kretsch, R.; Kladwang, W.; Zheludev, I.N.; Townley, J.; Rynge, M.; Thain, G.; Das, R. De Novo 3D Models of SARS-CoV-2 RNA Elements from Consensus Experimental Secondary Structures. Nucleic Acids Res. 2021, 49, 3092–3108. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.R.; Scaiola, A.; Loughran, G.; Leibundgut, M.; Kratzel, A.; Meurs, R.; Dreos, R.; O’Connor, K.M.; McMillan, A.; Bode, J.W.; et al. Structural Basis of Ribosomal Frameshifting during Translation of the SARS-CoV-2 RNA Genome. Science 2021, 372, 1306–1313. [Google Scholar] [CrossRef]
- Kappel, K.; Zhang, K.; Su, Z.; Watkins, A.M.; Kladwang, W.; Li, S.; Pintilie, G.; Topkar, V.V.; Rangan, R.; Zheludev, I.N.; et al. Accelerated Cryo-EM-Guided Determination of Three-Dimensional RNA-Only Structures. Nat. Methods 2020, 17, 699–707. [Google Scholar] [CrossRef]
- Tihova, M.; Dryden, K.A.; Le, T.L.; Harvey, S.C.; Johnson, J.E.; Yeager, M.; Schneemann, A. Nodavirus Coat Protein Imposes Dodecahedral RNA Structure Independent of Nucleotide Sequence and Length. J. Virol. 2004, 78, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, E.L.; Meshcheriakova, Y.; Dent, K.C.; Saxena, P.; Thompson, R.F.; Cockburn, J.J.; Lomonossoff, G.P.; Ranson, N.A. Mechanisms of Assembly and Genome Packaging in an RNA Virus Revealed by High-Resolution Cryo-EM. Nat. Commun. 2015, 6, 10113. [Google Scholar] [CrossRef] [PubMed]
- Beren, C.; Cui, Y.; Chakravarty, A.; Yang, X.; Rao, A.L.N.; Knobler, C.M.; Zhou, Z.H.; Gelbart, W.M. Genome Organization and Interaction with Capsid Protein in a Multipartite RNA Virus. Proc. Natl. Acad. Sci. USA 2020, 117, 10673–10680. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Bostock, R.; Mata, C.P.; Bingham, R.J.; Dykeman, E.C.; Meng, B.; Tuthill, T.J.; Rowlands, D.J.; Ranson, N.A.; Twarock, R.; Stockley, P.G. Assembly of Infectious Enteroviruses Depends on Multiple, Conserved Genomic RNA-Coat Protein Contacts. PLoS Pathog. 2020, 16, e1009146. [Google Scholar] [CrossRef]
- San Emeterio, J.; Pollack, L. Visualizing a Viral Genome with Contrast Variation Small Angle X-Ray Scattering. J. Biol. Chem. 2020, 295, 15923–15932. [Google Scholar] [CrossRef]
- Lin, T.; Cavarelli, J.; Johnson, J.E. Evidence for Assembly-Dependent Folding of Protein and RNA in an Icosahedral Virus. Virology 2003, 314, 26–33. [Google Scholar] [CrossRef]
- Morandi, E.; Manfredonia, I.; Simon, L.M.; Anselmi, F.; van Hemert, M.J.; Oliviero, S.; Incarnato, D. Genome-Scale Deconvolution of RNA Structure Ensembles. Nat. Methods 2021, 18, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Tomezsko, P.J.; Corbin, V.D.A.; Gupta, P.; Swaminathan, H.; Glasgow, M.; Persad, S.; Edwards, M.D.; Mcintosh, L.; Papenfuss, A.T.; Emery, A.; et al. Determination of RNA Structural Diversity and Its Role in HIV-1 RNA Splicing. Nature 2020, 582, 438–442. [Google Scholar] [CrossRef]
- Schmidt, N.; Lareau, C.A.; Keshishian, H.; Ganskih, S.; Schneider, C.; Hennig, T.; Melanson, R.; Werner, S.; Wei, Y.; Zimmer, M.; et al. The SARS-CoV-2 RNA-Protein Interactome in Infected Human Cells. Nat. Microbiol. 2021, 6, 339–353. [Google Scholar] [CrossRef]
- Weidmann, C.A.; Mustoe, A.M.; Jariwala, P.B.; Calabrese, J.M.; Weeks, K.M. Analysis of RNA-Protein Networks with RNP-MaP Defines Functional Hubs on RNA. Nat. Biotechnol. 2020, 39, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yu, C.-C.J.; Gao, L.; Piatkevich, K.D.; Neve, R.L.; Munro, J.B.; Upadhyayula, S.; Boyden, E.S. A Highly Homogeneous Polymer Composed of Tetrahedron-like Monomers for High-Isotropy Expansion Microscopy. Nat. Nanotechnol. 2021, 16, 698–707. [Google Scholar] [CrossRef]
- Ferrer, M.; Clerté, C.; Chamontin, C.; Basyuk, E.; Lainé, S.; Hottin, J.; Bertrand, E.; Margeat, E.; Mougel, M. Imaging HIV-1 RNA Dimerization in Cells by Multicolor Super-Resolution and Fluctuation Microscopies. Nucleic Acids Res. 2016, 44, 7922–7934. [Google Scholar] [CrossRef] [PubMed]
- Vahey, M.D.; Fletcher, D.A. Low-Fidelity Assembly of Influenza A Virus Promotes Escape from Host Cells. Cell 2020, 180, 205. [Google Scholar] [CrossRef] [PubMed]
- Sardo, L.; Hatch, S.C.; Chen, J.; Nikolaitchik, O.; Burdick, R.C.; Chen, D.; Westlake, C.J.; Lockett, S.; Pathak, V.K.; Hu, W.-S. Dynamics of HIV-1 RNA Near the Plasma Membrane during Virus Assembly. J. Virol. 2015, 89, 10832–10840. [Google Scholar] [CrossRef]
- Jouvenet, N.; Bieniasz, P.D.; Simon, S.M. Imaging the Biogenesis of Individual HIV-1 Virions in Live Cells. Nature 2008, 454, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Pénzes, Z.; Wroe, C.; Brown, T.D.; Britton, P.; Cavanagh, D. Replication and Packaging of Coronavirus Infectious Bronchitis Virus Defective RNAs Lacking a Long Open Reading Frame. J. Virol. 1996, 70, 8660–8668. [Google Scholar] [CrossRef]
- Li, Q.; Tong, Y.; Xu, Y.; Niu, J.; Zhong, J. Genetic Analysis of Serum-Derived Defective Hepatitis C Virus Genomes Revealed Novel Viral Cis Elements for Virus Replication and Assembly. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Fosmire, J.A.; Hwang, K.; Makino, S. Identification and Characterization of a Coronavirus Packaging Signal. J. Virol. 1992, 66, 3522–3530. [Google Scholar] [CrossRef]
- van der Most, R.G.; Bredenbeek, P.J.; Spaan, W.J. A Domain at the 3′ End of the Polymerase Gene Is Essential for Encapsidation of CoronSavirus Defective Interfering RNAs. J. Virol. 1991, 65, 3219–3226. [Google Scholar] [CrossRef]
- Makino, S.; Yokomori, K.; Lai, M.M. Analysis of Efficiently Packaged Defective Interfering RNAs of Murine Coronavirus: Localization of a Possible RNA-Packaging Signal. J. Virol. 1990, 64, 6045–6053. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Ambi, U.B.; Olguin-Nava, M.; Gribling-Burrer, A.-S.; Ahmad, S.; Bohn, P.; Weber, M.M.; Smyth, R.P. RNA Structures and Their Role in Selective Genome Packaging. Viruses 2021, 13, 1788. https://doi.org/10.3390/v13091788
Ye L, Ambi UB, Olguin-Nava M, Gribling-Burrer A-S, Ahmad S, Bohn P, Weber MM, Smyth RP. RNA Structures and Their Role in Selective Genome Packaging. Viruses. 2021; 13(9):1788. https://doi.org/10.3390/v13091788
Chicago/Turabian StyleYe, Liqing, Uddhav B. Ambi, Marco Olguin-Nava, Anne-Sophie Gribling-Burrer, Shazeb Ahmad, Patrick Bohn, Melanie M. Weber, and Redmond P. Smyth. 2021. "RNA Structures and Their Role in Selective Genome Packaging" Viruses 13, no. 9: 1788. https://doi.org/10.3390/v13091788
APA StyleYe, L., Ambi, U. B., Olguin-Nava, M., Gribling-Burrer, A.-S., Ahmad, S., Bohn, P., Weber, M. M., & Smyth, R. P. (2021). RNA Structures and Their Role in Selective Genome Packaging. Viruses, 13(9), 1788. https://doi.org/10.3390/v13091788






