Further Characterization of Rio Grande Virus and Potential for Cross Reactivity with Rift Valley Fever Virus Assays
Abstract
:1. Introduction
2. Materials and Methods
3. Results
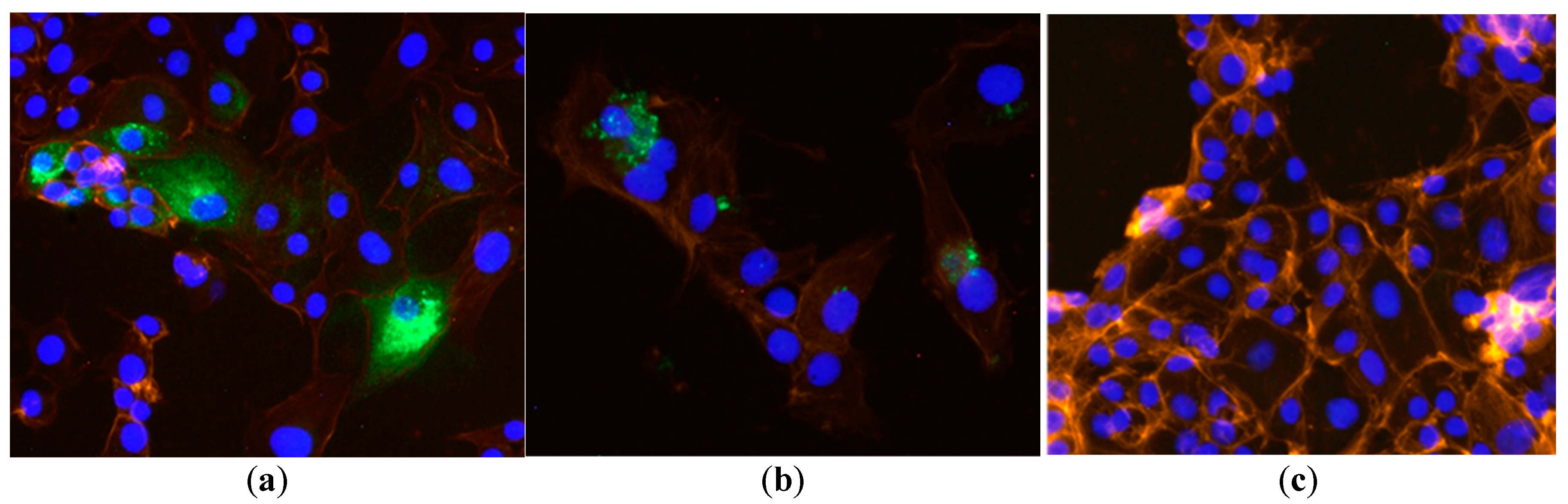
3.1. Immunocytochemistry
3.2. Protein Detection Using Western Blots
3.3. Whole-Genome Sequencing
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calisher, C.H.; McLean, R.G.; Smith, G.C.; Szmyd, D.M.; Muth, D.J.; Lazuick, J.S. Rio Grande—A new phlebotomus fever group virus from south Texas. Am. J. Trop. Med. Hyg. 1977, 26, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.G.; Szmyd, D.M.; Calisher, C.H. Experimental studies of Rio Grande virus in rodent hosts. Am. J. Trop. Med. Hyg. 1982, 31, 569–573. [Google Scholar] [CrossRef]
- Endris, R.G.; Tesh, R.B.; Young, D.G. Transovarial transmission of Rio Grande virus (Bunyaviridae: Phlebovirus) by the sand fly, Lutzomyia anthophora. Am. J. Trop. Med. Hyg. 1983, 32, 862–864. [Google Scholar] [CrossRef]
- Hughes, H.R.; Russell, B.J.; Lambert, A.J. Genetic Characterization of Frijoles and Chilibre Species Complex Viruses (Genus Phlebovirus; Family Phenuiviridae) and Three Unclassified New World Phleboviruses. Am. J. Trop. Med. Hyg. 2020, 102, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.K.; Mares, M.A. Neotoma micropus. Mamm. Species 1989, 1–9. [Google Scholar] [CrossRef]
- Herrero, M.V.; Yarnell, W.E.; Schmidtmann, E.T. Landscape associations of the sand fly, Lutzomyia (Heleocyrtomyia) apache (Diptera: Psychodidae), in the southwestern United States: A geographic information system analysis. J. Vector Ecol. 2004, 29, 205–211. [Google Scholar] [PubMed]
- Hartley, D.M.; Rinderknecht, J.L.; Nipp, T.L.; Clarke, N.P.; Snowder, G.D.; the National Center for Foreign Animal; Zoonotic Disease Defense Advisory Group on Rift Valley Fever. Potential effects of Rift Valley fever in the United States. Emerg. Infect. Dis. 2011, 17, e1. [Google Scholar] [CrossRef]
- Wilson, W.C.; Kim, I.J.; Trujillo, J.D.; Sunwoo, S.Y.; Noronha, L.E.; Urbaniak, K.; McVey, D.S.; Drolet, B.S.; Morozov, I.; Faburay, B.; et al. Susceptibility of White-Tailed Deer to Rift Valley Fever Virus. Emerg. Infect. Dis. 2018, 24, 1717–1719. [Google Scholar] [CrossRef] [Green Version]
- Tesh, R.B.; Peters, C.J.; Meegan, J.M. Studies on the antigenic relationship among phleboviruses. Am. J. Trop. Med. Hyg. 1982, 31, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.K.; Szymczak, M.S.; Burkhalter, K.L.; Miller, M.M. Laboratory Validation of the Sand Fly Fever Virus Antigen Assay. J. Am. Mosq. Control. Assoc. 2015, 31, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.M.; Bennett, K.E.; Drolet, B.S.; Lindsay, R.; Mecham, J.O.; Reeves, W.K.; Weingartl, H.M.; Wilson, W.C. Evaluation of the Efficacy, Potential for Vector Transmission, and Duration of Immunity of MP-12, an Attenuated Rift Valley Fever Virus Vaccine Candidate, in Sheep. Clin. Vaccine Immunol. 2015, 22, 930–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drolet, B.S.; Weingartl, H.M.; Jiang, J.; Neufeld, J.; Marszal, P.; Lindsay, R.; Miller, M.M.; Czub, M.; Wilson, W.C. Development and evaluation of one-step rRT-PCR and immunohistochemical methods for detection of Rift Valley fever virus in biosafety level 2 diagnostic laboratories. J. Virol. Methods 2012, 179, 373–382. [Google Scholar] [CrossRef]
- Burnette, W.N. "Western blotting": Electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, NY, USA, 1989; p. 1546. [Google Scholar]
- Boisvert, S.; Laviolette, F.; Corbeil, J. Ray: Simultaneous assembly of reads from a mix of high-throughput sequencing technologies. J. Comput. Biol. 2010, 17, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Marklewitz, M.; Dutari, L.C.; Paraskevopoulou, S.; Page, R.A.; Loaiza, J.R.; Junglen, S. Diverse novel phleboviruses in sandflies from the Panama Canal area, Central Panama. J. Gen. Virol. 2019, 100, 938–949. [Google Scholar] [CrossRef]
- Tesh, R.B. The genus Phlebovirus and its vectors. Annu. Rev. Entomol. 1988, 33, 169–181. [Google Scholar] [CrossRef]
- Paweska, J.T.; Smith, S.J.; Wright, I.M.; Williams, R.; Cohen, A.S.; Van Dijk, A.A.; Grobbelaar, A.A.; Croft, J.E.; Swanepoel, R.; Gerdes, G.H. Indirect enzyme-linked immunosorbent assay for the detection of antibody against Rift Valley fever virus in domestic and wild ruminant sera. Onderstepoort J. Vet. Res. 2003, 70, 49–64. [Google Scholar]
- Paweska, J.T.; Mortimer, E.; Leman, P.A.; Swanepoel, R. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J. Virol. Methods. 2005, 127, 10–18. [Google Scholar] [CrossRef]
- Yu, F.; Adungo, F.; Konongoi, S.L.; Inoue, S.; Sang, R.; Ashur, S.; Kwallah, A.O.; Uchida, L.; Buerano, C.C.; Mwau, M.; et al. Comparison of enzyme-linked immunosorbent assay systems using rift valley fever virus nucleocapsid protein and inactivated virus as antigens. Virol. J. 2018, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Wilson, W.C.; Secka, A.; Drolet, B.; McVey, D.S.; Richt, J.A. Evaluation of an Indirect Enzyme-Linked Immunosorbent Assay Based on Recombinant Baculovirus-Expressed Rift Valley Fever Virus Nucleoprotein as the Diagnostic Antigen. J. Clin. Microbiol. 2019, 57, e01058-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragan, I.K.; Davis, A.S.; McVey, D.S.; Richt, J.A.; Rowland, R.R.; Wilson, W.C. Evaluation of Fluorescence Microsphere Immunoassay for Detection of Antibodies to Rift Valley Fever Virus Nucleocapsid Protein and Glycoproteins. J. Clin. Microbiol. 2018, 56, e01626-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.G.; Perkins, P.V. Phlebotomine sand flies of North America (Diptera: Psychodidae). Mosq. News 1984, 44, 42. [Google Scholar]
- Konrad, S.K.; Miller, S.N.; Reeves, W.K. A spatially explicit degree-day model of Rift Valley fever transmission risk in the continental United States. GeoJournal 2011, 76, 257–266. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, M.S.; Reeves, W.K.; Miller, M.M. Further Characterization of Rio Grande Virus and Potential for Cross Reactivity with Rift Valley Fever Virus Assays. Viruses 2021, 13, 1719. https://doi.org/10.3390/v13091719
Szymczak MS, Reeves WK, Miller MM. Further Characterization of Rio Grande Virus and Potential for Cross Reactivity with Rift Valley Fever Virus Assays. Viruses. 2021; 13(9):1719. https://doi.org/10.3390/v13091719
Chicago/Turabian StyleSzymczak, Mitchell S., Will K. Reeves, and Myrna M. Miller. 2021. "Further Characterization of Rio Grande Virus and Potential for Cross Reactivity with Rift Valley Fever Virus Assays" Viruses 13, no. 9: 1719. https://doi.org/10.3390/v13091719
APA StyleSzymczak, M. S., Reeves, W. K., & Miller, M. M. (2021). Further Characterization of Rio Grande Virus and Potential for Cross Reactivity with Rift Valley Fever Virus Assays. Viruses, 13(9), 1719. https://doi.org/10.3390/v13091719




